Research Progress of Chromosome Doubling and 2n Gametes of Ornamental Plants
Abstract
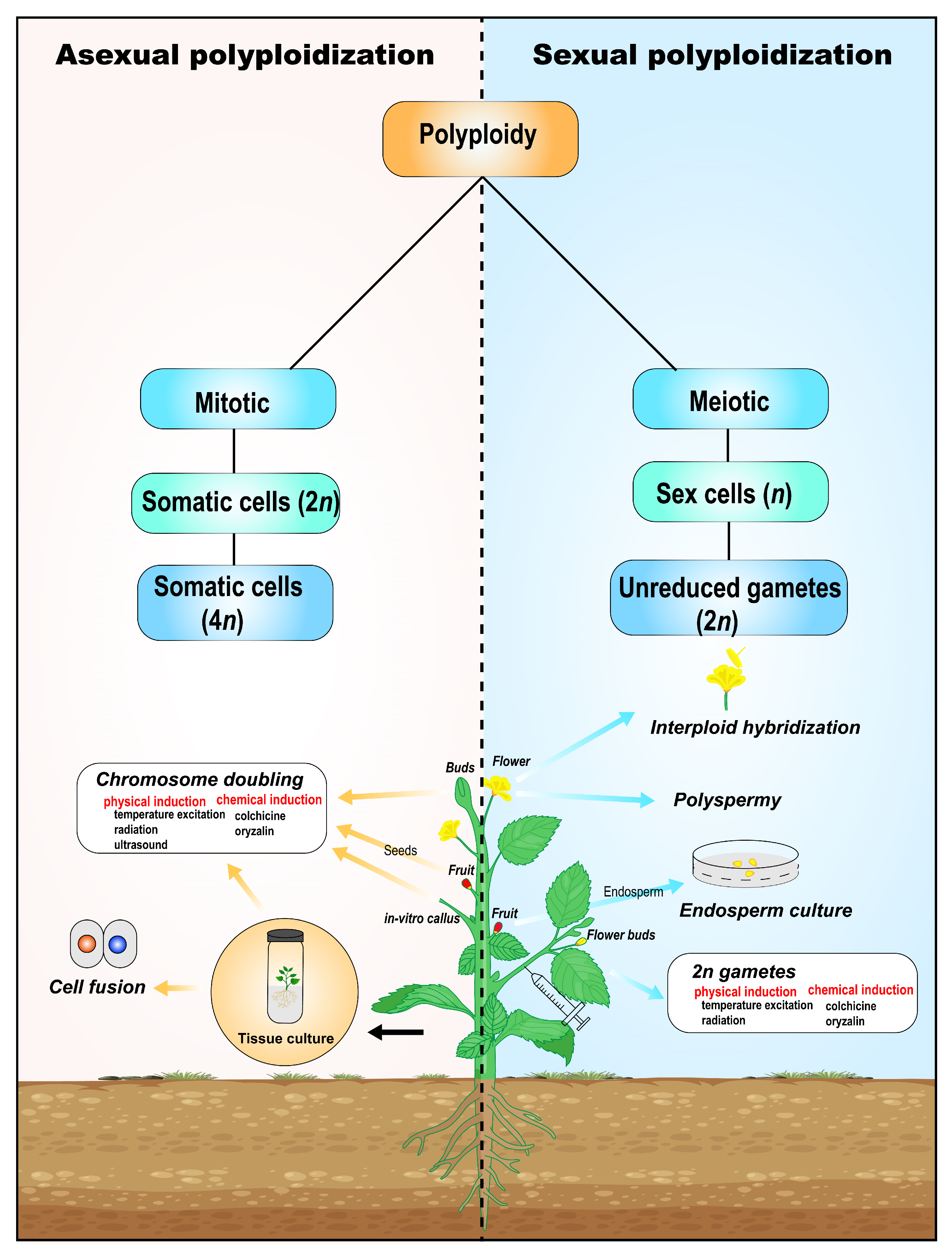
1. Introduction
2. Natural Occurrence of Polyploid Cells
3. Artificial Induction of Polyploidy in Ornamental Plant
3.1. Physical Induction Polyploidy/Mutation
3.2. Chemical Induction Polyploidy/Mutation
| Species | Explant | Induction Method | Method | Concentration, Duration | Most Successful Conversion rate | Reference |
|---|---|---|---|---|---|---|
| Actinidia arguta | hardwood | 60Co γ-ray | 60 Gy, 1 Gy·min−1 | 6.67% chimera | [53] | |
| Magnolia denudata | callus | γ-ray | 2.475 × 10−10 C/kg | chimera | [54] | |
| Cymbidium hybridum | protocorm-like bodies | colchicine | co-culture | 0.05% for 5 d | 23.7% | [56] |
| Cymbidium hybridum | young shoots | colchicine | immersion | 0.05% for 24 h | 28.2% | [55] |
| Lilium davidii var. unicolor | bulb | colchicine | immersion | 0.05% for 48 h | 33.3% | [57] |
| Asiatic Lilies | bulb | oryzalin | immersion | 0.005% for 5 h | 23% | [58] |
| Phalaenopsis amabilis | cluster bud | colchicine | co-culture | 0.05% for 10 d | 3% | [60] |
| Tagetes erecta | seeds | colchicine | immersion | 0.1% for 6 h | 100% | [61] |
| Impatiens walleriana | seeds | colchicine | immersion | 0.05% for 48 h | 1.5% | [62] |
| Gladiolus grandiflorus | corms | colchicine | immersion | 0.2% for 24 h | 18% | [63] |
| Chrysanthemum carinatum | apical buds | colchicine | absorbent cotton wrapping | 0.2% for 6 h/d, 3 d | 2.08% | [64] |
| Clematis heracleifolia | apical buds | colchicine | spraying | 0.2% for 2 d | 80% | [65] |
| Agastache foeniculum | seeds, apical buds | colchicine | immersion | 17.5 mM for 6 h | 16% | [66] |
| Agastache foeniculum | seeds, apical buds | oryzalin | immersion | 50 μM for 12 h | 14% | [66] |
| Agastache foeniculum | seeds, apical buds | trifluralin | immersion | 50 μM for 12 h | 12% | [66] |
| Hibiscus moscheutos | seedlings | colchicine | immersion | 0.025% for 12 h | 22.5% | [67] |
| Dendrobium officinale | seeds | colchicine | co-culture | 0.05% for 4 months | 50% | [59] |
| Dendrobium officinale | embryo | colchicine | immersion | 0.3% for 36 h | 40% | [59] |
| Dendrobium officinale | protocorm-like bodies | colchicine | immersion | 0.3% for 36 h | 40% | [59] |
| Dendrobium officinale | stems | colchicine | immersion | 0.3% for 36 h | 30% | [59] |
| Neolamarckia cadamba | nodal segments | colchicine | immersion | 0.3% for 48 h | 20% | [50] |
| Ziziphus jujuba | in vitro callus | colchicine + dimethylsulphoxide | absorbent cotton wrapping | 0.05% + 1% for 50 d | [53] | |
| Buddleja lindleyana | seeds | colchicine | immersion | 0.3% for 48 h | 3% | [68] |
3.3. Others
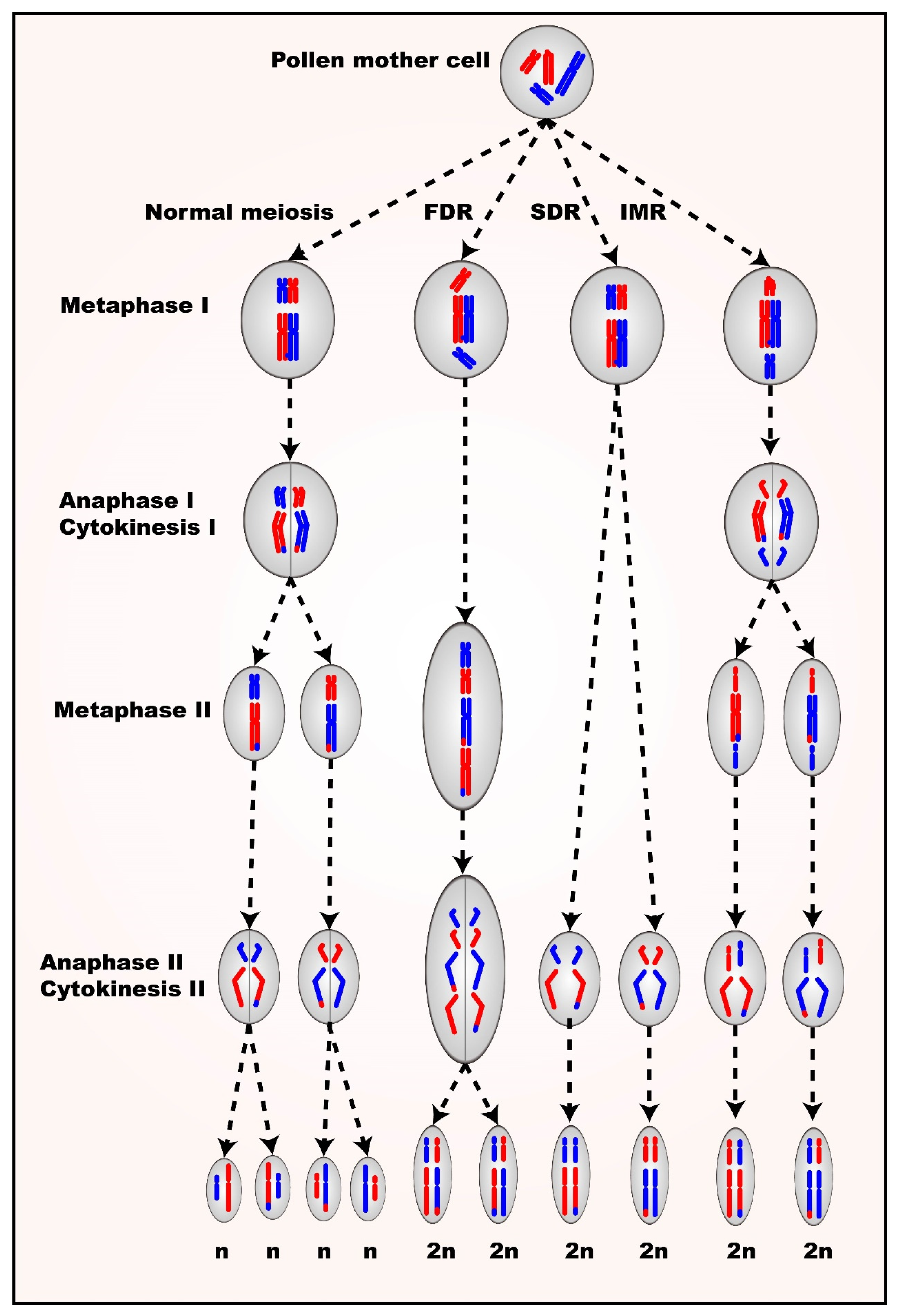
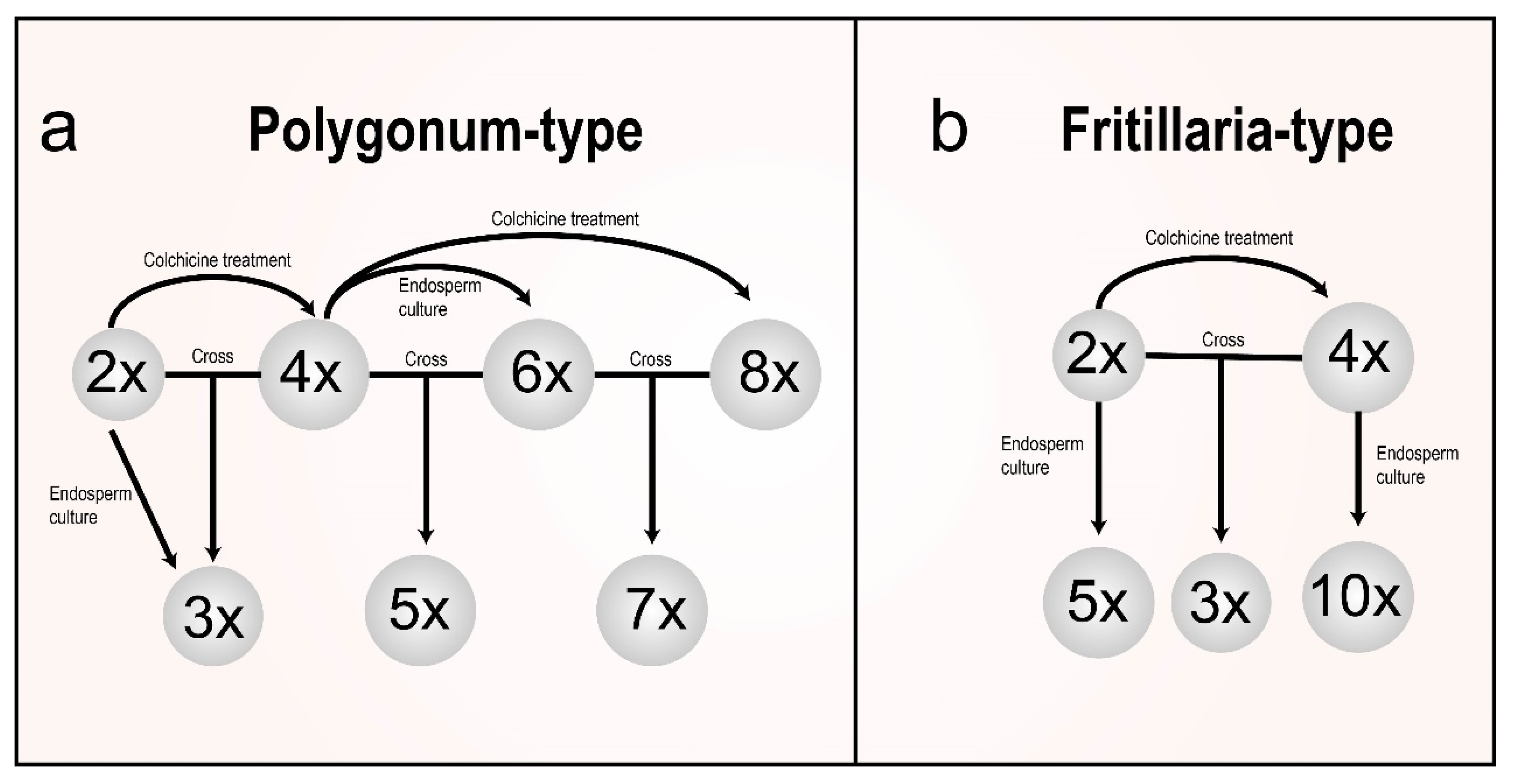
4. Sexual Polyploidization
4.1. 2n Gametes
4.2. Others
5. Identification of Ploidy
6. Conclusions and Further Prospects
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frawley, L.E.; Orr-Weaver, T.L. Polyploidy. Curr. Biol. 2015, 25, R353–R358. [Google Scholar] [CrossRef] [PubMed]
- Villa, S.; Montagna, M.; Pierce, S. Endemism in recently diverged angiosperms is associated with polyploidy. Plant Ecol. 2022, 223, 479–492. [Google Scholar] [CrossRef]
- Rice, A.; Šmarda, P.; Novosolov, M.; Drori, M.; Glick, L.; Sabath, N.; Meiri, S.; Belmaker, J.; Mayrose, I. The Global Biogeography of Polyploid Plants. Nat. Ecol. Evol. 2019, 3, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Soltis, D.E.; Soltis, P.S.; Tate, J.A. Advances in the Study of Polyploidy since Plant Speciation. New Phytol. 2004, 161, 173–191. [Google Scholar] [CrossRef]
- Wood, T.E.; Takebayashi, N.; Barker, M.S.; Mayrose, I.; Greenspoon, P.B.; Rieseberg, L.H. The Frequency of Polyploid Speciation in Vascular Plants. Proc. Natl. Acad. Sci. USA 2009, 106, 13875–13879. [Google Scholar] [CrossRef] [PubMed]
- Carta, A.; Bedini, G.; Peruzzi, L. A Deep dive into the ancestral chromosome number and genome size of flowering plants. New Phytol. 2020, 228, 1097–1106. [Google Scholar] [CrossRef]
- Parris, J.K. Magnolia: Impact of Interspecific Hybridization on Genetic Variation and Ongoing Breeding Initiatives. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2018. [Google Scholar]
- Lee, Y.I.; Tseng, Y.; Lee, Y.C.; Chung, M.C. Chromosome Constitution and Nuclear DNA Content of Phalaenopsis Hybrids. Sci. Hortic. 2020, 262, 109089. [Google Scholar] [CrossRef]
- Vilcherrez-Atoche, J.A.; Iiyama, C.M.; Cardoso, J.C. Polyploidization in Orchids: From Cellular Changes to Breeding Applications. Plants 2022, 11, 469. [Google Scholar] [CrossRef]
- Han, T.S.; Zheng, Q.J.; Onstein, R.E.; Rojas-Andrés, B.M.; Hauenschild, F.; Muellner-Riehl, A.N.; Xing, Y.W. Polyploidy Promotes Species Diversification of Allium through Ecological Shifts. New Phytol. 2020, 225, 571–583. [Google Scholar] [CrossRef]
- Hieu, P.V. Polyploid Gene Expression and Regulation in Polysomic Polyploids. Am. J. Plant Sci. 2019, 10, 1409–1443. [Google Scholar] [CrossRef]
- Van de Peer, Y.; Mizrachi, E.; Marchal, K. The Evolutionary Significance of Polyploidy. Nat. Rev. Genet. 2017, 18, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.D.; Yuan, D.Y.; Wang, W.; Xia, Q.M.; Wu, X.M.; Chen, C.W.; Guo, W.W. Citrus triploid recovery based on 2x × 4x crosses via an optimized embryo rescue approach. Sci. Hortic. 2019, 252, 104–109. [Google Scholar] [CrossRef]
- Park, Y.S.; Lee, J.C.; Jeong, H.N.; Um, N.Y.; Heo, J.Y. A Red Triploid Seedless Grape ‘Red Dream’. HortScience 2022, 57, 741–742. [Google Scholar] [CrossRef]
- Marasek-Ciolakowska, A.; Xie, S.L.; Arens, P.; van Tuyl, J.M. Ploidy Manipulation and Introgression Breeding in Darwin Hybrid Tulips. Euphytica 2014, 198, 389–400. [Google Scholar] [CrossRef]
- Arachchige, E.C.S.; Evans, L.J.; Samnegård, U.; Rader, R. Morphological Characteristics of Pollen from Triploid Watermelon and its fate on Stigmas in a Hybrid Crop Production System. Sci. Rep. 2022, 12, 3222. [Google Scholar] [CrossRef]
- Uwimana, B.; Mwanje, G.; Batte, M.; Akech, V.; Shah, T.; Vuylsteke, M.; Swennen, R. Continuous mapping identifies Loci associated with weevil resistance [Cosmopolites sordidus (Germar)] in a triploid banana population. Front. Plant Sci. 2021, 12, 753241. [Google Scholar] [CrossRef]
- Cui, L.M.; Sun, Y.N.; Xiao, K.Z.; Wan, L.; Zhong, J.; Liu, Y.M.; Xie, Q.L.; Zhou, S.J. Analysis on the Abnormal Chromosomal Behaviour and the Partial Female Fertility of Allotriploid Lilium—‘Triumphator’ (LLO) is Not Exceptional to the Hypothesis of Lily Interploid Hybridizations. Sci. Hortic. 2022, 293, 110746. [Google Scholar] [CrossRef]
- Xiao, K.Z.; Cui, L.M.; Wan, L.; Zhong, J.; Liu, Y.M.; Sun, Y.N.; Zhou, S.J. A New Way to Produce Odd-allotetraploid Lily (Lilium) through 2n Gametes. Plant Breed. 2021, 140, 711–718. [Google Scholar] [CrossRef]
- Zeng, J.; Sun, Y.N.; Wan, L.; Zhong, J.; Yu, S.Q.; Zou, N.; Cai, J.H.; Zhou, S.J. Analyzing Narcissus Genome Compositions Based on RDNA Loci on Chromosomes and Crossing-Compatibility of 16 Cultivars. Sci. Hortic. 2020, 267, 109359. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, M.; Li, S.; Chen, Q.; Teixeira da Silva, J.A.; Wang, A.; Yu, X.; Wang, L. Germplasm Resources and Genetic Breeding of Paeonia: A Systematic Review. Hortic. Res. 2020, 7, 107. [Google Scholar] [CrossRef]
- Van de Peer, Y.; Ashman, T.L.; Soltis, P.S.; Soltis, D.E. Polyploidy: An evolutionary and ecological force in stressful times. Plant Cell 2021, 33, 11–26. [Google Scholar] [CrossRef]
- Leitch, A.R.; Leitch, I.J. Genome Evolution: On the Nature of Trade-offs with Polyploidy and Endopolyploidy. Curr. Biol. 2022, 32, R952–R954. [Google Scholar] [CrossRef]
- Tate, J.A.; Joshi, P.; Soltis, K.A.; Soltis, P.S.; Soltis, D.E. On the Road to Diploidization? Homoeolog Loss in Independently Formed Populations of the Allopolyploid Tragopogon miscellus (Asteraceae). BMC Plant Biol. 2009, 9, 80. [Google Scholar] [CrossRef]
- Triplett, J.K.; Clark, L.G.; Fisher, A.E.; Wen, J. Independent Allopolyploidization Events Preceded Speciation in the Temperate and Tropical Woody Bamboos. New Phytol. 2014, 204, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Zhang, S.Z.; Li, Y.; Zhang, W.H. Chromosome Numbers of 13 Taxa and 12 Crossing Combinations in Magnoliaceae. Acta Phytotaxon. Sin. 2005, 43, 545–551, (In Chinese with an English Abstract). [Google Scholar] [CrossRef]
- Suzuki, T.; Yamagishi, M. Aneuploids without Bulbils Segregated in F1 Hybrids Derived from Triploid Lilium lancifolium and Diploid L. leichtlinii Crosses. Hortic. J. 2016, 85, 224–231. [Google Scholar] [CrossRef]
- Zhou, S.B.; Yu, B.Q.; Luo, Q.; Hu, J.R.; Bi, D. Karyotypes of Six Populations of Lycoris Radiata and Discovery of the Tetraploid. J. Syst. Evol. 2007, 45, 513. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.D.; Wu, Y.Y.; Cai, J.H.; Zhang, L. Karyotype Analysis of Seven Lycoris Species Based on Fluorescence in Situ Hybridization. Mol. Plant Breed. 2022, 1–9, (In Chinese with an English Abstract). [Google Scholar]
- Sakya, S.R.; Joshi, K.K. Karyomorphological Studies in Some Primula Species of Nepal Himalaya. Cytologia 1990, 55, 571–579. [Google Scholar] [CrossRef]
- Luo, Y.B. Cytological Studies on Some Representative Species of the Tribe Orchideae (Orchidaceae) from China. Bot. J. Linn Soc. 2004, 145, 231–238. [Google Scholar] [CrossRef]
- Liang, G.L.; Li, X.L. Supplementary Reports on the Chromosome Numbers of Malus in China. Southwest China J. Agric. Sci. 1991, 4, 25–29, (In Chinese with an English Abstract). [Google Scholar]
- Manshard, E. Chromosome Numbers in Styrax. Planta 1936, 25, 364. [Google Scholar] [CrossRef]
- Ruan, Y.Q. Taxonomic Studies on 13 Species of Styrax in China. Master’s Thesis, Jiangxi Agricultural University, Nanchang, China, 2020. (In Chinese with an English Abstract). [Google Scholar]
- Liu, B.; Davis, T.M. Conservation and Loss of Ribosomal RNA Gene Sites in Diploid and Polyploid Fragaria (Rosaceae). BMC Plant Biol. 2011, 11, 157. [Google Scholar] [CrossRef]
- Emshwiller, E.; Doyle, J.J. Origins of Domestication and Polyploidy in Oca (Oxalis tuberosa: Oxalidaceae). 2. Chloroplast-Expressed Glutamine Synthetase Data. Am. J. Bot. 2002, 89, 1042–1056. [Google Scholar] [CrossRef]
- Felix, W.J.P.; Felix, L.P.; Melo, N.F.; Dutilh, J.H.A.; Carvalho, R. Cytogenetics of Amaryllidaceae Species: Heterochromatin Evolution in Different Ploidy Levels. Plant Syst. Evol. 2011, 292, 215–221. [Google Scholar] [CrossRef]
- Ahuja, M.R.; Neale, D.B. Evolution of Genome Size in Conifers. Silvae Genet. 2005, 54, 126–137. [Google Scholar] [CrossRef]
- Hong, D. Peonies of the World Taxonomy and Phytogeography; Royal Botanic Gardens, Kew: London, UK, 2010. [Google Scholar]
- Gu, Z.J. The Discovery of Tetraploid Camellia Reticulata and its Implication in Studies on the Origin of This Species. Acta Phytotaxon. Sin. 1997, 35, 107–116 + 193–197, (In Chinese with an English Abstract). [Google Scholar]
- Jian, H.Y.; Zhang, H.; Tang, K.X.; Li, S.; Wang, Q.G.; Zhang, T.; Qiu, X.Q.; Yan, H.J. Decaploidy in Rosa Praelucens Byhouwer (Rosaceae) Endemic to Zhongdian Plateau, Yunnan, China. Caryologia 2010, 63, 162–167. [Google Scholar] [CrossRef]
- Lim, K.Y.; Werlemark, G.; Matyasek, R.; Bringloe, J.B.; Sieber, V.; El Mokadem, H.; Meynet, J.; Hemming, J.; Leitch, A.R.; Roberts, A.V. Evolutionary Implications of Permanent Odd Polyploidy in the Stable Sexual, Pentaploid of Rosa canina L. Heredity 2005, 94, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Tian, M.; Zhang, T.; Wang, Q.G.; Yan, H.J.; Qiu, X.Q.; Zhou, N.N.; Zhang, H.; Jian, H.Y.; Tang, K.X. Karyotype Analysis of Rosa praelucens and Its Closely Related Congeneric Species Based on FISH. Acta Hortic. Sin. 2020, 47, 503–516, (In Chinese with an English Abstract). [Google Scholar]
- Deepo, D.M.; Mazharul, I.M.; Hwang, Y.J.; Kim, H.Y.; Kim, C.K.; Lim, K.B. Chromosome and Ploidy Analysis of Winter Hardy Hibiscus Species by FISH and Flow Cytometry. Euphytica 2022, 218, 81. [Google Scholar] [CrossRef]
- Greizerstein, E.J.; Giberti, G.C.; Poggio, L. Cytogenetic Studies of Southern South-American Ilex. Caryologia 2004, 57, 19–23. [Google Scholar] [CrossRef]
- Wolin, C.L.; Galen, C.; Watkins, L. The Breeding System and Aspects of Pollination Effectiveness in Oenothera Speciosa (Onagraceae). Southwest. Nat. 1984, 29, 15–20. [Google Scholar] [CrossRef]
- Hembree, W.G.; Ranney, T.G.; Lynch, N.P.; Jackson, B.E. Identification, Genome Sizes, and Ploidy of Deutzia. J. Am. Soc. Hortic. Sci. 2020, 145, 88–94. [Google Scholar] [CrossRef]
- Yang, H.B.; Rao, L.B.; Guo, H.Y.; Chen, Y.S. Karyotyping of Five Species of Alnus in East Aisa Region. J. Plant Genet. Resour. 2013, 14, 1203–1207, (In Chinese with an English Abstract). [Google Scholar]
- Eng, W.H.; Ho, W.S.; Ling, K.H. In Vitro Induction and Identification of Polyploid Neolamarckia cadamba Plants by Colchicine Treatment. PeerJ 2021, 9, e12399. [Google Scholar] [CrossRef]
- Germana, M.A. Use of Irradiated Pollen to Induce Parthenogenesis and Haploid Production in Fruit Crops. In Plant Mutation Breeding and Biotechnology, CABI Books; CABI: Wallingford, UK, 2012; pp. 411–421. [Google Scholar]
- Manzoor, A.; Ahmad, T.; Bashir, M.A.; Hafiz, I.A.; Silvestri, C. Studies on Colchicine Induced Chromosome Doubling for Enhancement of Quality Traits in Ornamental Plants. Plants 2019, 8, 194. [Google Scholar] [CrossRef]
- Shi, Q.H.; Liu, P.; Wang, J.R.; Xu, J.; Ning, Q.; Liu, M.J. A Novel in Vivo Shoot Regeneration System via Callus in Woody Fruit Tree Chinese Jujube (Ziziphus Jujuba Mill.). Sci. Hortic. 2015, 188, 30–35. [Google Scholar] [CrossRef]
- Cao, J.W. Polyploid Induction and Ploidy Identification in Actinidia arguta. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2022. (In Chinese with an English Abstract). [Google Scholar]
- Jiang, C.Y.; Ning, S.X.; Yang, W.X.; Xiao, R.J. Selective Breeding of a New Breed of Magnolia denudata from Radiation-induced Mutation of Callus. Acta Hortic. Sin. 2002, 29, 473–476, (In Chinese with an English Abstract). [Google Scholar]
- Ji, B.X.; Chen, D.W.; Zhang, C.C.; Min, D.; Huang, W.; Wang, Y. High Efficient Polyploid Induction of Cymbidium hybridium. Bull. Bot. Res. 2011, 31, 558–562. [Google Scholar]
- Wang, M.G.; Zeng, R.Z.; Xie, L.; Li, Y.H.; Zeng, F.Y.; Zhang, Z. In Vitro Induction and Its Identification of Tetraploid Cymbidium hybridium. Acta Bot. Boreali-Occident. Sin. 2010, 31, 56–62. [Google Scholar]
- Feng, Y.Y.; Xu, L.F.; Yang, P.P.; Xu, H.; Cao, Y.W.; Tang, Y.C.; Yuan, S.X.; Ming, J. Production and Identification of a Tetraploid Germplasm of Edible Lilium davidii var. unicolor Salisb via Chromosome Doubling. HortScience 2017, 52, 946–951. [Google Scholar]
- Jian, J.; Fang, L.Q.; Tan, X.; Yuan, G.L.; Xu, P.; Zhou, S.J. Hybridization and Chromosome Doubling for Potted Asiatic Lilies (Lilium). J. Agric. Biotechnol. 2013, 21, 627–630, (In Chinese with an English Abstract). [Google Scholar]
- Zhang, J.J. Polyploid Induction and Identification of Dendrobium Officinale. Master’s Thesis, Zhejiang A&F University, Hangzhou, China, 2013. (In Chinese with an English Abstract). [Google Scholar]
- Putri, A.A.; Sukma, D.; Aziz, S.A.; dan Syukur, M. Komposisi Media Pertumbuhan Protokorm Sebelum Perlakuan Kolkisin Untuk Meningkatkan Poliploidi Pada Phalaenopsis amabilis (L.) Blume. Indones. J. Agric. 2018, 46, 306–313. [Google Scholar] [CrossRef]
- He, Y.H.; Sun, Y.L.; Zheng, R.R.; Ai, Y.; Cao, Z.; Bao, M.Z. Induction of Tetraploid Male Sterile Tagetes erecta by Colchicine Treatment and Its Application for Interspecific Hybridization. Hortic. Plant J. 2016, 2, 284–292. [Google Scholar] [CrossRef]
- Wang, W.N.; He, Y.H.; Cao, Z.; Deng, Z.A. Induction of Tetraploids in Impatiens (Impatiens walleriana) and Characterization of Their Changes in Morphology and Resistance to Downy Mildew. HortScience 2018, 53, 925–931. [Google Scholar] [CrossRef]
- Manzoor, A.; Ahmad, T.; Bashir, M.A.; Baig, M.M.Q.; Quresh, A.A.; Shah, M.K.N.; Hafiz, I.A. Induction and Identification of Colchicine Induced Polyploidy in ‘White Prosperity’. Folia Hortic. 2018, 30, 307–319. [Google Scholar] [CrossRef]
- Kushwah, K.; Verma, R.; Patel, S.; Jain, N.K. Colchicine Induced Polyploidy in Chrysanthemum carinatum L. J. Phylogenetic Evol. Biol. 2018, 6, 1. [Google Scholar] [CrossRef]
- Wu, Y.X.; Li, W.Y.; Dong, J.; Yang, N.; Zhao, X.M.; Yang, W.D. Tetraploid Induction and Cytogenetic Characterization for Clematis Heracleifolia. Caryologia 2013, 66, 215–220. [Google Scholar] [CrossRef]
- Talebi, S.F.; Saharkhiz, M.J.; Kermani, M.J.; Sharafi, Y.; Raouf Fard, F. Effect of Different Antimitotic Agents on Polyploid Induction of Anise Hyssop (Agastache foeniculum L.). Caryologia 2017, 70, 184–193. [Google Scholar] [CrossRef]
- Li, Z.T.; Ruter, J.M. Development and Evaluation of Diploid and Polyploid Hibiscus moscheutos. HortScience 2017, 52, 676–681. [Google Scholar] [CrossRef]
- Yan, Y.J.; Qin, S.S.; Zhou, N.Z.; Xie, Y.; He, Y. Effects of Colchicine on Polyploidy Induction of Buddleja lindleyana Seeds. Plant Cell Tissue Org. 2022, 149, 735–745. [Google Scholar] [CrossRef]
- Reyna-Llorens, I.; Ferro-Costa, M.; Burgess, S.J. Plant protoplasts in the age of synthetic biology. J. Exp. Bot. 2023, erad172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tan, F.Q.; Fan, Y.J.; Wang, T.T.; Song, X.; Xie, K.D.; Wu, X.M.; Zhang, F.; Deng, X.X.; Grosser, J.W.; et al. Acetylome Reprograming Participates in the Establishment of Fruit Metabolism during Polyploidization in Citrus. Plant Physiol. 2022, 190, 2519–2538. [Google Scholar] [CrossRef]
- Cui, H.F.; Sun, Y.; Deng, J.Y.; Wang, M.Q.; Xia, G.M. Chromosome Elimination and Introgression Following Somatic Hybridization between Bread Wheat and Other Grass Species. Plant Cell Tissue Org. 2015, 120, 203–210. [Google Scholar] [CrossRef]
- Galbraith, D.W.; Harkins, K.R.; Knapp, S. Systemic Endopolyploidy in Arabidopsis thaliana. Plant Physiol. 1991, 96, 985–989. [Google Scholar] [CrossRef]
- Smulders, M.J.M.; Rus-Kortekaas, W.; Gilissen, L.J.W. Development of Polysomaty during Differentiation in Diploid and Tetraploid Tomato (Lycopersicon esculentum) Plants. Plant Sci. 1994, 97, 53–60. [Google Scholar] [CrossRef]
- Chen, W.H.; Tang, C.Y.; Lin, T.Y.; Weng, Y.C.; Kao, Y.L. Changes in the Endopolyploidy Pattern of Different Tissues in Diploid and Tetraploid Phalaenopsis aphrodite Subsp. formosana (Orchidaceae). Plant Sci. 2011, 181, 31–38. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, S.Z.; Zhang, W.H. A Cytological Observation on Triploidy Hybridized Plant of Michelia. Acta Hortic. Sin. 2006, 1, 27, (In Chinese with an English Abstract). [Google Scholar]
- Kreiner, J.M.; Kron, P.; Husband, B.C. Frequency and Maintenance of Unreduced Gametes in Natural Plant Populations: Associations with Reproductive Mode, Life History and Genome Size. New Phytol. 2017, 214, 879–889. [Google Scholar] [CrossRef]
- Liu, Y.M.; Zhang, L.; Sun, Y.N.; Zhou, S.J. The Common Occurrence of 2n Eggs by Lily F1 Distant Hybrids and Its Significance on Lily Breeding: A Case of Analyzing OT Hybrids. Euphytica 2021, 217, 204. [Google Scholar] [CrossRef]
- Zhou, S.J.; Ramanna, M.S.; Visser, R.G.F.; van Tuyl, J.M. Genome Composition of Triploid Lily Cultivars Derived from Sexual Polyploidization of Longiflorum × Asiatic Hybrids (Lilium). Euphytica 2008, 160, 207–215. [Google Scholar] [CrossRef]
- Lobdell, M.S. Register of Magnolia Cultivars. HortScience 2021, 56, 1614–1675. [Google Scholar] [CrossRef]
- Harlan, J.R.; deWet, J.M.J.; On, Ö. Winge and a Prayer: The Origins of Polyploidy. Bot. Rev. 1975, 41, 361–390. [Google Scholar] [CrossRef]
- Zhou, S.J. Intergenomic Recombination and Introgression Breeding in Longiflorum × Asiatic lilies. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2007. [Google Scholar]
- Tian, M.D.; Zhang, Y.; Liu, Y.; Kang, X.Y.; Zhang, P.D. High Temperature Exposure Did Not Affect Induced 2n Pollen Viability in Populus. Plant Cell Environ. 2018, 41, 1383–1393. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Wang, P.Q.; Yang, J.; Kang, X.Y. Induction of Unreduced Megaspores in Eucommia ulmoides by High Temperature Treatment during Megasporogenesis. Euphytica 2016, 212, 515–524. [Google Scholar] [CrossRef]
- Li, H.M.; Gan, J.C.; Xiong, H.; Mao, X.D.; Li, S.W.; Zhang, H.Y.; Hu, G.B.; Liu, C.M.; Fu, J.X. Production of Triploid Germplasm by Inducing 2n Pollen in Longan. Horticulturae 2022, 8, 437. [Google Scholar] [CrossRef]
- Xiong, H.; Mao, X.D.; Gan, J.C.; Hu, G.B.; Liu, C.M.; Fu, J.X. Creation of Triploid Germplasm of Longan by Inducing 2n Male Gametes. Acta. Hortic. 2020, 1293, 113–120. [Google Scholar] [CrossRef]
- Lokker, A.C.; Barba-Gonzalez, R.; Lim, K.B.; Ramanna, M.S.; van Tuyl, J.M. Genotypic and Environmental Variation in Production of 2n-Gametes of Oriental × Asiatic Lily Hybrids. Acta. Hortic. 2004, 673, 453–456. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, J.; Sang, Y.R.; Zhao, Z.Y.; Zhang, P.D.; Liu, M.Q. Effects of Colchicine on Populus Canescens Ectexine Structure and 2n Pollen Production. Front Plant Sci. 2020, 11, 295. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zhou, Q.; Wu, J.; Zhang, P.D. Colchicine Did Not Affect the Viability of Induced 2n Pollen in Populus tomentosa. Silva Fenn. 2019, 53, 10132. [Google Scholar] [CrossRef]
- Yang, J.; Yao, P.Q.; Li, Y.; Mo, J.Y.; Wang, J.Z.I.; Kang, X.Y. Induction of 2n Pollen with Colchicine during Microsporogenesis in Eucalyptus. Euphytica 2016, 210, 69–78. [Google Scholar] [CrossRef]
- Wu, T.; Zhao, X.; Yang, S.H.; Yang, J.H.; Zhu, J.; Kou, Y.P.; Yu, X.; Ge, H.; Jia, R.D. Induction of 2n Pollen with Colchicine during Microsporogenesis in Phalaenopsis. Breed. Sci. 2022, 72, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.W.; Zhang, Y.Q.; Xing, G.M.; Cui, Y.H.; Xue, L.; Zhao, J.; Zhang, W.; Qu, L.; Lei, J.J. Inducing 2n Pollen to Obtain Polyploids in Tulip. Acta Hortic. 2019, 1237, 93–100. [Google Scholar] [CrossRef]
- Wongprichachan, P.; Huang, K.L.; Hsu, S.T.; Chou, Y.M.; Liu, T.; Okubo, H. Induction of Polyploid Phalaenopsis Amabilis by N2O Treatment. J. Fac. Agric. Kyushu Univ. 2013, 58, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kong, B.; Do, P.U.; Li, L.; Du, J.; Ma, L.; Sang, Y.; Wu, J.; Zhou, Q.; Cheng, X.; et al. Gibberellins as a Novel Mutagen for Inducing 2n Gametes in Plants. Front Plant Sci. 2023, 13, 1110027. [Google Scholar] [CrossRef]
- Zhu, W.; Dong, Z.J.; Chen, X.; Cao, J.X.; Zhang, W.; Sun, R.Z.; Teixeira da Silva, J.A.; Yu, X.N. Induction of 2n Pollen by Colchicine during Microsporogenesis to Produce Polyploids in Herbaceous Peony (Paeonia lactiflora Pall.). Sci. Hortic. 2022, 304, 111264. [Google Scholar] [CrossRef]
- Zhong, S.; Li, L.; Wang, Z.J.; Ge, Z.X.; Li, Q.Y.; Bleckmann, A.; Wang, J.Z.; Song, Z.H.; Shi, Y.H.; Liu, T.X.; et al. RALF Peptide Signaling Controls the Polytubey Block in Arabidopsis. Science 2022, 375, 290–296. [Google Scholar] [CrossRef]
- Okamoto, T.; Ohnishi, Y.; Toda, E. Development of Polyspermic Zygote and Possible Contribution of Polyspermy to Polyploid Formation in Angiosperms. J. Plant Res. 2017, 130, 485–490. [Google Scholar] [CrossRef]
- Góralski, G.; Popielarska-Konieczna, M.; Ślesak, H.; Siwińska, D.; Batycka, M. Organogenesis in Endosperm of Actinidia Deliciosa Cv. Hayward Cultured in Vitro. Acta Biol. Cracoviensia Ser. Bot. 2005, 47, 121–128. [Google Scholar]
- Miyashita, T.; Ohashi, T.; Shibata, F.; Araki, H.; Hoshino, Y. Plant Regeneration with Maintenance of the Endosperm Ploidy Level by Endosperm Culture in Lonicera caerulea var. emphyllocalyx. Plant Cell Tissue Org. 2009, 98, 291–301. [Google Scholar] [CrossRef]
- Gmitter, F.G.; Ling, X.B.; Deng, X.X. Induction of Triploid Citrus Plants from Endosperm Calli in Vitro. Theor. Appl. Genet. 1990, 80, 785–790. [Google Scholar] [CrossRef]
- Garg, L.; Bhandari, N.N.; Rani, V.; Bhojwani, S.S. Somatic Embryogenesis and Regeneration of Triploid Plants in Endosperm Cultures of Acacia nilotica. Plant Cell Rep. 1996, 15, 855–858. [Google Scholar] [CrossRef]
- Hoshino, Y.; Miyashita, T.; Thomas, T.D. In Vitro Culture of Endosperm and Its Application in Plant Breeding: Approaches to Polyploidy Breeding. Sci. Hortic. 2011, 130, 1–8. [Google Scholar] [CrossRef]
- Azmi, T.K.K.; Sukma, D.; Aziz, S.A.; Syukur, M. Polyploidy Induction of Moth Orchid (Phalaenopsis amabilis (L.) Blume) by Colchicine Treatment on Pollinated Flowers. J. Agric. Sci. 2016, 11, 62–73. [Google Scholar] [CrossRef]
- Rao, S.P.; Kang, X.Y.; Li, J.; Chen, J.H. Induction, Identification and Characterization of Tetraploidy in Lycium ruthenicum. Breed. Sci. 2019, 69, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, K.; Kareem, A.; Deng, Z. In Vivo Induction and Characterization of Polyploids in Gerbera Daisy. Sci. Hortic. 2021, 282, 110054. [Google Scholar] [CrossRef]
- Kang, X.Y.; Wei, H.R. Breeding Polyploid Populus: Progress and Perspective. For. Res. 2022, 2, 4. [Google Scholar] [CrossRef]
- Zhou, Q.; Cheng, X.T.; Kong, B.; Zhao, Y.F.; Li, Z.Q.; Sang, Y.R.; Wu, J.; Zhang, P.D. Heat Shock-Induced Failure of Meiosis I to Meiosis II Transition Leads to 2n Pollen Formation in a Woody Plant. Plant Physiol. 2022, 189, 2110–2127. [Google Scholar] [CrossRef]
- Chung, M.Y.; Kim, C.Y.; Min, J.S.; Lee, D.J.; Naing, A.H.; Chung, J.D.; Kim, C.K. In Vitro Induction of Tetraploids in an Interspecific Hybrid of Calanthe (Calanthe discolor × Calanthe sieboldii) through Colchicine and Oryzalin Treatments. Plant Biotechnol. Rep. 2014, 8, 251–257. [Google Scholar] [CrossRef]
- Zaker Tavallaie, F.; Kolahi, H. Induction of in Vitro Polyploidy in Ornamental Flowers of Orchid Species (Phalaenopsis amabilis). Iran. J. Rangel. For. Plant Breed. Genet. Res. 2017, 25, 259–270. [Google Scholar]
- Lan, Y.; Qu, L.W.; Xin, H.Y.; Gong, H.L.; Lei, J.J.; Xi, M.L. Physical Mapping of RDNA and Karyotype Analysis in Tulipa sinkiangensis and T. schrenkii. Sci. Hortic. 2018, 240, 638–644. [Google Scholar] [CrossRef]
- Xin, H.Y.; Zhang, T.; Wu, Y.F.; Zhang, W.L.; Zhang, P.D.; Xi, M.L.; Jiang, J.M. An Extraordinarily Stable Karyotype of the Woody Populus Species Revealed by Chromosome Painting. Plant J. 2020, 101, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Z.; Wang, Y.L.; He, Z.C.; Ejder, E. Genome Differentiation in Magonoliaceae as Revealed from Meiotic Pairing in Interspecific and Intergeneric Hybrids. J. Syst. Evol. 2011, 49, 518–527. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.M.; Zhang, Y.Y.; Zhang, Y.; Xu, S.C.; Lu, Y.M. The Application of Fluorescence in Situ Hybridization in Different Ploidy Levels Cross-Breeding of Lily. PLoS ONE 2015, 10, e0126899. [Google Scholar] [CrossRef]
- Zhang, X.X.; Ren, G.L.; Li, K.H.; Zhou, G.X.; Zhou, S.J. Genomic Variation of New Cultivars Selected from Distant Hybridization in Lilium: Genomic Variation of New Lilium Cultivars. Plant Breed. 2012, 131, 227–230. [Google Scholar] [CrossRef]
- Roux, N.; Toloza, A.; Radecki, Z.; Zapata-Arias, F.J.; Dolezel, J. Rapid Detection of Aneuploidy in Musa Using Flow Cytometry. Plant Cell Rep. 2003, 21, 483–490. [Google Scholar] [CrossRef]
- Henry, I.M.; Dilkes, B.P.; Comai, L. Molecular Karyotyping and Aneuploidy Detection in Arabidopsis Thaliana Using Quantitative Fluorescent Polymerase Chain Reaction. Plant J. 2006, 48, 307–319. [Google Scholar] [CrossRef]
- Chagné, D.; Kirk, C.; Whitworth, C.; Erasmuson, S.; Bicknell, R.; Sargent, D.J.; Kumar, S.; Troggio, M. Polyploid and Aneuploid Detection in Apple Using a Single Nucleotide Polymorphism Array. Tree Genet. Genomes 2015, 11, 94. [Google Scholar] [CrossRef]
| Species | Ploidy | Reference |
|---|---|---|
| Lilium lancifolium | 2n = 3x | [27] |
| Magnolia spp. | 2n = 4x, 6x, 8x | [7] |
| Lycoris spp. | 2n = 3x, 4x | [28,29] |
| Narcissus spp. | 2n = 3x | [20] |
| Primula spp. | 2n = 4x, 6x | [30] |
| Chrysanthemum spp. | 2n = 4x, 5x, 6x, 7x, 8x, 10x | [6] |
| Habenaria aitchisonii | 2n = 4x | [31] |
| Phalaenopsis amabilis | 2n = 4x | [8] |
| Malus spp. | 2n = 3x, 4x, 5x | [32] |
| Styrax spp. | 2n = 4x, 5x | [33,34] |
| Fragaria spp. | 2n = 4x, 6x, 8x, 10x | [35] |
| Oxalistuberosa | 2n = 8x | [36] |
| Zephyranthes grandiflora | 2n = 8x | [37] |
| Sequoia sempervirens | 2n = 6x | [38] |
| Paeonia mairei | 2n = 4x | [39] |
| Camellia reticulata | 2n = 4x, 6x | [40] |
| Rosa spp. | 2n = 4x, 5x, 6x, 10x | [41,42,43] |
| Hibiscus paramutabilis | 2n = 4x | [44] |
| Ilex theezans | 2n = 4x | [45] |
| Oenothera spp. | 2n = 4x, 6x, 8x | [46] |
| Deutzia spp. | 2n = 4x | [47] |
| Alnus spp. | 2n = 4x, 6x, 8x, 16x | [48] |
| Neolamarckia cadamba | 2n = 4x | [49] |
| Species | Explant | Induction Method | Method | Concentration, Duration | Result | Reference |
|---|---|---|---|---|---|---|
| Populus canescens | pollen | high temperature | detached heat treatment | 38~41 °C for 3 and 6 h | 42 triploids seedlings | [82] |
| Populus canescens | pollen | colchicine | injection | 0.5% for injection 11 times (2 h/time) | 30.27% 2n pollen | [87] |
| Populus tomentosa | pollen | colchicine | injection | 0.5% for injection 3, 5, 7 times (2 h/time) | 68 triploids seedlings | [88] |
| Dimocarpus longan | pollen | colchicine | absorbent cotton wrapping | 0.9% for 2 d | 19.46% 2n pollen | [85] |
| Dimocarpus longan | pollen | high temperature | 38 °C for 10 d | 5.7% 2n pollen | [84] | |
| Eucommia ulmoides | megasporogenesis | high temperature | bagging | 42~48 °C for 2~6 h | 23 triploids seedlings | [83] |
| Lily | pollen | high temperature | greenhouse | 30 °C for 4 h and 8 h | 1~5% 2n pollen | [86] |
| Eucalyptus urophylla | pollen | colchicine | injection | 0.5% for 3 and 6 h | 28.71% 2n pollen | [89] |
| Phalaenopsis | pollen | colchicine | absorbent cotton wrapping | 0.05% for 3 d | 0.9~1.78% 2n pollen | [90] |
| Tulipa | pollen | N2O | high-pressure container | 6 atm for 24 h | 16~26.7% 2n pollen | [91] |
| Phalaenopsis amabilis | pollen | N2O | high-pressure container | 48 h | 35.6% triploids seedlings | [92] |
| Populus | pollen | gibberellins | injection | 10 μM for 7 times | 21.37% 2n pollen | [93] |
| Paeonia lactiflora | pollen | colchicine | injection | 0.4% for 2 times | 7.79~47.39% 2n pollen | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, L.; Liu, Z.; Yin, Y.; Zou, Y.; Faizan, M.; Alam, P.; Yu, F. Research Progress of Chromosome Doubling and 2n Gametes of Ornamental Plants. Horticulturae 2023, 9, 752. https://doi.org/10.3390/horticulturae9070752
Cui L, Liu Z, Yin Y, Zou Y, Faizan M, Alam P, Yu F. Research Progress of Chromosome Doubling and 2n Gametes of Ornamental Plants. Horticulturae. 2023; 9(7):752. https://doi.org/10.3390/horticulturae9070752
Chicago/Turabian StyleCui, Luomin, Zemao Liu, Yunlong Yin, Yiping Zou, Mohammad Faizan, Pravej Alam, and Fangyuan Yu. 2023. "Research Progress of Chromosome Doubling and 2n Gametes of Ornamental Plants" Horticulturae 9, no. 7: 752. https://doi.org/10.3390/horticulturae9070752
APA StyleCui, L., Liu, Z., Yin, Y., Zou, Y., Faizan, M., Alam, P., & Yu, F. (2023). Research Progress of Chromosome Doubling and 2n Gametes of Ornamental Plants. Horticulturae, 9(7), 752. https://doi.org/10.3390/horticulturae9070752








