Metabolic Profiles of Pomegranate Juices during Fruit Development and the Redirection of Flavonoid Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Determination of Chemical Parameters of Pomegranate Juices
2.3. Analysis of Metabolome and Transcriptome
2.4. Statistical Analysis
3. Results
3.1. Chemical Parameters of Pomegranate Juices during Fruit Development
3.2. Metabolic Profiling of Pomegranate Juices during Fruit Development
3.3. Multivariate Analysis of Metabolic Profiling of Pomegranate Juices during Fruit Development
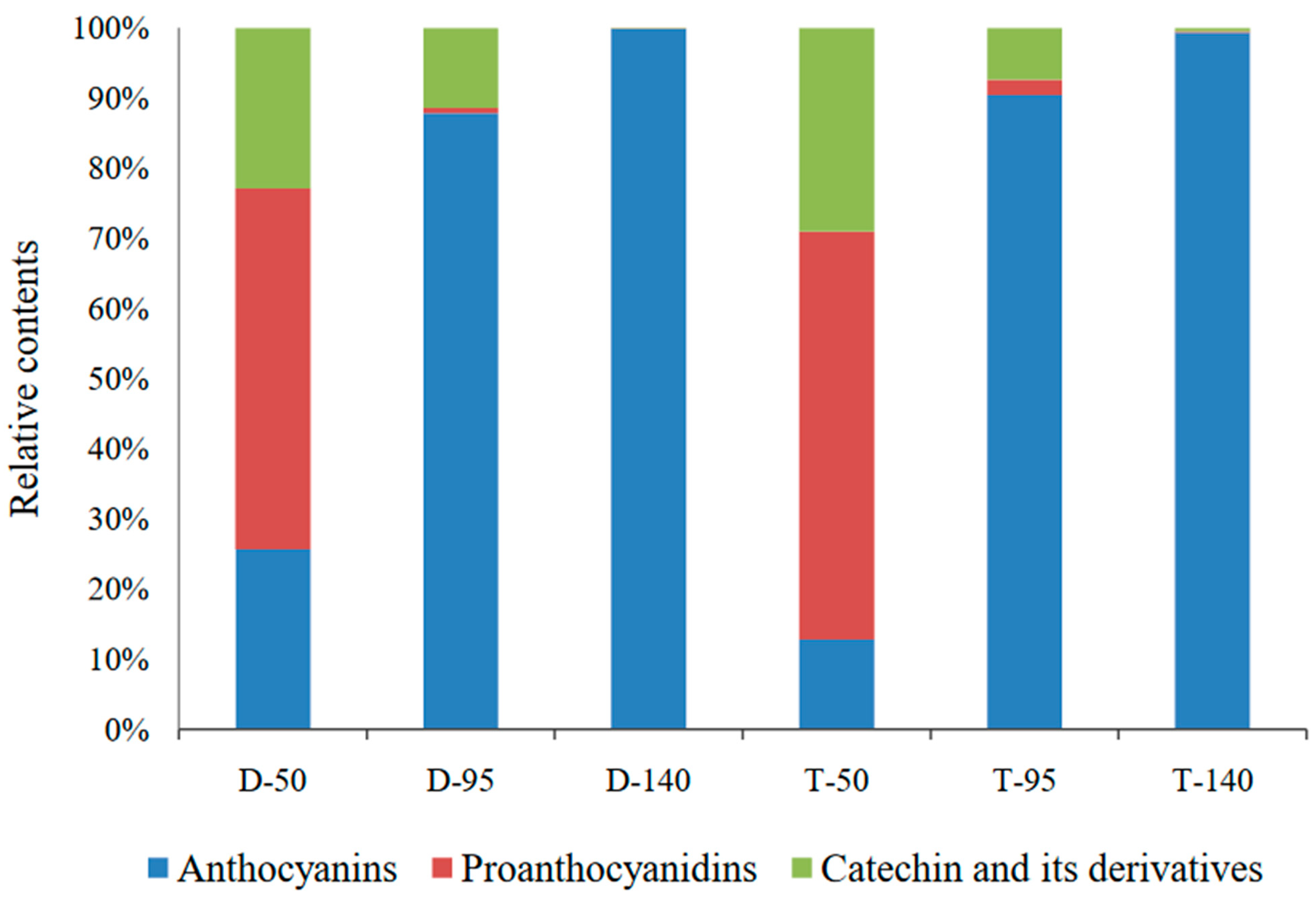
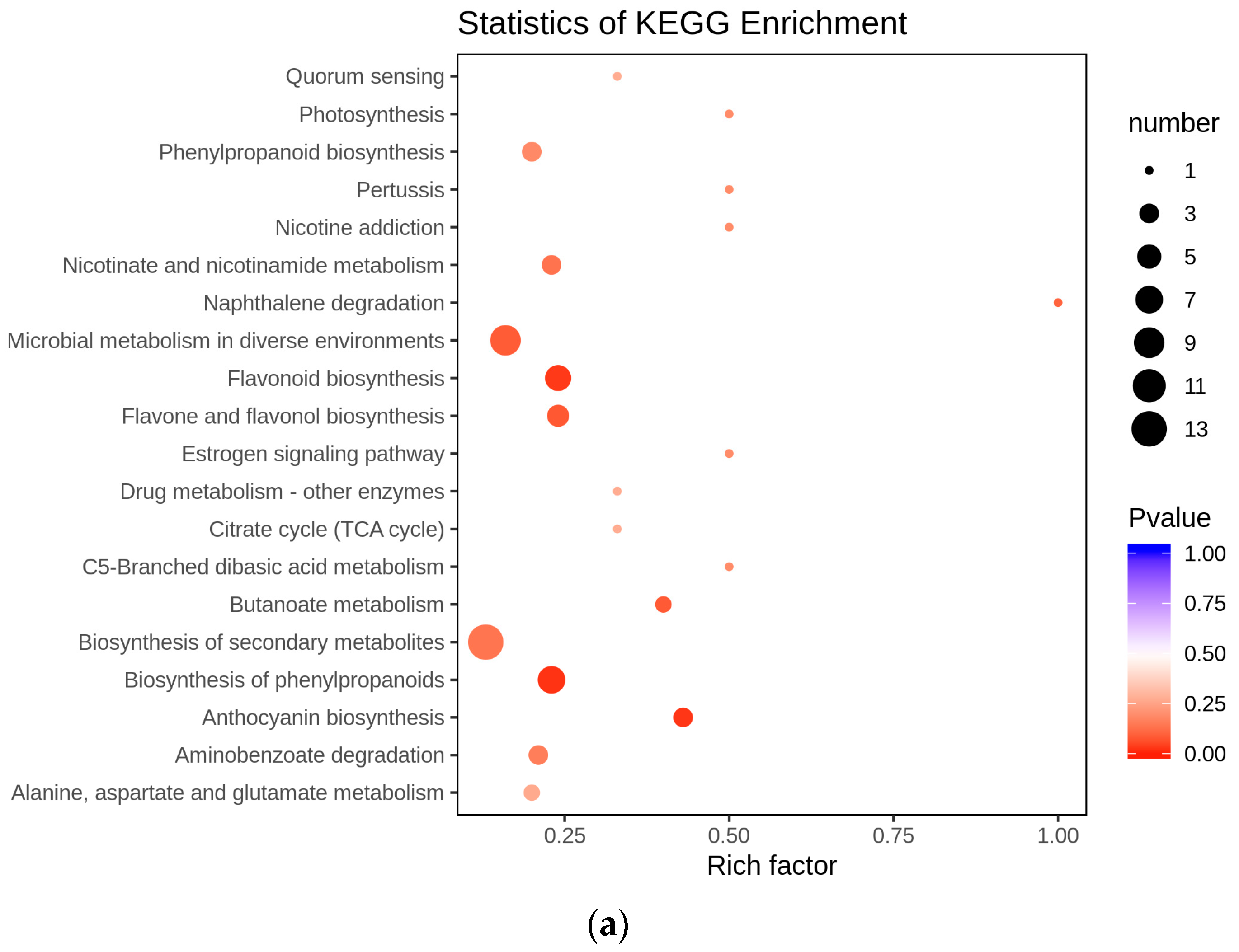
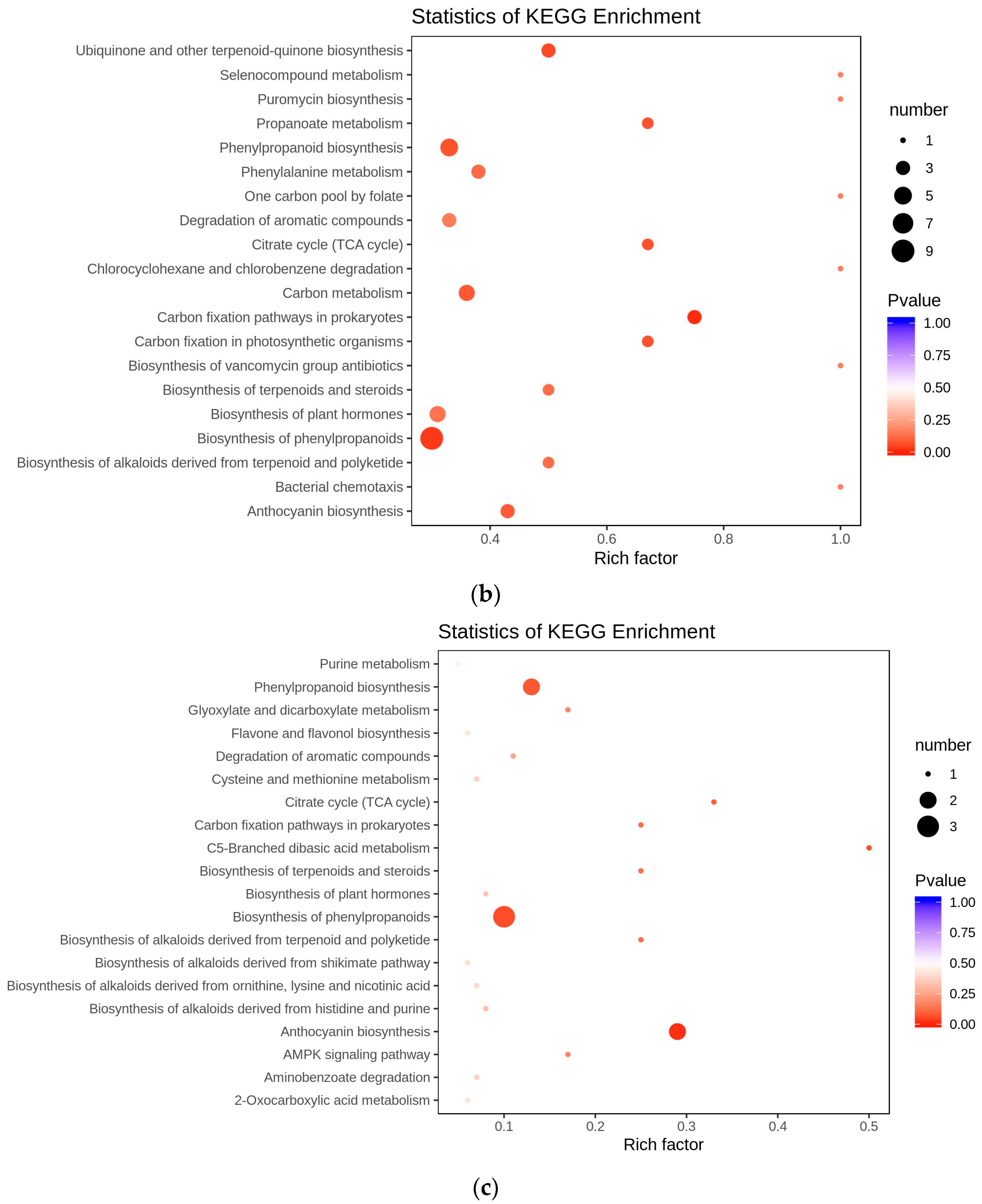
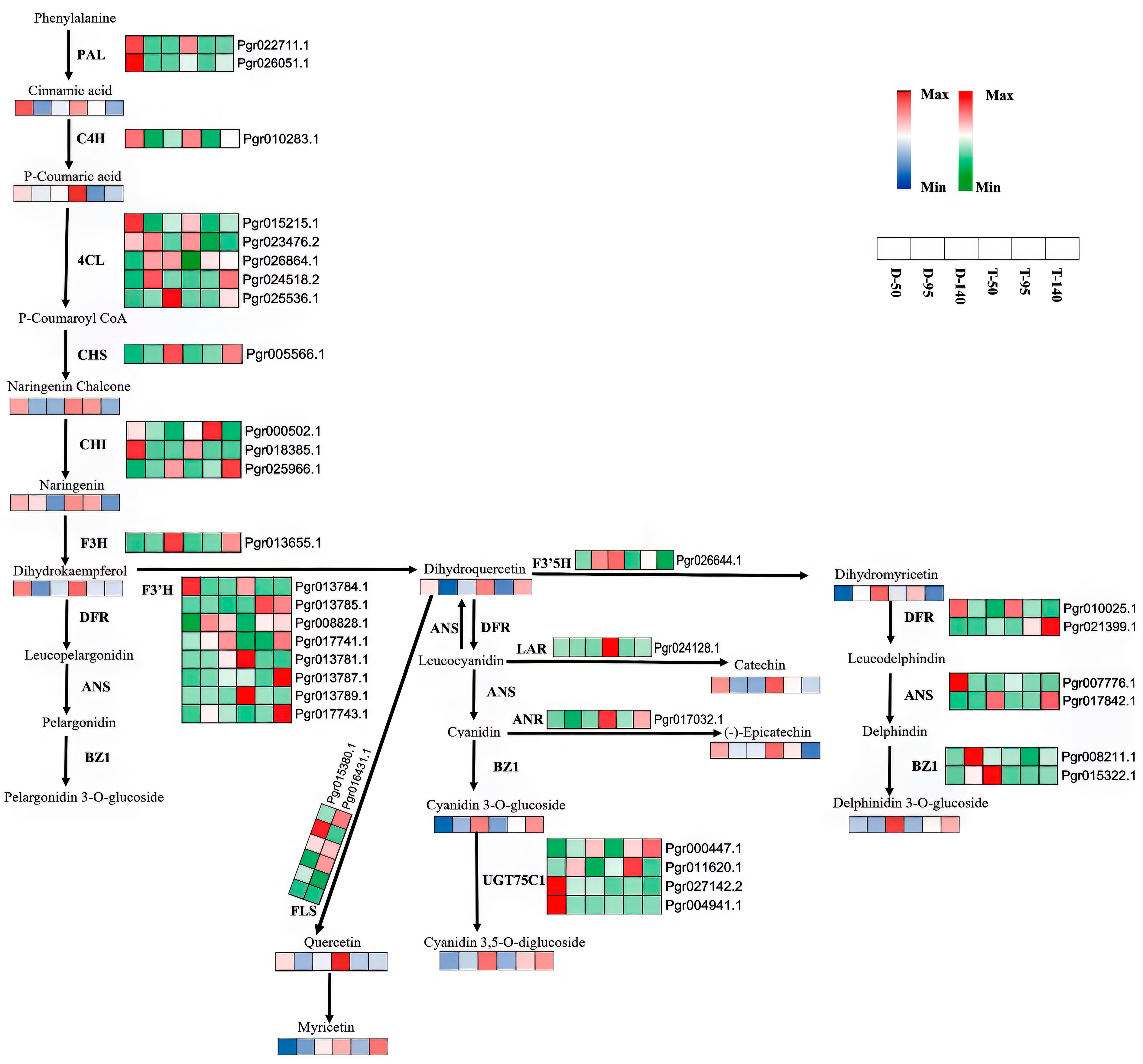
3.4. Redirection of Flavonoid Metabolism and the Underlying Genetic Mechanism
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, G.H.; Xu, C.Y.; Ming, R.; Tang, H.B.; Guyot, R.; Kramer, E.M.; Hu, Y.D.; Yi, X.K.; Qi, Y.J.; Xu, Y.L.; et al. The pomegranate (Punica granatum L.) genome and the genomics of punicalagin biosynthesis. Plant J. 2017, 91, 1108–1128. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Erim, F.B. Total phenolic contents, antioxidant activities, and bioactive ingredients of juices from pomegranate cultivars worldwide. Food Chem. 2017, 221, 496–507. [Google Scholar] [CrossRef]
- Wetzstein, H.Y.; Zhang, Z.B.; Ravid, N.; Wetzstein, M.E. Characterization of attributes related to fruit size in pomegranate. HortScience 2011, 46, 908–912. [Google Scholar] [CrossRef]
- Turrini, E.; Ferruzzi, L.; Fimoǵnari, C. Potential effects of pomegranate polyphenols in cancer prevention and therapy. Oxidative Med. Cell. Longev. 2015, 2015, 938475. [Google Scholar] [CrossRef]
- Asgary, S.; Keshvari, M.; Sahebkar, A.; Sarrafzadegan, N. Pomegranate consumption and blood pressure: A review. Curr. Pharm. Design 2017, 23, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Sumner, M.D.; Elliott-Eller, M.; Weidner, G.; Daubenmier, J.J.; Chew, M.H.; Marlin, R.; Raisin, C.J.; Ornish, D. Effects of pomegranate juice consumption on myocardial perfusion in patients with coronary heart disease. Am. J. Cardiol. 2005, 96, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.R.; Kang, S.J.; Kim, J.L.; Lee, Y.J.; Ku, S.K. Effects of pomegranate concentrate powder: Eucommiae cortex: Achyranthis radix 5:4:1 (w/w) mixed formula on monosodium iodoacetate-induced osteoarthritis in rats. Nat. Prod. Commun. 2020, 15, 1–18. [Google Scholar] [CrossRef]
- Kim, H.; Banerjee, N.; Sirven, M.A.; Minamoto, Y.; Markel, M.E.; Suchodolski, J.S.; Talcott, S.T.; Mertens-Talcott, S.U. Pomegranate polyphenolics reduce inflammation and ulceration in intestinal colitis-involvement of the miR-145/p70S6K1/HIF1α axis in vivo and in vitro. J. Nutr. Biochem. 2017, 43, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, Z.; Ferrante, M.; Tadi, M.; Ansari, F.; Heydari, A.; Hosseini, M.S.; Conti, G.O.; Sadrabad, E.K. Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food Chem. Toxicol. 2018, 114, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Lamar, A.; Fonseca, G.; Fuentes, J.L.; Cozzi, R.; Cundari, E.; Fiore, M.; Ricordy, R.; Perticone, P.; Degrassi, F.; Salvia, R.D. Assessment of the genotoxic risk of Punica granatum L.(Punicaceae) whole fruit extracts. J. Ethnopharmacol. 2008, 115, 416–422. [Google Scholar] [CrossRef]
- Glevitzky, I.; Dumitrel, G.A.; Mirel, G.; Pasca, B.; Otřísal, P.; Bungau, G.; Cioca, G.; Pantis, C.; Popa, M. Statistical analysis of the relationship between antioxidant activity and the structure of flavonoid compounds. Rev. Chim. 2019, 70, 3104–3107. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Qin, G.H.; Liu, C.Y.; Li, J.Y.; Qi, Y.J.; Gao, Z.H.; Zhang, X.L.; Yi, X.K.; Pan, H.F.; Ming, R.; Xu, Y.L. Diversity of metabolite accumulation patterns in inner and outer seed coats of pomegranate: Exploring their relationship with genetic mechanisms of seed coat development. Hortic. Res. 2020, 7, 10. [Google Scholar] [CrossRef]
- Li, X.X.; Li, J.Z. Determination of the content of soluble sugar in sweet corn with optimized anthrone colorimetric method. Storage Process 2013, 13, 24–27. [Google Scholar] [CrossRef]
- Ranjbari, F.; Moradinezhad, F.; Khayyat, M. Effect of nitric oxide on biochemical and antioxidant properties of pomegranate fruit cv. Shishe-kab during cold storage. Int. J. Hortic. Sci. Technol. 2016, 3, 211–219. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Pilerood, S.A.; Prakash, J. Evaluation of nutritional composition and antioxidant activity of Borage (Echium amoenum) and Valerian (Valerian officinalis). J. Food Sci. Technol. 2014, 51, 845–854. [Google Scholar] [CrossRef]
- Lee, H.S.; Wicker, L. Anthocyanin pigments in the skin of lychee fruit. J. Food Sci. 1991, 56, 466–468. [Google Scholar] [CrossRef]
- Albertini, M.V.; Carcouet, E.; Pailly, O.; Gambotti, C.; Luro, F.; Berti, L. Changes in organic acids and sugars during early stages of development of acidic and acidless citrus fruit. J. Agric. Food Chem. 2006, 54, 8335–8339. [Google Scholar] [CrossRef]
- Horbowicz, M.; Kosson, R.; Grzesiuk, A.; Debski, H. Anthocyanins of fruit and vegetables -their occurrence, analysis and role in human nutrition. J. Fruit Ornam. Plant Res. 2008, 68, 5–22. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.A. Pomegranate and its manyfunctional components as related to human health: A review. Compr. Rev. Food Sci. 2010, 9, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Yaich, H.; Cheikhrouhou, S.; Khemakhem, I.; Bouaziz, M.; Attia, H.; Ayadi, M.A. Antioxidant properties and phenolic profile characterization by LC-MS/MS of selected Tunisian pomegranate peels. J. Food Sci. Technol. 2017, 54, 2890–2901. [Google Scholar] [CrossRef]
- Bar-Ya’akov, I.; Tian, L.; Amir, R.; Holland, D. Primary metabolites, anthocyanins, and hydrolyzable tannins in the pomegranate fruit. Front. Plant Sci. 2019, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Segers, K.; Declerck, S.; Mangelings, D.; Heyden, Y.V.; Eeckhaut, A.V. Analytical techniques for metabolomic studies: A review. Bioanalysis 2019, 11, 2297–2318. [Google Scholar] [CrossRef] [PubMed]
- Antunes, A.C.; Acunha, T.D.S.; Perin, E.C.; Rombaldi, C.V.; Galli, V.; Chaves, F.C. Untargeted metabolomics of strawberry (Fragaria x ananassa ‘Camarosa’) fruit from plants grown under osmotic stress conditions. J. Sci. Food Agric. 2019, 99, 6973–6980. [Google Scholar] [CrossRef]
- Lim, V.; Gorji, S.G.; Daygon, V.D.; Fitzgerald, M. Untargeted and targeted metabolomics profiling of Australian indigenous fruits. Metabolites 2020, 10, 114. [Google Scholar] [CrossRef]
- Bao, L.J.; Gao, H.P.; Zheng, Z.L.; Zhao, X.X.; Zhang, M.J.; Jiao, F.; Su, C.; Qian, Y.H. Integrated transcriptomic and un-targeted metabolomics analysis reveals mulberry fruit (Morus atropurpurea) in response to sclerotiniose pathogen ciboria shiraiana infection. Int. J. Mol. Sci. 2020, 21, 1789. [Google Scholar] [CrossRef]
- Dasenaki, M.E.; Drakopoulou, S.K.; Aalizadeh, R.; Thomaidis, N.S. Targeted and untargeted metabolomics as an enhanced tool for the detection of pomegranate juice adulteration. Foods 2019, 8, 212. [Google Scholar] [CrossRef]
- He, Q.; Xue, Y.H.; Wang, Y.X.; Zhang, N.N.; Zhang, L.G. Metabolic profiling and transcriptomic data providing critical flavonoid biosynthesis mechanisms disclose color differences of purple heading Chinese cabbages (Brassica rapa L.). LWT-Food Sci. Technol. 2022, 168, 113885. [Google Scholar] [CrossRef]
- Liang, Y.Q.; Han, C.Z.; Yun, L.; Yang, Y.; Cao, Y.P. Transcriptomic and metabolomic analysis of the mechanism of temperature-regulated anthocyanin biosynthesis in purple asparagus spears. Sci. Hortic. 2022, 295, 110858. [Google Scholar] [CrossRef]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Wang, B.; He, G.; Wang, Y.; Tang, X.F.; Wang, S.M.; Ma, Y.B.; Fu, C.X.; Chai, G.H.; Zhou, G.K. Metabolomics integrated with transcriptomics reveals redirection of the phenylpropanoids metabolic flux in Ginkgo biloba. J. Agric. Food Chem. 2019, 67, 3284–3291. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.Y.; Lei, Y.; Yan, L.Y.; Liu, Y.; Pandey, M.K.; Wan, X.; Varshney, R.K.; Fang, J.H.; Liao, B.S. Transcriptome and metabolome reveal redirection of flavonoids in a white testa peanut mutant. BMC Plant Biol. 2020, 20, 161. [Google Scholar] [CrossRef]
- Fukusaki, E.; Kawasaki, K.; Kajiyama, S.; An, C.; Suzuki, K.; Tanaka, Y.; Kobayashi, A. Flower color modulations of Torenia hybrida by downregulation of chalcone synthase genes with RNA interference. J. Biotechnol. 2004, 111, 229–240. [Google Scholar] [CrossRef]
- Ben-Simhon, Z.; Judeinstein, S.; Trainin, T.; Harel-Beja, R.; Bar-Ya’akov, I.; Borochov-Neori, H.; Holland, D. A “White” anthocyanin-less pomegranate (Punica granatum L.) caused by an insertion in the coding region of the leucoanthocyanidin dioxygenase (LDOX; ANS) Gene. PLoS ONE 2015, 10, e0142777. [Google Scholar] [CrossRef]
- Jiao, F.C.; Zhao, L.; Wu, X.F.; Song, Z.B.; Li, Y.P. Metabolome and transcriptome analyses of the molecular mechanisms of flower color mutation in tobacco. BMC Genom. 2020, 21, 611. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, Z.; Maximova, S.; Payne, M.J.; Guiltinan, M.J. Proanthocyanidin synthesis in Theobroma cacao: Genes encoding anthocyanidin synthase, anthocyanidin reductase, and leucoanthocyanidin reductase. BMC Plant Biol. 2013, 13, 202. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.S.; Cui, L.; Li, Y.B.; Liang, Y.H.; Wang, S.S.; Chen, Y.B.; Zhou, L.; Zhang, Y.B.; Li, F. Transcriptome and metabolome analyses reveal anthocyanins pathways associated with fruit color changes in plum (Prunus salicina Lindl. ) Peer J. 2022, 10, e14413. [Google Scholar] [CrossRef]


| P.granatum ‘Dabenzi’ | P. granatum ‘Tunisia’ | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabolite Name | 35 d | 50 d | 70 d | 95 d | 105 d | 120 d | 140 d | 35 d | 50 d | 70 d | 95 d | 105 d | 120 d | 140 d |
| Soluble sugar (μg/mL) | 8.21 ± 0.34 | 10.68 ± 0.05 | 12.28 ± 0.02 | 13.45 ± 0.51 | 16.26 ± 0.89 | 18.50 ± 0.19 | 20.19 ± 0.68 | 7.27 ± 0.96 | 9.52 ± 0.51 | 13.26 ± 1.24 | 15.62 ± 0.53 | 16.86 ± 0.69 | 18.62 ± 0.22 | 19.37 ± 0.66 |
| Total titratable acidity (%) | 0.65 ± 0.02 | 0.61 ± 0.03 | 0.62 ± 0.01 | 0.47 ± 0.35 | 0.47 ± 0.01 | 0.47 ± 0.01 | 0.40 ± 0.02 | 0.48 ± 0.02 | 0.38 ± 0.01 | 0.29 ± 0.02 | 0.28 ± 0.01 | 0.27 ± 0.03 | 0.27 ± 0.03 | 0.27 ± 0.03 |
| Total anthocyanin (mg/L) | 5.33 ± 0.17 | 10.10 ± 0.01 | 16.70 ± 1.21 | 39.07 ± 5.40 | 104.03 ± 2.90 | 138.87 ± 10.82 | 173.60 ± 7.46 | 5.33 ± 0.16 | 48.40 ± 7.00 | 106.40 ± 7.41 | 168.27 ± 1.53 | 167.17 ± 2.85 | 182.50 ± 5.38 | 195.17 ± 1.25 |
| Total phenolics (mg/mL) | 5.81 ± 0.35 | 5.13 ± 0.32 | 4.21 ± 0.07 | 2.55 ± 0.03 | 2.37 ± 0.04 | 2.18 ± 0.03 | 2.02 ± 0.11 | 3.66 ± 0.11 | 2.60 ± 0.04 | 2.16 ± 0.04 | 1.82 ± 0.04 | 1.47 ± 0.04 | 1.34 ± 0.06 | 1.51 ± 0.04 |
| Tannins (mg/mL) | 5.20 ± 0.08 | 5.14 ± 0.09 | 5.33 ± 0.03 | 3.03 ± 0.07 | 2.84 ± 0.02 | 2.58 ± 0.06 | 2.41 ± 0.02 | 5.09 ± 0.08 | 3.63 ± 0.09 | 3.35 ± 0.03 | 2.56 ± 0.07 | 2.41 ± 0.02 | 1.57 ± 0.06 | 1.23 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Qi, X.; Li, J.; Cao, Z.; Liu, X.; Yu, Q.; Xu, Y.; Qin, G. Metabolic Profiles of Pomegranate Juices during Fruit Development and the Redirection of Flavonoid Metabolism. Horticulturae 2023, 9, 881. https://doi.org/10.3390/horticulturae9080881
Zhao J, Qi X, Li J, Cao Z, Liu X, Yu Q, Xu Y, Qin G. Metabolic Profiles of Pomegranate Juices during Fruit Development and the Redirection of Flavonoid Metabolism. Horticulturae. 2023; 9(8):881. https://doi.org/10.3390/horticulturae9080881
Chicago/Turabian StyleZhao, Jianrong, Xiaoxiao Qi, Jiyu Li, Zhen Cao, Xin Liu, Qing Yu, Yiliu Xu, and Gaihua Qin. 2023. "Metabolic Profiles of Pomegranate Juices during Fruit Development and the Redirection of Flavonoid Metabolism" Horticulturae 9, no. 8: 881. https://doi.org/10.3390/horticulturae9080881
APA StyleZhao, J., Qi, X., Li, J., Cao, Z., Liu, X., Yu, Q., Xu, Y., & Qin, G. (2023). Metabolic Profiles of Pomegranate Juices during Fruit Development and the Redirection of Flavonoid Metabolism. Horticulturae, 9(8), 881. https://doi.org/10.3390/horticulturae9080881







