Changes in Antioxidant and Color Properties of Raisins According to Variety and Drying Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Pretreatment Solution Preparation and Treatment
2.3. Measurement of Raisin Weight and Physicochemicals
2.4. Total Phenolics and DPPH Scavenging Assay
2.5. Soluble Protein and Total Monomeric Anthocyanin Assays
2.6. Colorimetric Properties and Color Density Determination
2.7. Statistical Evaluations
3. Results
3.1. Raisin Weight and Physicochemicals
3.2. Total Phenolics and DPPH Scavenging
3.3. Changes in Protein and Anthocyanin Content
3.4. Changes in Raisin Color Properties
3.5. Statistical Approaches
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Güler, E. Polyphenols, organic acids, and their relationships in red grapes of Vitis vinifera and Isabella (Vitis labrusca) under arid conditions. Eur. Food Res. Technol. 2022, 249, 913–921. [Google Scholar] [CrossRef]
- Güler, E. Current status of grape production and trade in Turkey and the world: A data analysis. In Proceedings of the Conferința “Ştiinţă, Educaţie, Cultură” Conferinţă ştiinţifico-Practică Internaţională, Comrat, Moldova, 12 February 2021; Volume 1, pp. 269–272. [Google Scholar]
- Sério, S.; Rivero-Pérez, M.D.; Correia, A.C.; Jordão, A.M.; González-SanJosé, M.L. Analysis of commercial grape raisins: Phenolic content, antioxidant capacity and radical scavenger activity. Ciência Téchnica Vitivinícola 2014, 29, 1–8. [Google Scholar] [CrossRef]
- Keskin, N.; Kaya, O.; Ates, F.; Turan, M.; Gutiérrez-Gamboa, G. Drying Grapes after the Application of Different Dipping Solutions: Effects on Hormones, Minerals, Vitamins, and Antioxidant Enzymes in Gök Üzüm (Vitis vinifera L.) Raisins. Plants 2022, 11, 529. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.; Peinado, R.A.; Medina, M.; Moreno, J. Off-Vine Grape Drying Effect on Volatile Compounds and Aromatic Series in Must from Pedro Ximénez Grape Variety. J. Agric. Food Chem. 2004, 52, 3905–3910. [Google Scholar] [CrossRef]
- Panagopoulou, E.A.; Chiou, A.; Nikolidaki, E.K.; Christea, M.; Karathanos, V.T. Corinthian raisins (Vitis vinifera L., var. Apyrena) antioxidant and sugar content as affected by the drying process: A 3 year study. J. Sci. Food Agric. 2019, 99, 915–922. [Google Scholar] [CrossRef]
- Esmaiili, M.; Sotudeh-Gharebagh, R.; Cronin, K.; Mousavi, M.A.E.; Rezazadeh, G. Grape Drying: A Review. Food Rev. Int. 2007, 23, 257–280. [Google Scholar] [CrossRef]
- Guler, A.; Candemir, A. Çekirdeksiz Kuru Üzüm Üretiminde Alternatif Çeşitler ve Kuru Üzüm Karakteristiklerinin Belirlenmesi. Selcuk. Tarım Gıda Bilim. Derg. A 2013, 27, 348–358. [Google Scholar]
- Dong, Y.; Duan, S.; Xia, Q.; Liang, Z.; Dong, X.; Margaryan, K.; Musayev, M.; Goryslavets, S.; Zdunić, G.; Bert, P.-F.; et al. Dual domestications and origin of traits in grapevine evolution. Science 2023, 379, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Carughi, A. Polyphenol content and health benefits of raisins. Nutr. Res. 2010, 30, 511–519. [Google Scholar] [CrossRef]
- Patidar, A.; Vishwakarma, S.; Meena, D. Traditional and recent development of pretreatment and drying process of grapes during raisin production: A review of novel pretreatment and drying methods of grapes. Food Front. 2021, 2, 46–61. [Google Scholar] [CrossRef]
- Imlak, M.; Randhawa, M.A.; Hassan, A.; Ahmad, N.; Nadeem, M. Post-harvest Shelf Life Extension and Nutritional Profile of Thompson Seedless Table Grapes Under Calcium Chloride and Modified Atmospheric Storage. J. Food Process. Technol. 2017, 7, 648. [Google Scholar] [CrossRef]
- De Simone, N.; Pace, B.; Grieco, F.; Chimienti, M.; Tyibilika, V.; Santoro, V.; Capozzi, V.; Colelli, C.; Spano, G.; Russo, P. Botrytis cinerea and table grapes: A review of the main physical, chemical, and bio-based control treatments in post-harvest. Foods 2020, 9, 1138. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kalita, P. Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Gundogdu, M.; Güler, E.; Ağlar, E.; Arslan, T.; Kan, T.; Çelik, K. Use of spermidine to preserve organic acids, polyphenols, and quality of cold stored plum fruits. J. Food Compos. Anal. 2023, 121, 105411. [Google Scholar] [CrossRef]
- Yilmaz, A.; Yildirim, E.; Yilmaz, H.; Soydemir, H.E.; Güler, E.; Ciftci, V.; Yaman, M. Use of Arbuscular Mycorrhizal Fungi for Boosting Antioxidant Enzyme Metabolism and Mitigating Saline Stress in Sweet Basil (Ocimum basilicum L.). Sustainability 2023, 15, 5982. [Google Scholar] [CrossRef]
- Güler, E.; Karadeniz, T.; Özer, G.; Uysal, T. Diversity and association mapping assessment of an untouched native grapevine genetic resource by iPBS retrotransposon markers. Genet. Resour. Crop. Evol. 2023; in press. [Google Scholar] [CrossRef]
- Guler, A.; Candemir, A.; Ozaltin, K.E.; Asiklar, F.B.; Saygac, S. Determination of Biochemical Characteristics, Antioxidant Activities, and Individual Phenolic Compounds of 13 Native Turkish Grape Juices. Erwerbs-Obstbau 2022, 64 (Suppl. 1), 25–35. [Google Scholar] [CrossRef]
- Keskin, N.; Kunter, B.; Celik, H.; Kaya, O.; Keskin, S. Evaluation of Clonal Variability of Berry Phenolics in Vitis vinifera L. Cv. Kalecik Karası. Erwerbs-Obstbau 2022, 64 (Suppl. 1), 65–72. [Google Scholar] [CrossRef]
- Kısaca, G.; Sensoy, R.I.G. Phenolic contents, organicacids and antioxidant capacities of twenty grape (Vitis vinifera L.) cultivars having different berry colors. J. Food Meas. Charact. 2022, 17, 1354–1370. [Google Scholar] [CrossRef]
- Wang, J.; Mujumdar, A.S.; Wang, H.; Fang, X.-M.; Xiao, H.-W.; Raghavan, V. Effect of drying method and cultivar on sensory attributes, textural profiles, and volatile characteristics of grape raisins. Dry. Technol. 2020, 39, 495–506. [Google Scholar] [CrossRef]
- Candemir, A.; Koç, G.; Dirim, S.N.; Pandiselvam, R. Effect of ultrasound pretreatment and drying air temperature on the drying characteristics, physicochemical properties, and rehydration capacity of raisins. Biomass Convers. Biorefinery 2023, 1–13. [Google Scholar] [CrossRef]
- Ortega-Regules, A.; Ros-García, J.M.; Bautista-Ortín, A.B.; López-Roca, J.M.; Gómez-Plaza, E. Changes in skin cell wall com-position during the maturation of four premium wine grape varieties. J. Sci. Food Agric. 2008, 88, 420–428. [Google Scholar] [CrossRef]
- Keller, M. The Science of Grapevines: Anatomy and Physiology; Macmillan Publishers Ltd.: London, UK, 2010; Volume 7, ISBN 9789968663038. [Google Scholar]
- Abuajah, C.I.; Ogbonna, A.C.; Osuji, C.M. Functional components and medicinal properties of food: A review. J. Food Sci. Technol. 2015, 52, 2522–2529. [Google Scholar] [CrossRef]
- Papadaki, A.; Kachrimanidou, V.; Lappa, I.K.; Eriotou, E.; Sidirokastritis, N.; Kampioti, A.; Kopsahelis, N. Medi-terranean raisins/currants as traditional superfoods: Processing, health benefits, food applications and future trends within the bio-economy era. Appl. Sci. 2021, 11, 1605. [Google Scholar] [CrossRef]
- Breksa, A.P.; Takeoka, G.R.; Hidalgo, M.B.; Vilches, A.; Vasse, J.; Ramming, D.W. Antioxidant activity and phenolic content of 16 raisin grape (Vitis vinifera L.) cultivars and selections. Food Chem. 2010, 121, 740–745. [Google Scholar] [CrossRef]
- Güzel, D.U.; Doğan, A. Erciş (Van) Yöresinde Üzüm (Vitis spp.) Yetiştirmeye Uygun Potansiyel Alanların Coğrafi Bilgi Sistemleri (CBS) Teknikleri Kullanılarak İklim, Toprak ve Topoğrafya Faktörlerine Göre Belirlenmesi. Yuz. Yıl Univ. J. Agric. Sci. 2020, 30, 672–687. [Google Scholar] [CrossRef]
- Coelho, M.; Pereira, R.; Rodrigues, A.; Teixeira, J.; Pintado, M. The use of emergent technologies to extract added value compounds from grape by-products. Trends Food Sci. Technol. 2020, 106, 182–197. [Google Scholar] [CrossRef]
- Akdeniz, B. Geleneksel usullerde sultani çekirdeksiz üzüm çeşidinin kurutulması. Gıda Teknol. Elektron. Derg. 2011, 6, 13–22. [Google Scholar]
- Unal, M.S.; Saglam, H.; Kirkaya, H. Şırnak ili İdil ilçesinde yetiştirilen mahalli üzüm çeşitlerinin değerlendirilme şekilleri. Bahri Dagdas Bitkisel Arastırma Derg 2019, 8, 159–162. [Google Scholar]
- Uzun, T.; Hallac, B.; Altin, A.; Kaya, G. Midyat/Mardin ve Beşiri/Batman İlçelerinde Satışa Sunulan Bazı Kuru Üzüm Çeşitlerinin Fizikokimyasal Özelliklerinin Karşılaştırıl-ması. Turk. J. Agric. Eng. Res. 2020, 1, 404–414. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of total phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1-1. [Google Scholar]
- Kruger, N.J. The Bradford method for protein quantitation. In The Protein Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 17–24. [Google Scholar]
- Fuleki, T.; Francis, F.J. Quantitative methods for anthocyanins. 2. Determination of total anthocyanin and degra-dation index for cranberry juice. J. Food Sci. 1968, 33, 78–83. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Winkler, A.J. General Viticulture; University of California Press: Berkeley, CA, USA, 1974. [Google Scholar]
- Kok, D.; Celik, S. Determination of Characteristics of Grape Berry Skin in Some Table GrapeCultivars (V. vinifera L.). J. Agron. 2004, 3, 141–146. [Google Scholar]
- Ohlhorst, S.D.; Russell, R.; Bier, D.; Klurfeld, D.M.; Li, Z.; Mein, J.R.; Milner, J.; Ross, A.C.; Stover, P.; Konopka, E. Nutrition research to affect food and a healthy lifespan. Adv. Nutr. Int. Rev. J. 2013, 4, 579–584. [Google Scholar] [CrossRef]
- Shangguan, S.; Afshin, A.; Shulkin, M.; Ma, W.; Marsden, D.; Smith, J.; Saheb-Kashaf, M.; Shi, P.; Micha, R.; Imamura, F.; et al. A Meta-Analysis of Food Labeling Effects on Consumer Diet Behaviors and Industry Practices. Am. J. Prev. Med. 2018, 56, 300–314. [Google Scholar] [CrossRef]
- Unal, M.S. Effects of Pretreatment Solutions on Drying Yield and Bioactive Compounds of Local Grape Raisin. Erwerbs-Obstbau 2022, 1–7. [Google Scholar] [CrossRef]
- Jadhav, P.B.; Kakade, D.K.; Suryawanshi, G.B.; Ruggue, V.C.; Chavan, N.D.; Kumar, V.V.S. Effect of different pre-treatments on physico-chemical parameters of raisins prepared from variety Thompson Seedless. Asian J. Hortic. 2010, 5, 237–239. [Google Scholar]
- Mandal, G.; Thakur, A.K. Preparation of raisin from grapes varieties grown in Punjab with different processing treatments. Int. J. Bio-Res. Environ. Agric. Sci. 2015, 1, 25–31. [Google Scholar]
- Yalçınkaya, E. Kuru Besni Üzümünde Bandırma Eriyiğinin (Potasa Çözeltisi) Aroma Profili Üzerine Etkileri. Master’s Thesis, İnönü University, Institute of Science, Elazığ, Türkiye, 2016. [Google Scholar]
- Foshanji, A.S.; Krishna, H.C.; Vasudeva, K.R.; Ramegowda, G.K.; Shankarappa, T.H.; Bhuvaneswar, S.; Sahel, N.A. Effects of pre-treatments and drying methods on nutritional and sensory quality of raisin. J. Pharmacogn. Phytochem. 2018, 7, 3079–3083. [Google Scholar]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.-M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Khiari, R.; Zemni, H.; Mihoubi, D. Raisin processing: Physicochemical, nutritional and microbiological quality characteristics as affected by drying process. Food Rev. Int. 2018, 35, 246–298. [Google Scholar] [CrossRef]
- Zemni, H.; Sghaier, A.; Khiari, R.; Chebil, S.; Ben Ismail, H.; Nefzaoui, R.; Hamdi, Z.; Lasram, S. Physicochemical, Phyto-chemical and Mycological Characteristics of Italia Muscat Raisins Obtained Using Different Pre-Treatment and Drying Techniques. Food Bioproc. Technol. 2017, 10, 479–490. [Google Scholar] [CrossRef]
- Kamizake, N.K.; Gonçalves, M.M.; Zaia, C.T.; Zaia, D.A. Determination of total proteins in cow milk powder samples: A comparative study between the Kjeldahl method and spectrophotometric methods. J. Food Compos. Anal. 2003, 16, 507–516. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Color measurement and analysis in fresh and processed foods: A review. Food Bioproc. Technol. 2012, 6, 36–60. [Google Scholar] [CrossRef]
- Guiné, R.P.F. The Drying of Foods and Its Effect on the Physical-Chemical, Sensorial and Nutritional Properties. Int. J. Food Eng. 2018, 2, 93–100. [Google Scholar] [CrossRef]
- Ashtiani, S.-H.M.; Rafiee, M.; Morad, M.M.; Khojastehpour, M.; Khani, M.R.; Rohani, A.; Shokri, B.; Martynenko, A. Impact of gliding arc plasma pretreatment on drying efficiency and physicochemical properties of grape. Innov. Food Sci. Emerg. Technol. 2020, 63, 102381. [Google Scholar] [CrossRef]
- Bahabaad, G.A.; Esmaiili, M. Effects of different dipping solutions and storage conditions on the color properties of raisin. Am. J. Agric Environ. Sci. 2012, 12, 1311–1315. [Google Scholar]
- Farahbakhsh, E.; Pakbin, B.; Mahmoudi, R.; Katiraee, F.; Kohannia, N.; Valizade, S. Microbiological Quality of Raisin Dried by Different Methods. Int. J. Food Nutr. Saf. 2015, 6, 62–66. [Google Scholar]
- Muradoğlu, S.; Gürsoy, E. Güler Multivariate analysis revealed the morphological variability among Crataegus species. YYU J. Agric. Sci. 2021, 31, 961–972. [Google Scholar]
- Güler, E.; Karadeniz, T. Discrimination of an untouched autochthonous grapevine (Vitis vinifera L.) population by morphological markers and multivariate analyses. Erwerbs-Obstbau, 2023; in press. [Google Scholar]

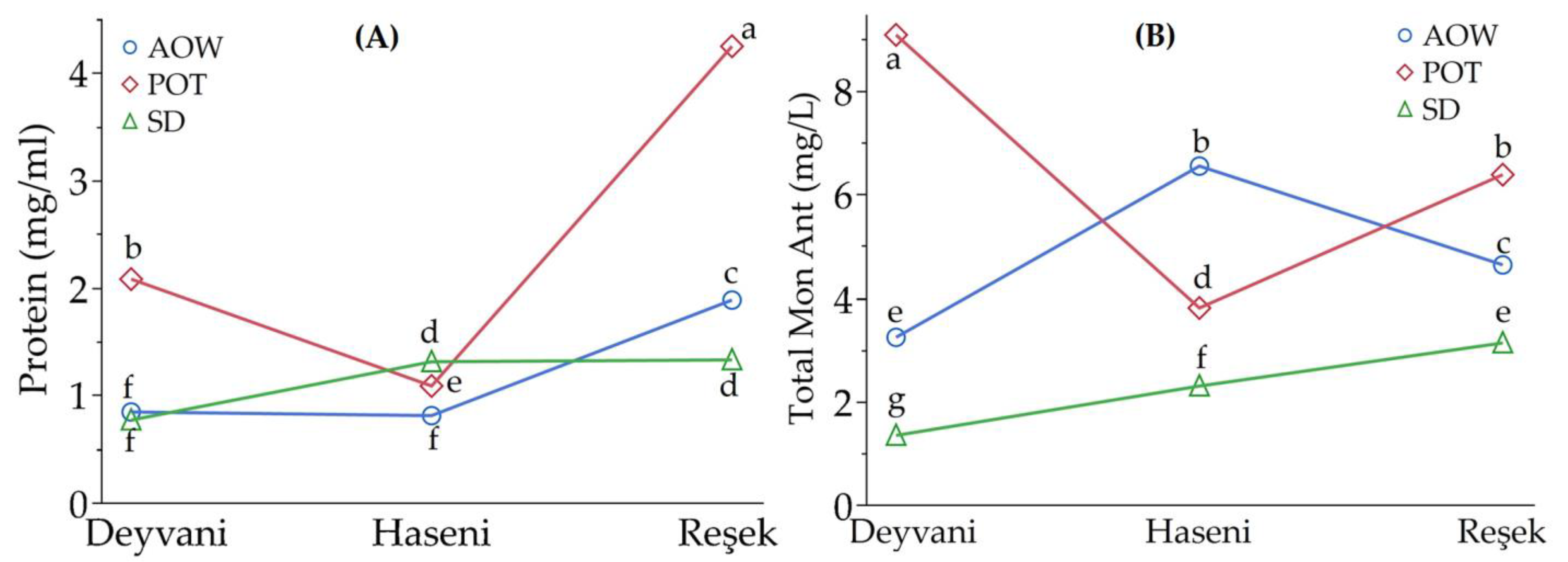
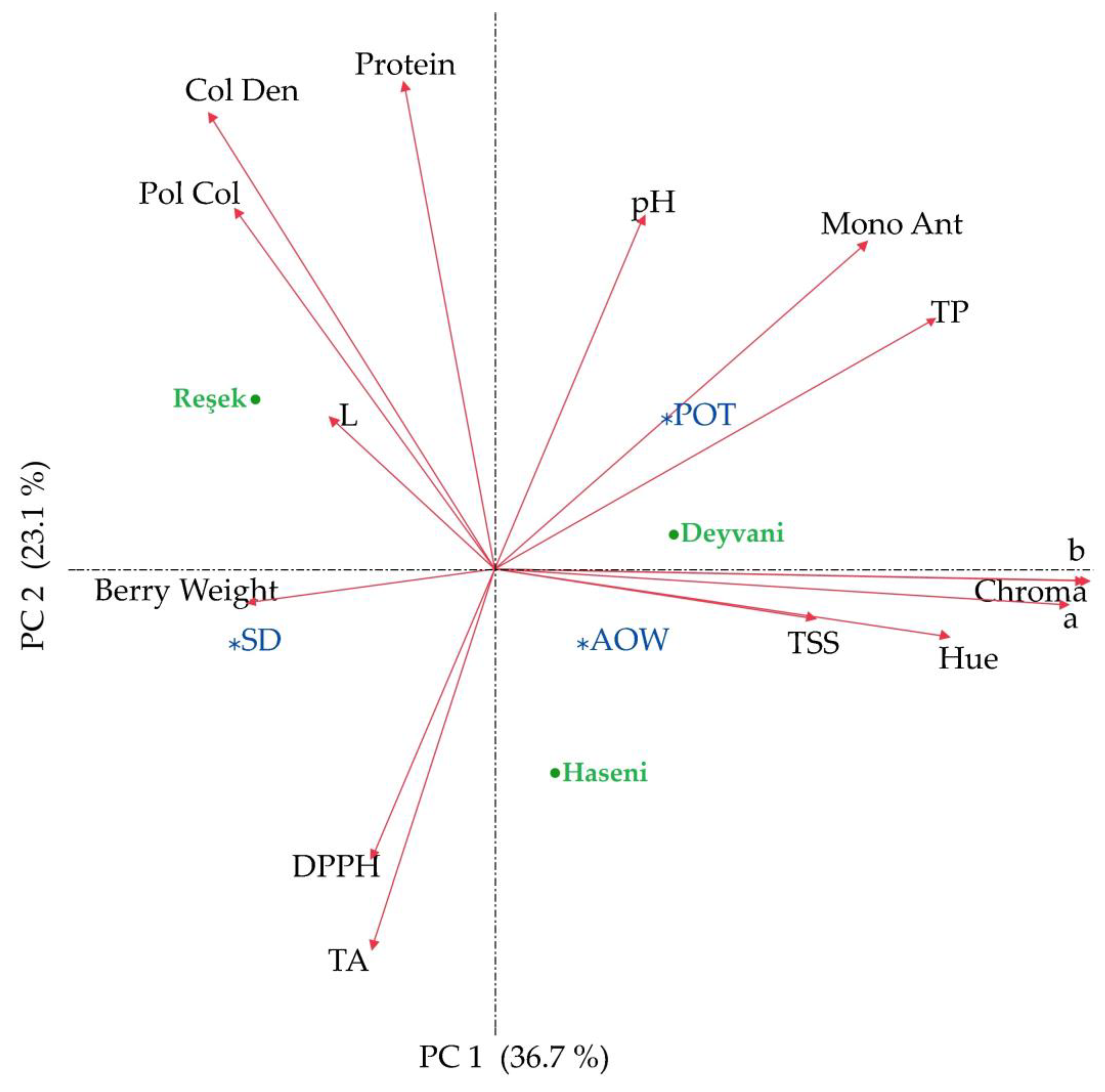
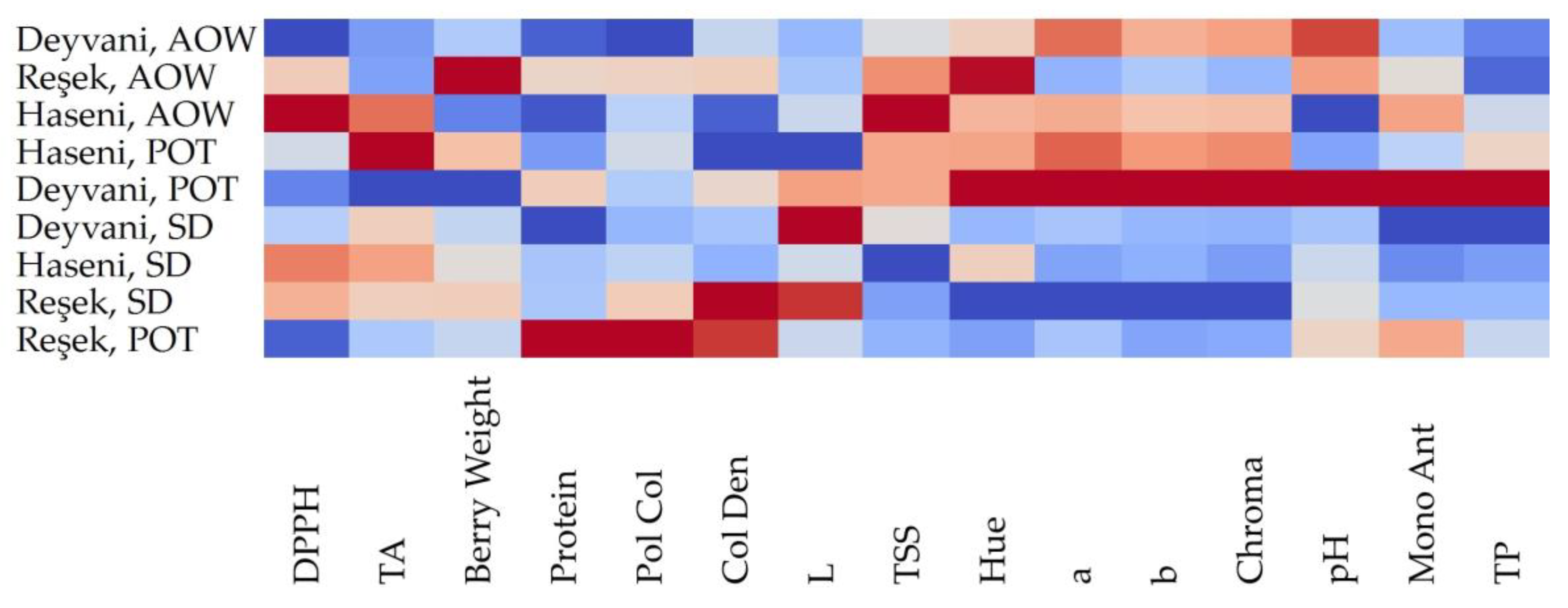
| Variety | Raisin Weight (g) | TSS (%) | pH | TA (mg/L) | |
| Deyvani | 0.541 ± 0.051 c | 68.86 ± 3.60 a | 5.14 ± 0.44 a | 0.52 ± 0.07 b | |
| Haseni | 0.586 ± 0.066 b | 68.59 ± 6.22 a | 4.53 ± 0.33 b | 0.67 ± 0.04 a | |
| Reşek | 0.662 ± 0.096 a | 66.91 ± 4.56 a | 4.99 ± 0.27 a | 0.55 ± 0.05 b | |
| Drying method | |||||
| AOW | 0.618 ± 1.026 a | 70.86 ± 4.26 a | 4.91 ± 0.56 a | 0.56 ± 0.09 b | |
| POT | 0.569 ± 0.076 b | 68.63 ± 4.28 ab | 4.97 ± 0.46 a | 0.57 ± 0.11 b | |
| SD | 0.602 ± 0.037 ab | 64.88 ± 4.24 b | 4.78 ± 0.26 a | 0.62 ± 0.04 a | |
| Variety × drying method | |||||
| Deyvani | AOW | 0.563 ± 0.032 de | 68.01 ± 3.90 abc | 5.34 ± 0.31 ab | 0.51 ± 0.03 de |
| POT | 0.483 ± 0.028 f | 70.35 ± 4.04 ab | 5.43 ± 0.31 a | 0.46 ± 0.03 e | |
| SD | 0.577 ± 0.033 cd | 68.24 ± 3.92 abc | 4.66 ± 0.27 cde | 0.60 ± 0.03 c | |
| Haseni | AOW | 0.512 ± 0.029 ef | 73.52 ± 4.22 a | 4.25 ± 0.24 e | 0.67 ± 0.04 ab |
| POT | 0.646 ± 0.037 b | 70.35 ± 4.04 ab | 4.52 ± 0.26 de | 0.71 ± 0.04 a | |
| SD | 0.601 ± 0.034 bcd | 61.91 ± 3.55 c | 4.81 ± 0.28 cd | 0.64 ± 0.04 bc | |
| Reşek | AOW | 0.779 ± 0.045 a | 71.05 ± 4.08 ab | 5.14 ± 0.29 abc | 0.51 ± 0.03 de |
| POT | 0.579 ± 0.033 cd | 65.19 ± 3.74 bc | 4.95 ± 0.28 abcd | 0.54 ± 0.03 d | |
| SD | 0.629 ± 0.036 bc | 64.49 ± 3.70 bc | 4.88 ± 0.28 bcd | 0.60 ± 0.03 c | |
| ANOVA | |||||
| FVariety | 28.18 *** | 0.66 ns | 11.82 *** | 50.17 *** | |
| FDrying method | 4.64 * | 5.37 * | 1.00 ns | 6.74 ** | |
| FVariety × drying method | 20.31 *** | 2.28 ns | 4.65 ** | 8.46 *** | |
| Variety | L* | a* | b* | Chroma | Hueo | ColDen | PolCol | |
| Deyvani | 19.79 ± 1.98 a | 3.75 ± 1.41 a | 4.75 ± 2.52 a | 6.09 ± 2.81 a | 48.72 ± 7.39 a | 2.12 ± 0.16 b | 1.30 ± 0.23 c | |
| Haseni | 17.74 ± 1.57 b | 3.15 ± 1.36 b | 3.82 ± 1.70 b | 4.96 ± 2.18 b | 50.14 ± 2.76 a | 1.75 ± 0.16 c | 1.57 ± 0.09 b | |
| Reşek | 19.26 ± 1.84 a | 1.33 ± 0.71 c | 1.22 ± 0.99 c | 2.00 ± 0.94 c | 42.59 ± 11.11 b | 2.70 ± 0.32 a | 2.17 ± 0.48 a | |
| Drying method | ||||||||
| AOW | 18.04 ± 0.98 b | 3.20 ± 1.21 b | 3.83 ± 1.23 b | 5.00 ± 1.72 b | 51.92 ± 4.47 a | 2.04 ± 0.30 b | 1.46 ± 0.37 c | |
| POT | 18.23 ± 2.02 b | 3.79 ± 1.42 a | 4.81 ± 2.71 a | 6.18 ± 2.96 a | 48.92 ± 8.47 b | 2.25 ± 0.54 a | 1.97 ± 0.63 a | |
| SD | 20.52 ± 1.72 a | 1.25 ± 0.68 c | 1.15 ± 0.87 c | 1.87 ± 0.87 c | 40.61 ± 7.29 c | 2.29 ± 0.50 a | 1.60 ± 0.24 b | |
| Variety × drying method | ||||||||
| Deyvani | AOW | 17.65 ± 1.01 cd | 4.30 ± 0.25 b | 4.87 ± 0.28 c | 6.51 ± 0.37 b | 48.68 ± 2.79 b | 2.11 ± 0.12 bc | 1.02 ± 0.06 e |
| POT | 20.15 ± 1.16 ab | 5.00 ± 0.29 a | 7.58 ± 0.43 a | 9.09 ± 0.52 a | 56.80 ± 3.26 a | 2.26 ± 0.13 b | 1.49 ± 0.09 cd | |
| SD | 21.57 ± 1.24 a | 1.93 ± 0.11 d | 1.80 ± 0.10 f | 2.68 ± 0.15 de | 40.70 ± 2.34 c | 2.00 ± 0.11 c | 1.39 ± 0.08 d | |
| Haseni | AOW | 18.54 ± 1.06 bc | 3.64 ± 0.21 c | 4.39 ± 0.25 d | 5.71 ± 0.33 c | 50.41 ± 2.89 b | 1.69 ± 0.10 d | 1.53 ± 0.09 cd |
| POT | 16.01 ± 0.92 d | 4.41 ± 0.25 b | 5.42 ± 0.31 b | 7.00 ± 0.40 b | 51.38 ± 2.95 b | 1.64 ± 0.09 d | 1.63 ± 0.09 c | |
| SD | 18.68 ± 1.07 bc | 1.41 ± 0.08 e | 1.66 ± 0.10 f | 2.18 ± 0.13 e | 48.65 ± 2.79 b | 1.92 ± 0.11 c | 1.54 ± 0.09 cd | |
| Reşek | AOW | 17.92 ± 1.03 c | 1.65 ± 0.09 de | 2.23 ± 0.13 e | 2.78 ± 0.16 d | 56.69 ± 3.25 a | 2.32 ± 0.13 b | 1.84 ± 0.11 b |
| POT | 18.54 ± 1.06 bc | 1.95 ± 0.11 d | 1.44 ± 0.08 f | 2.45 ± 0.14 de | 38.58 ± 2.21 c | 2.85 ± 0.16 a | 2.79 ± 0.16 a | |
| SD | 21.31 ± 1.22 a | 0.40 ± 0.02 f | 0.10 ± 0.01 g | 0.76 ± 0.04 f | 32.50 ± 1.86 d | 2.94 ± 0.17 a | 1.89 ± 0.11 b | |
| ANOVA | ||||||||
| FVariety | 8.52 ** | 439.40 *** | 575.49 *** | 472.80 *** | 19.30 *** | 126.04 *** | 180.41 *** | |
| FDrying method | 14.42 *** | 490.21 *** | 618.58 *** | 523.00 *** | 41.06 *** | 9.86 ** | 62.58 *** | |
| FVariety × drying method | 4.69 ** | 18.26 *** | 75.99 *** | 51.99 *** | 24.36 *** | 8.99 *** | 22.18 *** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ünal, M.S.; Güler, E.; Yaman, M. Changes in Antioxidant and Color Properties of Raisins According to Variety and Drying Method. Horticulturae 2023, 9, 771. https://doi.org/10.3390/horticulturae9070771
Ünal MS, Güler E, Yaman M. Changes in Antioxidant and Color Properties of Raisins According to Variety and Drying Method. Horticulturae. 2023; 9(7):771. https://doi.org/10.3390/horticulturae9070771
Chicago/Turabian StyleÜnal, Mehmet Settar, Emrah Güler, and Mehmet Yaman. 2023. "Changes in Antioxidant and Color Properties of Raisins According to Variety and Drying Method" Horticulturae 9, no. 7: 771. https://doi.org/10.3390/horticulturae9070771
APA StyleÜnal, M. S., Güler, E., & Yaman, M. (2023). Changes in Antioxidant and Color Properties of Raisins According to Variety and Drying Method. Horticulturae, 9(7), 771. https://doi.org/10.3390/horticulturae9070771






