From Fresh to Dried Lavender Flower: Changes in Phytochemical Profile According to Drying Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Drying Method
2.3. Ultrasound-Assisted Extraction (UAE)
2.4. Bioactive Compounds
2.4.1. Total Phenolic Content, Total Anthocyanin Content, and Antioxidant Activity
2.4.2. Phenolic Profile
2.5. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic Content, Total Anthocyanin Content, and Antioxidant Activity
3.2. Phenolic Profile
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernandes, L.; Ramalhosa, E.; Baptista, P.; Saraiva, J.A.; Casal, S.I.P. Nutritional and Nutraceutical Composition of Pansies (Viola × wittrockiana) During Flowering. J. Food Sci. 2019, 84, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.I.; Barros, L.; Dueñas, M.; Pereira, E.; Carvalho, A.M.; Alves, R.C.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical composition of wild and commercial Achillea millefolium L. and bioactivity of the methanolic extract, infusion and decoction. Food Chem. 2013, 141, 4152–4160. [Google Scholar] [CrossRef] [PubMed]
- Różyło, R. Recent trends in methods used to obtain natural food colorants by freeze-drying. Trends Food Sci. Technol. 2020, 102, 39–50. [Google Scholar] [CrossRef]
- Rop, O.; Mlcek, J.; Jurikova, T.; Neugebauerova, J.; Vabkova, J. Edible Flowers—A New Promising Source of Mineral Elements in Human Nutrition. Molecules 2012, 17, 6672–6683. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Edible flowers: A review of the nutritional, antioxidant, antimicrobial properties and effects on human health. J. Food Compos. Anal. 2017, 60, 38–50. [Google Scholar] [CrossRef]
- Ceccanti, C.; Landi, M.; Benvenuti, S.; Pardossi, A.; Guidi, L. Mediterranean Wild Edible Plants: Weeds or “ New Functional Crops ”? Molecules 2018, 23, 2299. [Google Scholar] [CrossRef]
- Fernandes, L.; Saraiva, J.A.; Pereira, J.A.; Casal, S.; Ramalhosa, E. Post-harvest technologies applied to edible flowers: A review. Food Rev. Int. 2019, 35, 132–154. [Google Scholar] [CrossRef]
- Brito, C.; Bertotti, T.; Primitivo, M.J.; Neves, M.; Pires, C.L.; Cruz, P.F.; Martins, P.A.T.; Rodrigues, C.A.; Moreno, M.J.; Brito, R.M.M.; et al. Corema album spp: Edible wild crowberries with a high content in minerals and organic acids. Food Chem. 2021, 345, 128732. [Google Scholar] [CrossRef]
- Devecchi, A.; Demasi, S.; Saba, F.; Rosato, R.; Gambino, R.; Ponzo, V.; De Francesco, A.; Massarenti, P.; Bo, S.; Scariot, V. Compositional Characteristics and Antioxidant Activity of Edible Rose Flowers and Their Effect on Phenolic Urinary Excretion. Polish J. Food Nutr. Sci. 2021, 71, 383–392. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Tenuta, M.C.; Menichini, F.; Xiao, J.; Tundis, R. Edible Flowers: A Rich Source of Phytochemicals with Antioxidant and Hypoglycemic Properties. J. Agric. Food Chem. 2016, 64, 2467–2474. [Google Scholar] [CrossRef]
- Scariot, V.; Gaino, W.; Demasi, S.; Caser, M.; Ruffoni, B. Flowers for edible gardens: Combinations of species and colours for northwestern Italy. Acta Hortic. 2018, 1215, 363–367. [Google Scholar] [CrossRef]
- Zheng, J.; Yu, X.; Maninder, M.; Xu, B. Total phenolics and antioxidants profiles of commonly consumed edible flowers in China. Int. J. Food Prop. 2018, 21, 1524–1540. [Google Scholar] [CrossRef]
- Demasi, S.; Falla, N.M.; Caser, M.; Scariot, V. Postharvest aptitude of Begonia semperflorens and Viola cornuta edible flowers. Adv. Hortic. Sci. 2020, 34, 13–20. [Google Scholar] [CrossRef]
- Demasi, S.; Caser, M.; Donno, D.; Enri, S.R.; Lonati, M.; Scariot, V. Exploring wild edible flowers as a source of bioactive compounds: New perspectives in horticulture. Folia Hortic. 2021, 33, 27–48. [Google Scholar] [CrossRef]
- Falla, N.M.; Demasi, S.; Caser, M.; Scariot, V. Preliminary observations on viola calcarata as a source of bioactive compounds: Antioxidant activity and phytochemical profile of two alpine subspecies. Agronomy 2021, 11, 2241. [Google Scholar] [CrossRef]
- Demasi, S.; Mellano, M.G.; Falla, N.M.; Caser, M.; Scariot, V. Sensory profile, shelf life, and dynamics of bioactive compounds during cold storage of 17 edible flowers. Horticulturae 2021, 7, 166. [Google Scholar] [CrossRef]
- Demasi, S.; Caser, M.; Lonati, M.; Gaino, W.; Scariota, V. Ornamental traits of Lavandula angustifolia Mill. are affected by geographical origin and cultivation substrate composition. Acta Hortic. 2021, 1331, 49–55. [Google Scholar] [CrossRef]
- Prusinowska, R.; Smigielski, K.B. Composition, biological properties and therapeutic effects of lavender (Lavandula angustifolia L.) A review. Herba Pol. 2014, 60, 56–66. [Google Scholar] [CrossRef]
- Lyczko, J.; Jałoszynski, K.; Surma, M.; Garcia-Garvì, J.M.; Carbonell-Barrachina, A.A.; Szumny, A. Determination of Various Drying Methods’ Impact on Odour Quality of True Lavender (Lavandula angustifolia Mill.) Flowers. Molecules 2019, 24, 2900. [Google Scholar] [CrossRef]
- Najar, B.; Demasi, S.; Caser, M.; Gaino, W.; Cioni, P.L.; Pistelli, L.; Scariot, V. Cultivation Substrate Composition Influences Morphology, Volatilome and Essential Oil of Lavandula angustifolia Mill. Agronomy 2019, 9, 411. [Google Scholar] [CrossRef]
- Slimani, C.; Sqalli, H.; Rais, C.; Farah, A.; Lazraq, A.; El Ghadraoui, L.; Belmalha, S.; Echchgadda, G. Chemical composition and evaluation of biological effects of essential oil and aqueous extract of Lavandula angustifolia L. Not. Sci. Biol. 2022, 14, 1172. [Google Scholar] [CrossRef]
- Demasi, S.; Caser, M.; Lonati, M.; Cioni, P.L.; Pistelli, L.; Najar, B.; Scariot, V. Latitude and altitude influence secondary metabolite production in peripheral alpine populations of the mediterranean species Lavandula angustifolia Mill. Front. Plant Sci. 2018, 9, 983. [Google Scholar] [CrossRef] [PubMed]
- Dorozko, J.; Kunkulberga, D.; Sivicka, I.; Kruma, Z. The influence of various drying methods on the quality of edible flower petals. In Proceedings of the 13th Baltic Conference on Food Science and Technology “Food. Nutrition. Well-Being”, Jelgava, Latvia, 2–3 May 2019; pp. 182–187. [Google Scholar]
- Mirjalili, H.M.; Salehi, P.; Vala, M.M.; Ghorbanpour, M. The effect of drying methods on yield and chemical constituents of the essential oil in Lavandula angustifolia Mill. (Lamiaceae). Indian J. Plant Physiol. 2019, 24, 96–103. [Google Scholar] [CrossRef]
- Zhao, L.; Fan, H.; Zhang, M.; Chitrakar, B.; Bhandari, B.; Wang, B. Edible flowers: Review of flower processing and extraction of bioactive compounds by novel technologies. Food Res. Int. 2019, 126, 108660. [Google Scholar] [CrossRef]
- Patel, K.K.; Kar, A. Heat pump assisted drying of agricultural produce—An overview. J. Food Sci. Technol. 2012, 49, 142–160. [Google Scholar] [CrossRef]
- Boyar, S.; Dikmen, E.; Erbaş, S. Drying of Edible Flowers. In Handbook of Drying of Vegetables and Vegetable Products; Zhang, M., Bhandari, B., Fang, Z., Eds.; Taylor & Francis: Abingdon, UK, 2017; pp. 195–234. [Google Scholar]
- Youssef, K.M.; Mokhtar, S.M. Effect of Drying Methods on the Antioxidant Capacity, Color and Phytochemicals of Portulaca oleracea L. Leaves. Nutr. Food Sci. 2014, 4, 6. [Google Scholar] [CrossRef]
- Méndez-lagunas, L.; Rodríguez-Ramírez, J.; Cruz-Gracida, M.; Sandoval-Torres, S.; Barriada-Bernal, G. Convective drying kinetics of strawberry (Fragaria ananassa): Effects on antioxidant activity, anthocyanins and total phenolic content. Food Chem. 2017, 230, 174–181. [Google Scholar] [CrossRef]
- Orphanides, A.; Goulas, V.; Gekas, V. Effect of drying method on the phenolic content and antioxidant capacity of spearmint. Czech J. Food Sci. 2013, 31, 509–513. [Google Scholar] [CrossRef]
- Geng, W.G.; Li, Z.C.; Yuan, L.D.; Sun, R.F. An improvement of rose flowers drying process recovering volatile compounds by heat pump systems. Earth Environ. Sci. 2020, 594, 012006. [Google Scholar] [CrossRef]
- Demasi, S.; Caser, M.; Scariot, V. Hot and cold drying of edible flowers affects metabolite pattern in extracts and decoctions. Folia Hortic. 2023, 35, 1–15. [Google Scholar] [CrossRef]
- Falla, N.M.; Caser, M.; Demasi, S.; Scariot, V. Heat Pump Drying of Lavender Flowers Leads to Decoctions Richer in Bioactive Compounds. Agronomy 2022, 12, 3162. [Google Scholar] [CrossRef]
- Zheng, J.; Lu, B.; Xu, B. An update on the health benefits promoted by edible flowers and involved mechanisms. Food Chem. 2021, 340, 127940. [Google Scholar] [CrossRef] [PubMed]
- Weremfo, A.; Adulley, F.; Dabie, K.; Abassah-Oppong, S.; Peprah-Yamoah, E. Optimization of ultrasound-assisted extraction of phenolic antioxidants from turkey berry (Solanum torvum Sw) fruits using response surface methodology. J. Appl. Res. Med. Aromat. Plants 2022, 30, 100387. [Google Scholar] [CrossRef]
- Sobhani, A.; Noormohammadi, N.; Moradi, K.; Ebrahimi, M.; Khanahmadi, M. Optimization of heat and ultrasound assisted extraction of bioactive compounds from Echinacea purpurea using response surface methodology. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100399. [Google Scholar] [CrossRef]
- Stelluti, S.; Caser, M.; Demasi, S.; Scariot, V. Sustainable processing of floral bio-residues of saffron (Crocus sativus L.) for valuable biorefinery products. Plants 2021, 10, 523. [Google Scholar] [CrossRef]
- Caser, M.; Demasi, S.; Stelluti, S.; Donno, D.; Scariot, V. Crocus sativus L. Cultivation in Alpine Environments: Stigmas and Tepals as Source of Bioactive Compounds. Agronomy 2020, 10, 1473. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin- Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Sánchez-Rangel, J.C.; Benavides Lozano, J.A.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin-Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Wong, S.P.; Leong, L.P.; William Koh, J.H. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006, 99, 775–783. [Google Scholar] [CrossRef]
- Tawaha, K.; Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007, 104, 1372–1378. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Caser, M.; Demasi, S.; Victorino, M.M.I.; Donno, D.; Faccio, A.; Lumini, E.; Bianciotto, V.; Scariot, V. Arbuscular Mycorrhizal Fungi Modulate the Crop Performance and Metabolic Profile of Saffron in Soilless Cultivation. Agronomy 2019, 9, 232. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; Riondato, I.; De Biaggi, M.; Andriamaniraka, H.; Gamba, G.; Beccaro, G.L. Traditional and Unconventional Dried Fruit Snacks as a Source of Health-Promoting Compounds. Antioxidants 2019, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Stelluti, S.; Caser, M.; Demasi, S.; Rodriguez Herrero, E.; García-González, I.; Lumini, E.; Bianciotto, V.; Scariot, V. Beneficial microorganisms: A sustainable horticultural solution to improve the quality of saffron in hydroponics. Sci. Hortic. 2023, 319, 112155. [Google Scholar] [CrossRef]
- Sun, T.; Tanumihardjo, S.A. An integrated approach to evaluate food antioxidant capacity. J. Food Sci. 2007, 72, 159–165. [Google Scholar] [CrossRef]
- Van Leeuw, R.; Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Antioxidant capacity and phenolic composition of red wines from various grape varieties: Specificity of Pinot Noir. J. Food Compos. Anal. 2014, 36, 40–50. [Google Scholar] [CrossRef]
- Radu, D.; Râpeanu, G.; Bahrim, G.E.; Stânciuc, N. Investigations on thermal degradation of phytochemicals from lavender extract. Food Technol. 2019, 43, 33–47. [Google Scholar]
- Calín-Sánchez, Á.; Lipan, L.; Cano-Lamadrid, M.; Kharaghani, A.; Masztalerz, K.; Carbonell-Barrachina, Á.A.; Figiel, A. Comparison of Traditional and Novel Drying Techniques and Its Effect on Quality of Fruits, Vegetables and Aromatic Herbs. Foods 2020, 9, 1261. [Google Scholar] [CrossRef]
- Dobros, N.; Zawada, K.; Paradowska, K. Phytochemical Profile and Antioxidant Activity of Lavandula angustifolia and Lavandula x intermedia Cultivars Extracted with Different Methods. Antioxidants 2022, 11, 711. [Google Scholar] [CrossRef] [PubMed]
- Duda, S.C.; Marghitas, L.A.; Dezmirean, D.; Duda, M.; Margaoan, R.; Bobis, O. Changes in major bioactive compounds with antioxidant activity of Agastache foeniculum, Lavandula angustifolia, Melissa officinalis and Nepeta cataria: Effect of harvest time and plant species. Ind. Crop. Prod. 2015, 77, 499–507. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Toydemir, G.; Boyacioglu, D.; Beekwilder, J.; Hall, R.D.; Capanoglu, E. A Review on the Effect of Drying on Antioxidant Potential of Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2016, 56, S110–S129. [Google Scholar] [CrossRef] [PubMed]
- Leporini, M.; Bonesi, M.; Loizzo, M.R.; Passalacqua, N.G.; Tundis, R. The essential oil of Salvia rosmarinus Spenn. from Italy as a source of health-promoting compounds: Chemical profile and antioxidant and cholinesterase inhibitory activity. Plants 2020, 9, 798. [Google Scholar] [CrossRef]
- Petrović, M.; Sužnjević, D.; Pastor, F.; Veljović, M.; Pezo, L.; Antić, M.; Gorjanović, S. Antioxidant Capacity Determination of Complex Samples and Individual Phenolics—Multilateral Approach. Comb. Chem. High Throughput Screen. 2016, 19, 58–65. [Google Scholar] [CrossRef]
- Ananingsih, V.K.; Sharma, A.; Zhou, W. Green tea catechins during food processing and storage: A review on stability and detection. Food Res. Int. 2013, 50, 469–479. [Google Scholar] [CrossRef]
- Cao, H.; Saroglu, O.; Karadag, A.; Diaconeasa, Z.; Zoccatelli, G.; Conte-Junior, C.A.; Gonzalez-Aguilar, G.A.; Ou, J.; Bai, W.; Zamarioli, C.M.; et al. Available technologies on improving the stability of polyphenols in food processing. Food Front. 2021, 2, 109–139. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Ozdemir, F.A.; Kołodziej, B. In vitro bioaccessibility and activity of basil (Ocimum basilicum L.) phytochemicals as affected by cultivar and postharvest preservation method—Convection drying, freezing, and freeze-drying. Food Chem. 2022, 382, 132363. [Google Scholar] [CrossRef]
- Alasalvar, H.; Yildirim, Z. Ultrasound-assisted extraction of antioxidant phenolic compounds from Lavandula angustifolia flowers using natural deep eutectic solvents: An experimental design approach. Sustain. Chem. Pharm. 2021, 22, 100492. [Google Scholar] [CrossRef]
| Classes of Compounds | Standard | Retention Time (t R) (min) | Mobile Phase | Elution Conditions | Wavelength (nm) | LOD (mg L−1) | LOQ (mg L−1) |
|---|---|---|---|---|---|---|---|
| Cinnamic acids | Caffeic acid | 4.54 | A: 10 mM KH2PO4/H3PO4 pH = 2.8 B: CH3CN | 5% B to 21% B in 17 min + 21% B in 3 min (2 min conditioning time); flow: 1.5 mL min−1 | 330 | 0.305 | 1.016 |
| Chlorogenic acid | 3.89 | 0.940 | 3.134 | ||||
| Coumaric acid | 6.74 | 2.907 | 9.690 | ||||
| Ferulic acid | 7.99 | 1.245 | 4.150 | ||||
| Flavonols | Hyperoside | 10.89 | 3.372 | 11.241 | |||
| Isoquercitrin | 11.24 | 0.252 | 0.840 | ||||
| Quercetin | 17.67 | 4.055 | 13.518 | ||||
| Quercitrin | 13.28 | 5.456 | 18.187 | ||||
| Rutin | 12.95 | 2.937 | 9.790 | ||||
| Benzoic acids | Ellagic acid | 18.65 | A: H2O/CH3OH/HCOOH (5:95:0.1 v/v/v), pH = 2.5 B: CH3OH/HCOOH (100:0.1 v/v) | 3% B to 85% B in 22 min + 85% B in 1 min (2 min conditioning time); flow: 0.6 mL min−1 | 280 | 0.611 | 2.035 |
| Gallic acid | 4.26 | 0.435 | 1.451 | ||||
| Catechins | Catechin | 10.31 | 2.343 | 7.809 | |||
| Epicatechin | 14.3 | 0.763 | 2.543 |
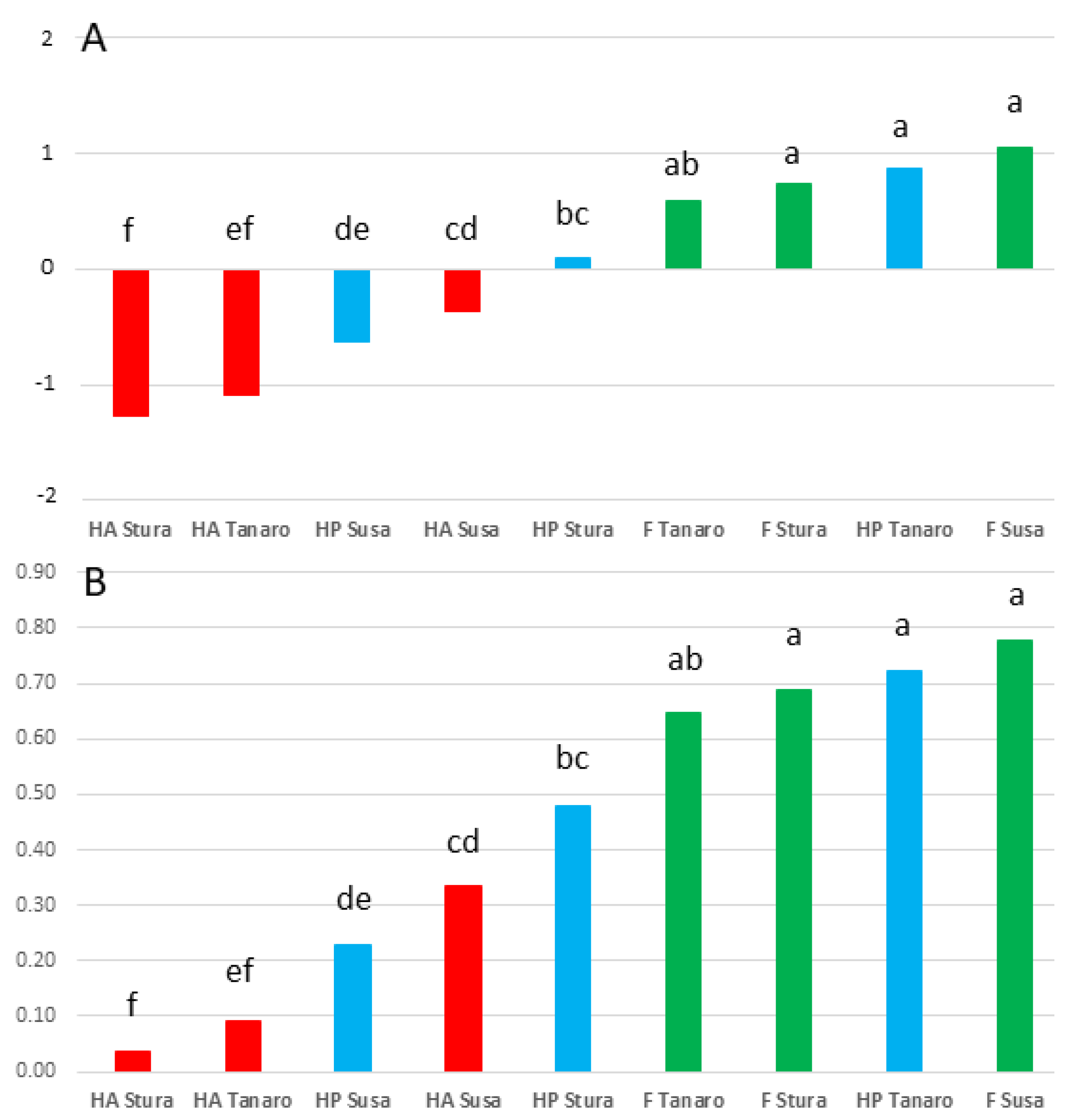
| SELECTION (A) | TPC (mg GAE/100 g DM) | TAC (mg C3G/100 g DM) | FRAP (mmol Fe2+/kg DM) | DPPH (µmol TE/g DM) | ABTS (µmol TE/g DM) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Susa | 662.29 | 44.70 | c | 210.41 | ab | 34.95 | 29.08 | |||
| Stura | 777.56 | 66.99 | b | 198.44 | b | 26.82 | 29.30 | |||
| Tanaro | 781.01 | 95.75 | a | 262.06 | a | 27.49 | 33.00 | |||
| p | ns | *** | * | ns | ns | |||||
| PROCESSING METHOD (B) | TPC (mg GAE/100 g DM) | TAC (mg C3G/100 g DM) | FRAP (mmol Fe2+/kg DM) | DPPH (µmol TE/g DM) | ABTS (µmol TE/g DM) | |||||
| F | 1268.43 | a | 12.51 | c | 286.63 | a | 47.36 | a | 44.81 | a |
| HA | 357.08 | c | 74.34 | b | 79.35 | b | 16.06 | c | 17.89 | c |
| HP | 595.35 | b | 120.59 | a | 304.93 | a | 25.84 | b | 28.68 | b |
| p | *** | *** | *** | *** | *** | |||||
| INTERACTION | TPC | TAC | FRAP | DPPH | ABTS | |||||
| p | ||||||||||
| A × B | *** | *** | ** | *** | *** | |||||
| SELECTION (A) | TPC (mg GAE/100 g DM) | TAC (mg C3G/100 g DM) | FRAP (mmol Fe2+/kg DM) | DPPH (µmol TE/g DM) | ABTS (µmol TE/g DM) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| F Susa | 1133.36 | abc | 13.65 | cd | 260.55 | ab | 66.98 | a | 45.02 | a |
| F Stura | 1427.94 | a | 13.65 | d | 326.19 | ab | 39.30 | ab | 43.68 | ab |
| F Tanaro | 1244.00 | ab | 10.24 | d | 273.15 | ab | 35.81 | ab | 45.73 | ab |
| HA Susa | 597.53 | cde | 69.56 | bc | 77.10 | cd | 32.82 | ab | 29.68 | bcd |
| HA Stura | 220.74 | f | 32.08 | cd | 61.59 | d | 6.58 | c | 11.06 | e |
| HA Tanaro | 252.95 | ef | 121.40 | ab | 99.35 | bcd | 8.77 | bc | 12.93 | cde |
| HP Susa | 255.98 | def | 50.89 | bcd | 293.58 | ab | 5.06 | c | 12.54 | de |
| HP Stura | 684.00 | bcd | 155.25 | a | 207.54 | abc | 34.57 | ab | 33.16 | abc |
| HP Tanaro | 846.07 | a–d | 155.62 | a | 413.68 | a | 37.89 | ab | 40.34 | abc |
| p | ** | ** | ** | ** | ** | |||||
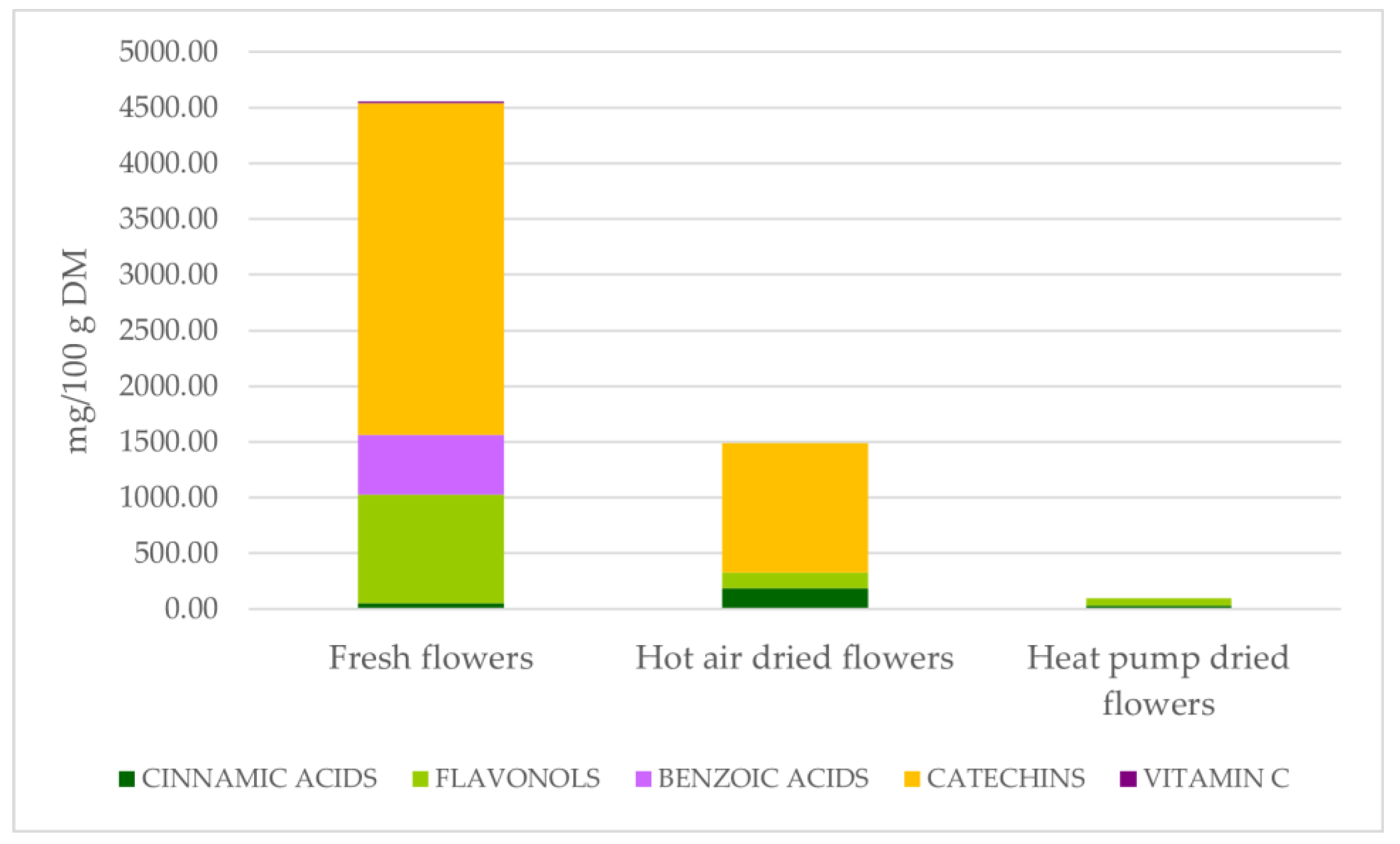
| Processing Method | Cinnamic Acids | Flavonols | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Caffeic Acid | Ferulic Acid | Hyperoside | Quercetin | Quercitrin | ||||||
| F | 37.40 | a | 0.00 | b | 55.26 | 664.06 | a | 0.00 | b | |
| HA | 0.00 | c | 62.37 | a | 36.71 | 0.00 | b | 10.69 | a | |
| HP | 9.60 | b | 0.00 | b | 22.65 | 0.00 | b | 0.00 | b | |
| p | *** | ** | ns | ** | ** | |||||
| Processing Method | Benzoic Acids | CATECHINS | Vitamin C | |||||||
| Ellagic Acid | Catechin | Epicatechin | Dehydroascorbic Acid | |||||||
| F | 393.39 | a | 1203.21 | a | 982.09 | a | 8.94 | a | ||
| HA | 0.00 | b | 0.00 | b | 387.49 | b | 0.00 | b | ||
| HP | 0.00 | b | 0.00 | b | 0.00 | c | 0.00 | b | ||
| p | ** | ** | ** | *** | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caser, M.; Falla, N.M.; Demasi, S.; Scariot, V. From Fresh to Dried Lavender Flower: Changes in Phytochemical Profile According to Drying Method. Horticulturae 2023, 9, 700. https://doi.org/10.3390/horticulturae9060700
Caser M, Falla NM, Demasi S, Scariot V. From Fresh to Dried Lavender Flower: Changes in Phytochemical Profile According to Drying Method. Horticulturae. 2023; 9(6):700. https://doi.org/10.3390/horticulturae9060700
Chicago/Turabian StyleCaser, Matteo, Nicole Mélanie Falla, Sonia Demasi, and Valentina Scariot. 2023. "From Fresh to Dried Lavender Flower: Changes in Phytochemical Profile According to Drying Method" Horticulturae 9, no. 6: 700. https://doi.org/10.3390/horticulturae9060700
APA StyleCaser, M., Falla, N. M., Demasi, S., & Scariot, V. (2023). From Fresh to Dried Lavender Flower: Changes in Phytochemical Profile According to Drying Method. Horticulturae, 9(6), 700. https://doi.org/10.3390/horticulturae9060700










