The Effects of Soybean Meal on Growth, Bioactive Compounds, and Antioxidant Activity of Hericium erinaceus
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Analyses of the Physical Properties and Nutrient Composition of a Mushroom Cultivation Substrate
2.3. Samples Preparation
2.4. Preparation of Extracts
2.5. Determination of Total Triterpenoid Content
2.6. Determination of Total Phenolic Content
2.7. Scavenging Activity on DPPH Radicals
2.8. Statistical Analysis
3. Results and Discussion
3.1. Study of the Physical Properties and Nutrient Composition of the Mushroom Cultivation Substrate
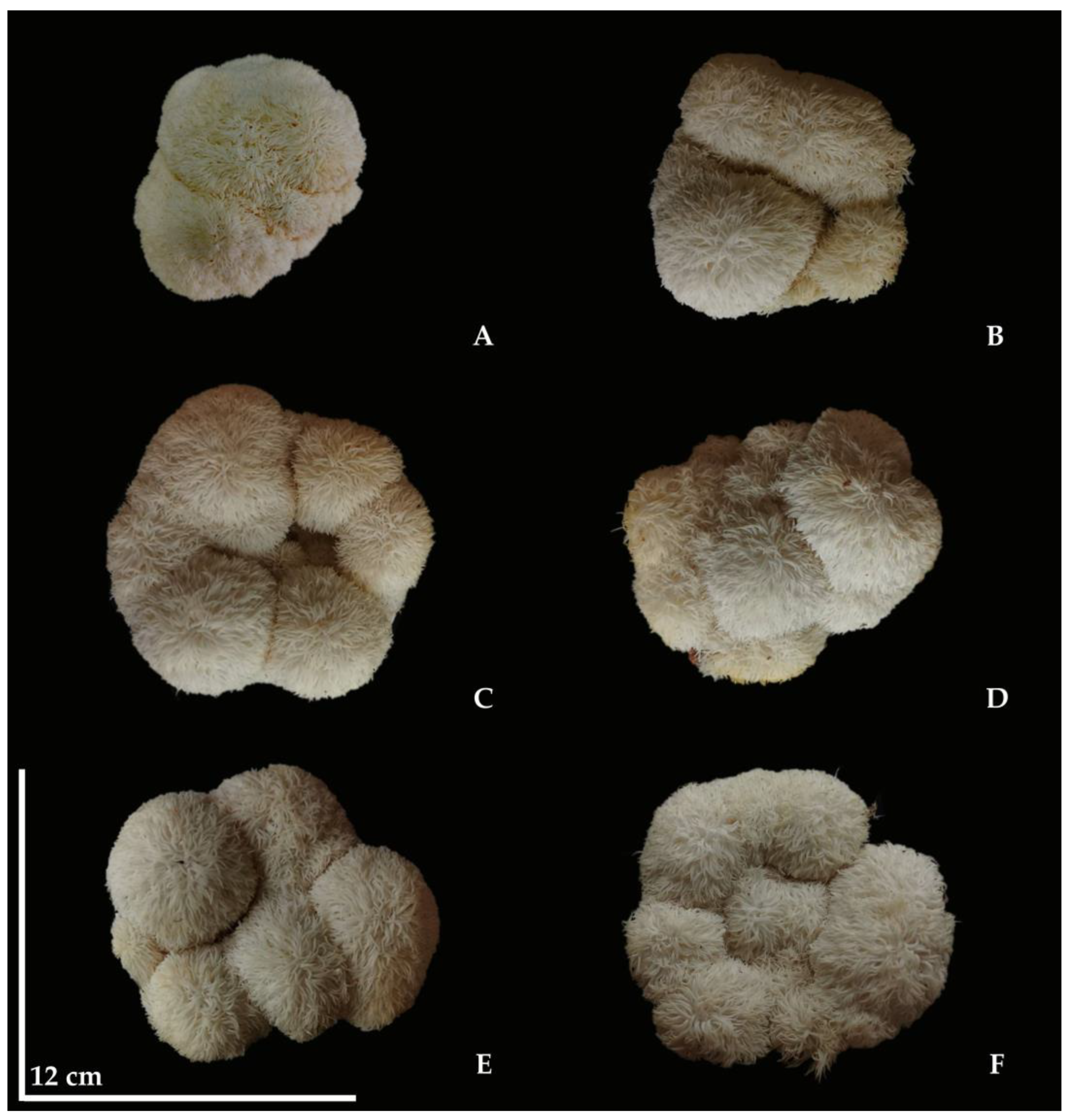
3.2. Growth of H. erinaceus
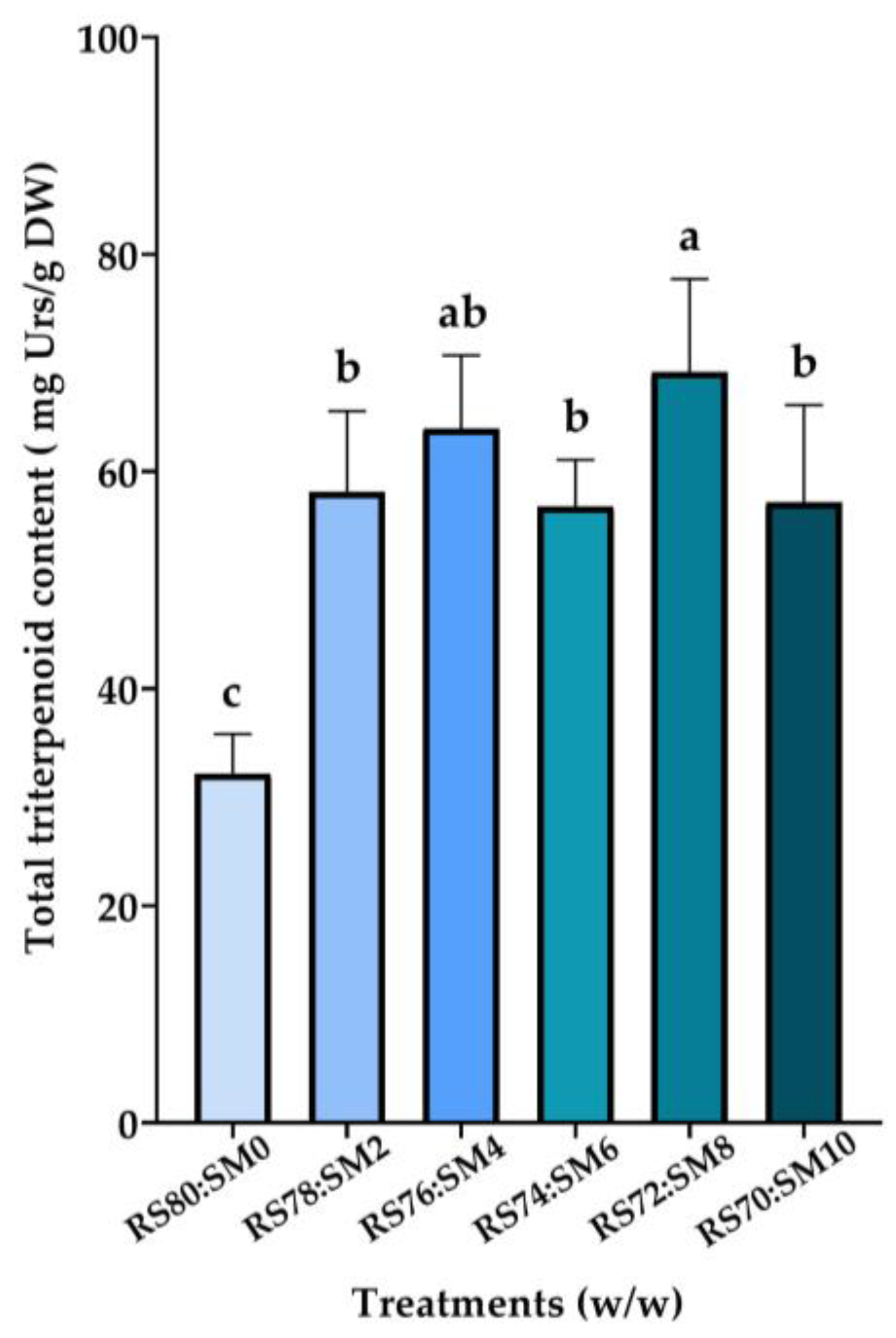
3.3. Determination of Total Triterpenoid Content of H. erinaceus
3.4. Determination of Total Phenolic Content of H. erinaceus
3.5. DPPH Radical Scavenging Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, S.T.; Wasser, S.P. The Cultivation and Environmental Impact of Mushrooms. In Oxford Research Encyclopedia of Environmental Science; Oxford University Press: Oxford, UK, 2017. [Google Scholar] [CrossRef]
- Gong, W.; Wang, Y.; Xie, C.; Zhou, Y.; Zhu, Z.; Peng, Y. Whole genome sequence of an edible and medicinal mushroom, Hericium erinaceus (Basidiomycota, Fungi). Genomics 2020, 112, 2393–2399. [Google Scholar] [CrossRef] [PubMed]
- Sabaratnam, V.; Kah-Hui, W.; Naidu, M.; Rosie David, P. Neuronal health-can culinary and medicinal mushrooms help? J. Tradit. Complement. Med. 2013, 3, 62–68. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, H.; Zhao, C.; Ren, L.; Wang, C.; Georgiev, M.I.; Xiao, J.; Zhang, T. Value added immunoregulatory polysaccharides of Hericium erinaceus and their effect on the gut microbiota. Carbohydr. Polym. 2021, 262, 117668. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, Nutrition, and Health-Promoting Properties of Hericium erinaceus (Lion’s Mane) Mushroom Fruiting Bodies and Mycelia and Their Bioactive Compounds. J. Agric. Food Chem. 2015, 63, 7108–7123. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.-G.; Agar, N.; Jones, G. Oxidative insult to human red blood cells induced by free radical initiator AAPH and its inhibition by a commercial antioxidant mixture. Life Sci. 2001, 69, 75–86. [Google Scholar] [CrossRef]
- Marimuthu, C.; Sudha, G.; Balakrishnan, P. Detection of antioxidant activity and bioactive constituents in the fruiting bodies of hericium erinaceus pers-an edible mushroom. Int. J. Pharm. Pharm. Sci. 2016, 8, 152–156. [Google Scholar]
- Wong, J.-Y.; Abdulla, M.; Raman, J.; Phan, C.-W.; Kuppusamy, U.R.; Golbabapour, S.; Sabaratnam, V. Gastroprotective effects of Lion’s Mane Mushroom Hericium erinaceus (Bull.:Fr.) Pers. (Aphyllophoromycetideae) extract against ethanol-induced ulcer in rats. Evid.-Based Complement. Altern. Med. 2013, 2013, 492976. [Google Scholar] [CrossRef]
- Phan, C.-W.; Guan-Serm, L.; Hong, S.L.; Wong, Y.-T.; Brkljača, R.; Urban, S.; Abd Malek, S.N.; Sabaratnam, V. Hericium erinaceus (Bull.:Fr) Pers. cultivated in tropical conditions: Isolation of hericenones and demonstration of NGF-mediated neurite outgrowth in PC12 cells via MEK/ERK and PI3K-Akt signaling pathways. Food Funct. 2014, 5, 3160–3169. [Google Scholar] [CrossRef]
- Grimm, D.; Wosten, H. Mushroom cultivation in the circular economy. Appl. Microbiol. Biotechnol. 2018, 102, 7795–7803. [Google Scholar] [CrossRef]
- Malinowska, E.; Krzyczkowski, W.; Łapienis, G.; Herold, F. Improved simultaneous production of mycelial biomass and polysaccharides by submerged culture of Hericium erinaceum: Optimization using a central composite rotatable design (CCRD). J. Ind. Microbiol. Biotechnol. 2009, 36, 1513–1527. [Google Scholar] [CrossRef]
- Lin, E.-S.; Chen, Y.-H. Factors affecting mycelial biomass and exopolysaccharide production in submerged cultivation of Antrodia cinnamomea using complex media. Bioresour. Technol. 2007, 98, 2511–2517. [Google Scholar] [CrossRef] [PubMed]
- Fewell, J.; Gustafson, C. Economic Analysis of Using Soybean Meal as a Mushroom Growing Substrate. In Agribusiness & Applied Economics Report; North Dakota State University, Department of Agribusiness and Applied Economics: Fargo, ND, USA, 2007. [Google Scholar]
- Udelhoven, T.; Emmerling, C.; Jarmer, T. Quantitative analysis of soil chemical properties with diffuse reflectance spectrometry and partial least-square regression: A feasibility study. Plant Soil 2003, 251, 319–329. [Google Scholar] [CrossRef]
- Ni, Q.; Xu, G.; Wang, Z.; Gao, Q.; Wang, S.; Zhang, Y. Seasonal Variations of the Antioxidant Composition in Ground Bamboo Sasa argenteastriatus Leaves. Int. J. Mol. Sci. 2012, 13, 2249–2262. [Google Scholar] [CrossRef] [PubMed]
- Folin, O.; Ciocalteu, V. On Tyrosine and Tryptophane Determinations in Proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; van Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Seephonkai, P.; Samchai, S.; Thongsom, A.; Sunaart, S.; Kiemsanmuang, B.; Chakuton, K. DPPH Radical Scavenging Activity and Total Phenolics of Phellinus Mushroom Extracts Collected from Northeast of Thailand. Chin. J. Nat. Med. 2011, 9, 441–445. [Google Scholar] [CrossRef]
- Karr-Lilienthal, L.K.; Bauer, L.L.; Utterback, P.L.; Zinn, K.E.; Frazier, R.L.; Parsons, C.M.; Fahey, G.C. Chemical Composition and Nutritional Quality of Soybean Meals Prepared by Extruder/Expeller Processing for Use in Poultry Diets. J. Agric. Food Chem. 2006, 54, 8108–8114. [Google Scholar] [CrossRef]
- Costa, C.; Geraldo, M.; Arroteia, C.; Kemmelmeier, C. In vitro activity of neem oil [Azadirachta indica A. Juss (Meliaceae)] on Aspergillus flavus growth, sporulation, viability of spores, morphology and Aflatoxins B1 and B2 production. Adv. Biosci. Biotechnol. 2010, 1, 292–299. [Google Scholar] [CrossRef]
- Imtiaj, A.; Jayasinghe, C.; Lee, G.; Shim, M.; Ro, H.-S.; Lee, H.S.; Hur, H.; Lee, M.; Lee, U.Y.; Lee, T.-S. Vegetative Growth of Four Strains of Hericium erinaceus Collected from Different Habitats. Mycobiology 2008, 36, 88–92. [Google Scholar] [CrossRef]
- Pardo Giménez, A.; Pardo-González, J.E. Evaluation of casing materials made from spent mushroom substrate and coconut fibre pith for use in production of Agaricus bisporus (Lange) Imbach. Span. J. Agric. Res. 2008, 6, 683–690. [Google Scholar] [CrossRef]
- Owaid, M.N.; Abed, I.A.; Al-Saeedi, S.S.S. Applicable properties of the bio-fertilizer spent mushroom substrate in organic systems as a byproduct from the cultivation of Pleurotus spp. Inf. Process. Agric. 2017, 4, 78–82. [Google Scholar] [CrossRef]
- Wood, D.A. Extracellular enzymes as targets for strain improvement in Agaricus bisporus. In Proceedings of the First International Congress on Cultivated Mushroom, The Chinese University, Hong Kong, 23–26 August 1993; p. 1993. [Google Scholar]
- Xie, C.; Gong, W.; Yan, L.; Zhu, Z.; Hu, Z.; Peng, Y. Biodegradation of ramie stalk by Flammulina velutipes: Mushroom production and substrate utilization. AMB Express 2017, 7, 171. [Google Scholar] [CrossRef]
- Harith, N.; Abdullah, N.; Sabaratnam, V. Cultivation of Flammulina velutipes mushroom using various agro-residues as a fruiting substrate. Pesqui. Agropecuária Bras. 2014, 49, 181–188. [Google Scholar] [CrossRef]
- Dulay, R.M.R.; Cabrera, E.C.; Kalaw, S.P.; Reyes, R.G. Optimization of submerged culture conditions for mycelial biomass production of fourteen Lentinus isolates from Luzon Island, Philippines. Biocatal. Agric. Biotechnol. 2021, 38, 102226. [Google Scholar] [CrossRef]
- Bellettini, M.B.; Fiorda, F.A.; Maieves, H.A.; Teixeira, G.L.; Ávila, S.; Hornung, P.S.; Júnior, A.M.; Ribani, R.H. Factors affecting mushroom Pleurotus spp. Saudi J. Biol. Sci. 2019, 26, 633–646. [Google Scholar] [CrossRef]
- Lin, Q.; Long, L.; Wu, L.; Zhang, F.; Wu, S.; Zhang, W.; Sun, X. Evaluation of different agricultural wastes for the production of fruiting bodies and bioactive compounds by medicinal mushroom Cordyceps militaris. J. Sci. Food Agric. 2017, 97, 3476–3480. [Google Scholar] [CrossRef]
- Ashrafi, R.; Mian, M.H.; Rahman, M.M.; Jahiruddin, M. Recycling of Spent Mushroom Substrate for the Production of Oyster Mushroom. Res. Biotechnol. 2014, 5, 13–21. [Google Scholar]
- Attaran Dowom, S.; Rezaeian, S.; Pourianfar, H. Agronomic and environmental factors affecting cultivation of the winter mushroom or Enokitake: Achievements and prospects. Appl. Microbiol. Biotechnol. 2019, 103, 2469–2481. [Google Scholar] [CrossRef]
- Wang, D.; Sakoda, A.; Suzuki, M. Biological efficiency and nutritional value of Pleurotus ostreatus cultivated on spent beer grain. Bioresour. Technol. 2001, 78, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Estrada, A.E.; Jimenez-Gasco, M.d.M.; Royse, D.J. Improvement of yield of Pleurotus eryngii var. eryngii by substrate supplementation and use of a casing overlay. Bioresour. Technol. 2009, 100, 5270–5276. [Google Scholar] [CrossRef] [PubMed]
- Royse, D.J. Recycling of spent shiitake substrate for production of the oyster mushroom, Pleurotus sajor-caju. Appl. Microbiol. Biotechnol. 1992, 38, 179–182. [Google Scholar] [CrossRef]
- Yang, L.-C.; Fu, T.-J.; Yang, F.-C. Biovalorization of soybean residue (okara) via fermentation with Ganoderma lucidum and Lentinus edodes to attain products with high anti-osteoporotic effects. J. Biosci. Bioeng. 2020, 129, 514–518. [Google Scholar] [CrossRef]
- Guo, S.; Zhou, Y.; Shen, Q.; Zhang, F. Effect of ammonium and nitrate nutrition on some physiological processes in higher plants-growth, photosynthesis, photorespiration, and water relations. Plant Biol. 2006, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-Y.; Burgos, A.; Zhang, Q.; Tang, D.; Shi, Y.; Ma, L.; Yi, X.; Ruan, J. Analyses of transcriptome profiles and selected metabolites unravel the metabolic response to NH4+ and NO3− as signaling molecules in tea plant (Camellia sinensis L.). Sci. Hortic. 2017, 218, 293–303. [Google Scholar] [CrossRef]
- Deng, B.; Li, Y.; Lei, G.; Liu, G. Effects of nitrogen availability on mineral nutrient balance and flavonoid accumulation in Cyclocarya paliurus. Plant Physiol. Biochem. 2019, 135, 111–118. [Google Scholar] [CrossRef]
- Qin, J.; Yue, X.; Shang, X.; Fang, S. Nitrogen Forms Alter Triterpenoid Accumulation and Related Gene Expression in Cyclocarya paliurus (Batalin) Iljinsk. Seedlings. Forests 2020, 11, 631. [Google Scholar] [CrossRef]
- Shi, L.; Qin, L.; Xu, Y.; Ren, A.; Fang, X.; Mu, D.; Tan, Q.; Zhao, M. Molecular cloning, characterization, and function analysis of a mevalonate pyrophosphate decarboxylase gene from Ganoderma lucidum. Mol. Biol. Rep. 2012, 39, 6149–6159. [Google Scholar] [CrossRef] [PubMed]
- Zied, D.; Savoie, J.-M.; Pardo-Giménez, A. Soybean the Main Nitrogen Source in Cultivation Substrates of Edible and Medicinal Mushrooms. Soybean Nutr. 2011, 22, 433–452. [Google Scholar]
- Darmasiwi, S.; Aramsirirujiwet, Y.; Kimkong, I. Evaluation of the nutritional value, mycochemicals, and antioxidant activities of Hericium erinaceus cultivated using jasmine rice. Asian J. Agric. Biol. 2022, 2022, 202108309. [Google Scholar] [CrossRef]
- Ikeda, Y.; Murakami, A.; Ohigashi, H. Ursolic acid: An anti- And pro-inflammatory triterpenoid. Mol. Nutr. Food Res. 2008, 52, 26–42. [Google Scholar] [CrossRef]
- Máñez, S.; Recio, M.C.; Giner, R.M.; Ríos, J.-L. Effect of selected triterpenoids on chronic dermal inflammation. Eur. J. Pharmacol. 1997, 334, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Ntougias, S.; Baldrian, P.; Ehaliotis, C.; Nerud, F.; Antoniou, T.; Merhautová, V.; Zervakis, G.I. Biodegradation and detoxification of olive mill wastewater by selected strains of the mushroom genera Ganoderma and Pleurotus. Chemosphere 2012, 88, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Zervakis, G.; Koutrotsios, G.; Katsaris, P. Composted versus Raw Olive Mill Waste as Substrates for the Production of Medicinal Mushrooms: An Assessment of Selected Cultivation and Quality Parameters. BioMed Res. Int. 2013, 2013, 546830. [Google Scholar] [CrossRef] [PubMed]
- Koutrotsios, G.; Mountzouris, K.C.; Chatzipavlidis, I.; Zervakis, G.I. Bioconversion of lignocellulosic residues by Agrocybe cylindracea and Pleurotus ostreatus mushroom fungi—Assessment of their effect on the final product and spent substrate properties. Food Chem. 2014, 161, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Shetty, K. Role of proline-linked pentose phosphate pathway in biosynthesis of plant phenolics for functional food and environmental applications: A review. Process Biochem. 2004, 39, 789–804. [Google Scholar] [CrossRef]
- Vamanu, E. In vitro antioxidant and antimicrobial activities of two edible mushroom mycelia obtained in the presence of different nitrogen sources. J. Med. Food 2013, 16, 155–166. [Google Scholar] [CrossRef]
- Singh, G.; Tiwari, A.; Rathore, H.; Prasad, S.; Hariprasad, P.; Sharma, S. Valorization of Paddy Straw Using De-oiled Cakes for P. ostreatus Cultivation and Utilization of Spent Mushroom Substrate for Biopesticide Development. Waste Biomass Valorization 2021, 12, 333–346. [Google Scholar] [CrossRef]
- Halliwell, B.; Rafter, J.; Jenner, A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: Direct or indirect effects? Antioxidant or not? Am. J. Clin. Nutr. 2005, 81, 268S–276S. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113 (Suppl. S9), 71s–88s. [Google Scholar] [CrossRef]
- Yan, J.-K.; Ding, Z.-C.; Gao, X.; Wang, Y.-Y.; Yang, Y.; Wu, D.; Zhang, H.-N. Comparative study of physicochemical properties and bioactivity of Hericium erinaceus polysaccharides at different solvent extractions. Carbohydr. Polym. 2018, 193, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Otieno, O.D.; Mulaa, F.J.; Obiero, G.; Midiwo, J. Utilization of fruit waste substrates in mushroom production and manipulation of chemical composition. Biocatal. Agric. Biotechnol. 2022, 39, 102250. [Google Scholar] [CrossRef]
- Yildiz, O.; Can, Z.; Laghari, A.; Sahin, H.; Malkoç, M. Wild Edible Mushrooms as a Natural Source of Phenolics and Antioxidants. J. Food Biochem. 2015, 39, 148–154. [Google Scholar] [CrossRef]
- Lau, C.-C.; Abdullah, N.; Shuib, A.S.; Aminudin, N. Proteomic Analysis of Antihypertensive Proteins in Edible Mushrooms. J. Agric. Food Chem. 2012, 60, 12341–12348. [Google Scholar] [CrossRef] [PubMed]
| Treatments (w/w) | Physical Properties | Nutrient Composition | C/N Ratio | |||||
|---|---|---|---|---|---|---|---|---|
| pH | EC (dS m−1) | Organic Carbon (%) | Organic Matter (%) | Total N (%) | Total P (%) | Total K (%) | ||
| RS80:SM0 (control) | 5.93 | 2.07 | 46.73 | 80.56 | 0.65 | 0.10 | 0.51 | 71.78 |
| RS78:SM2 | 7.62 | 1.72 | 46.47 | 80.11 | 0.74 | 0.09 | 0.58 | 62.81 |
| RS76:SM4 | 7.77 | 1.39 | 46.00 | 79.30 | 0.76 | 0.10 | 0.61 | 60.53 |
| RS74:SM6 | 7.55 | 1.74 | 46.59 | 80.32 | 0.70 | 0.10 | 0.53 | 66.51 |
| RS72:SM8 | 7.76 | 1.31 | 46.28 | 79.78 | 0.77 | 0.10 | 0.63 | 59.74 |
| RS70:SM10 | 7.44 | 1.49 | 46.35 | 79.91 | 1.25 | 0.10 | 0.61 | 37.14 |
| Treatments (w/w) | Mycelial Growth (Day) | Diameter of Cap (cm) | Biological Efficiency (%) | % Yield |
|---|---|---|---|---|
| RS80:SM0 | 19.30 ± 0.45 d | 8.32 ± 0.47 c | 15.57 ± 0.71 d | 14.94 ± 0.30 |
| RS78:SM2 | 18.00 ± 0.35 c | 9.36 ± 0.42 b | 27.07 ± 1.03 c | 12.88 ± 1.34 |
| RS76:SM4 | 16.30 ± 0.45 ab | 10.56 ± 0.40 a | 35.60 ± 1.08 a | 14.80 ± 1.73 |
| RS74:SM6 | 17.80 ± 0.57 c | 9.58 ± 0.34 b | 27.72 ± 1.00 c | 13.58 ± 0.83 |
| RS72:SM8 | 16.90 ± 0.22 b | 10.36 ± 0.32 a | 32.86 ± 1.26 b | 12.74 ± 0.29 |
| RS70:SM10 | 15.70 ± 0.84 a | 10.78 ± 0.28 a | 33.27 ± 1.13 b | 14.24 ± 2.19 |
| F-test | ** | ** | ** | ns |
| C.V.% | 2.81 | 3.38 | 2.74 | 7.98 |
| Treatments (w/w) | Total Phenolic Content (mg GAE/g DW) | Antioxidant Activity DPPH Scavenging Activity (IC50, mg/mL) |
|---|---|---|
| RS80:SM0 | 7.75 ± 5.06 b | 1.08 ± 0.23 c |
| RS78:SM2 | 15.26 ± 9.75 a | 0.84 ± 0.13 b |
| RS76:SM4 | 16.07 ± 3.54 a | 0.67 ± 0.04 a |
| RS74:SM6 | 15.52 ± 8.40 a | 0.89 ± 0.06 b |
| RS72:SM8 | 15.62 ± 9.54 a | 0.80 ± 0.10 ab |
| RS70:SM10 | 15.59 ± 4.90 a | 0.73 ± 0.04 ab |
| F-test | ** | ** |
| C.V.% | 4.96 | 10.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chutimanukul, P.; Sukdee, S.; Prajuabjinda, O.; Thepsilvisut, O.; Panthong, S.; Athinuwat, D.; Chuaboon, W.; Poomipan, P.; Vachirayagorn, V. The Effects of Soybean Meal on Growth, Bioactive Compounds, and Antioxidant Activity of Hericium erinaceus. Horticulturae 2023, 9, 693. https://doi.org/10.3390/horticulturae9060693
Chutimanukul P, Sukdee S, Prajuabjinda O, Thepsilvisut O, Panthong S, Athinuwat D, Chuaboon W, Poomipan P, Vachirayagorn V. The Effects of Soybean Meal on Growth, Bioactive Compounds, and Antioxidant Activity of Hericium erinaceus. Horticulturae. 2023; 9(6):693. https://doi.org/10.3390/horticulturae9060693
Chicago/Turabian StyleChutimanukul, Preuk, Siripong Sukdee, Onmanee Prajuabjinda, Ornprapa Thepsilvisut, Sumalee Panthong, Dusit Athinuwat, Wilawan Chuaboon, Phakpen Poomipan, and Vorapat Vachirayagorn. 2023. "The Effects of Soybean Meal on Growth, Bioactive Compounds, and Antioxidant Activity of Hericium erinaceus" Horticulturae 9, no. 6: 693. https://doi.org/10.3390/horticulturae9060693
APA StyleChutimanukul, P., Sukdee, S., Prajuabjinda, O., Thepsilvisut, O., Panthong, S., Athinuwat, D., Chuaboon, W., Poomipan, P., & Vachirayagorn, V. (2023). The Effects of Soybean Meal on Growth, Bioactive Compounds, and Antioxidant Activity of Hericium erinaceus. Horticulturae, 9(6), 693. https://doi.org/10.3390/horticulturae9060693








