Genetic Diversity Analysis of Guangxi Kumquat (Fortunella Swing) Germplasm Using SRAP Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. DNA Isolation
2.3. PCR Amplification for SRAP Markers
2.4. Agarose Gel Electrophoresis
2.5. Parameters Used for Analysis of SRAP Markers
3. Results and Analysis
3.1. Polymorphism Analysis Using SRAP Markers
3.2. Principal Coordinate Analysis
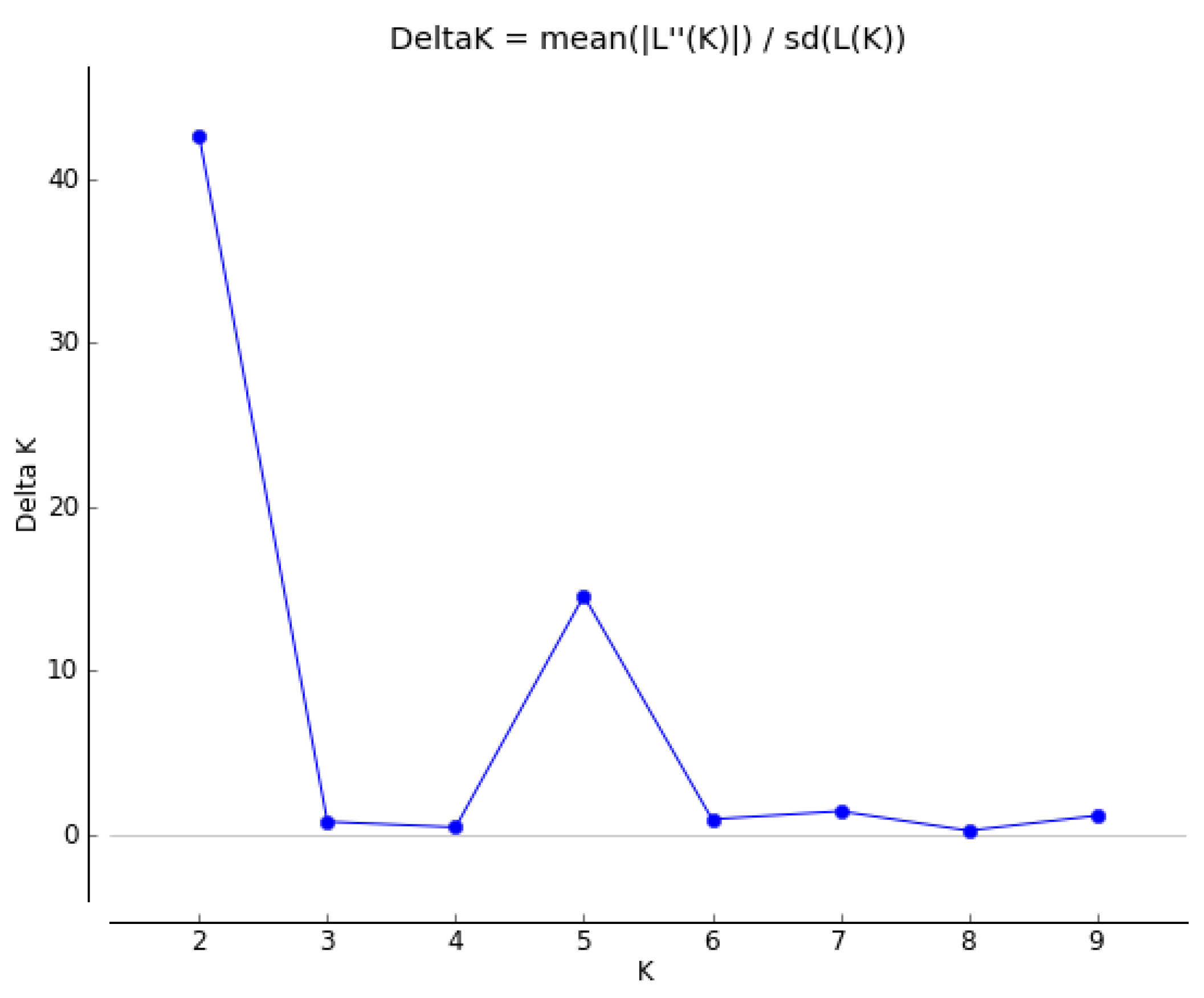
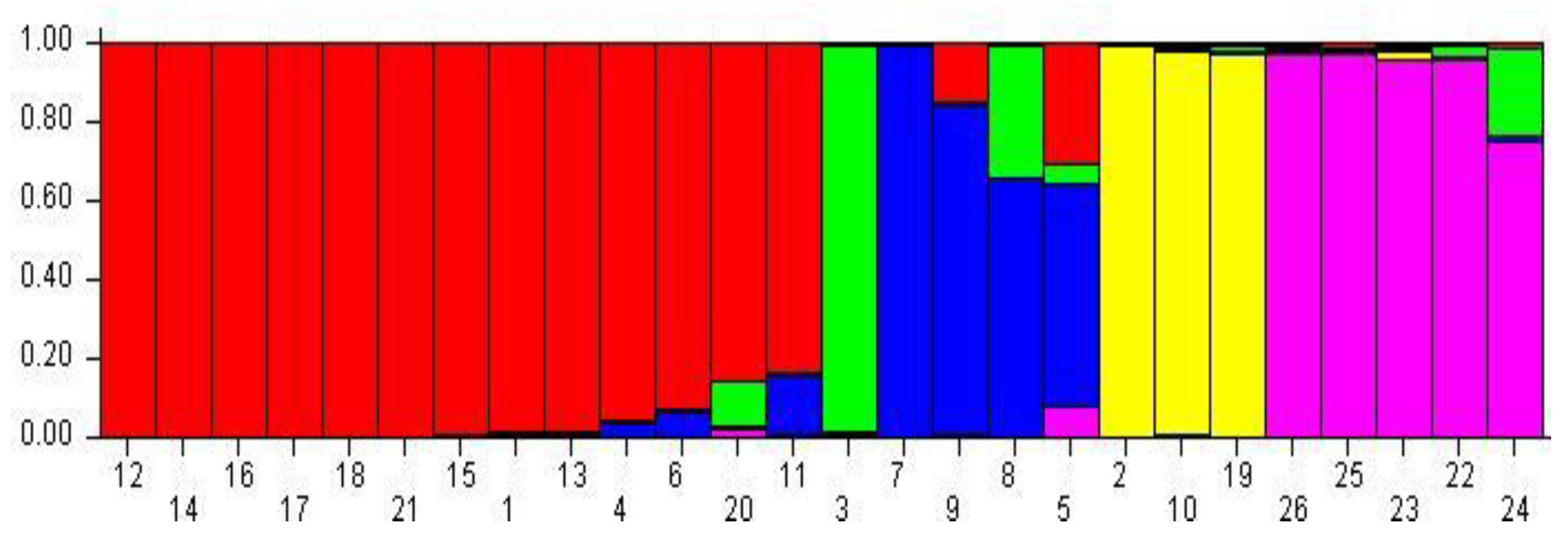
3.3. Population Structure Analysis
3.4. Hierarchical Cluster Analysis (HCA)
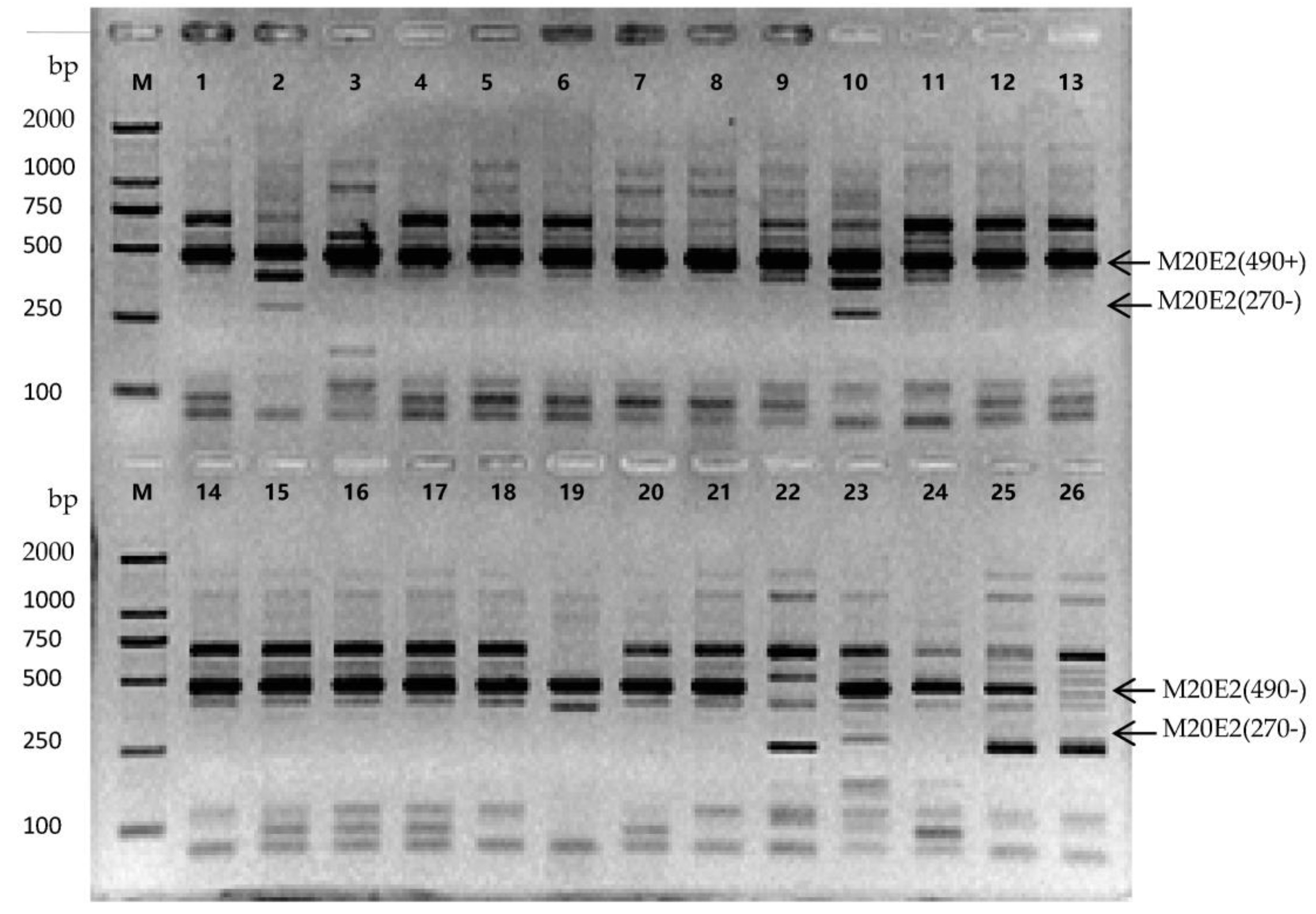
3.5. Screening of Genotype-Specific Markers and Identification od Kumquat Accessions
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, B.H.; Deng, C.L.; Chen, C.W.; Deng, G.Z.; Ding, P.; Niu, Y.; Tang, Y.; Fu, H.M. ISSR analysis of local citrus resources in Guangxi. J. Fruit Sci. 2015, 32, 1001–1006. [Google Scholar]
- Zhou, K.L.; Ye, Y.M. Chinese Record of Fruit Trees—Citrus Volume; Beijing Forestry Publishing House: Beijing, China, 2010; Volume 126–130, pp. 427–433. [Google Scholar]
- Lou, S.N.; Ho, P. Compounds and biological activities of small-size citrus: Kumquat and calamondin. J. Food Drug Anal. 2017, 25, 162–175. [Google Scholar] [CrossRef]
- Sadek, E.S.; Makris, D.P.; Kefalas, P. Polyphenolic composition and antioxidant characteristics of kumquat (Fortunella margarita) peel fractions. Plant Foods Hum. Nutr. 2009, 64, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Terao, R.; Murata, A.; Sugamoto, K.; Watanabe, T.; Nagahama, K.; Nakahara, K.; Kondo, T.; Murakami, N.; Keiichi, F.; Hattoriab, H.; et al. Immunostimulatory effect of kumquat (Fortunella crassifolia) and its constituents, β-cryptoxanthin and R-limonene. Food Funct. 2019, 10, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.H.; Deng, G.Z.; Deng, C.L.; Chen, C.W.; Tang, Y.; Fu, H.M.; Ding, P. Selection of kumquat new cultivar ‘Guijingan No. 1’. J. Fruit Sci. 2016, 33, 762–765. [Google Scholar]
- Deng, C.L.; Deng, G.Z.; Deng, X.X.; Liu, B.H.; Tang, Y.; Chen, C.W.; Deng, J.Y. Breeding report of a new late ripening kumquat cultivar ‘Guijingan No.2’. J. Fruit Sci. 2017, 34, 1357–1360. [Google Scholar]
- Tang, Z.P.; Gao, X.; Qin, R.Y.; Sun, N.J.; Lan, H.G.; Wei, R.J.; Deng, G.Z.; Liu, B.H. A new Fortunella crassifiolia cultivar ‘Cuimi Kumquat’. J. Fruit Sci. 2018, 35, 131–134. [Google Scholar]
- Liu, B.H.; Deng, G.Z.; Tang, Z.P.; Qin, R.Y.; Wei, R.J.; Xia, L.H.; Qin, Q. A new kumquat cultivar ‘Fuyuan Jingan’. Acta Hortic. Sin. 2022, 49, 71–72. [Google Scholar]
- Li, G.G.; Liu, Y.X.; Chai, L.J.; Ye, J.L.; Mai, C.S.; Ou, Z.T.; Chen, X.L. Ploidy analysis and SSR molecular identification of Gui Wild Shanjingan. Southwest China J. Agric. Sci. 2017, 30, 1872–1876. [Google Scholar]
- Huang, G.X.; Guo, L.Y.; Zhang, S.W.; He, X.H.; Zhou, R.Y.; Chen, H.; Yang, C.J. Genetic relationship analysis of Fortunella germplasm resources from China and Vietnam by ISSR markers. J. Fruit Sci. 2011, 28, 563–567. [Google Scholar]
- Zhang, D.X.; Feng, Z.Y.; Liu, C.R.; Yu, H.P.; Deng, Y.F.; Hartey, T.G.; Mabberley, D.J. Flora of China, Kumquat; Science Press: Beijing, China, 1997; pp. 169–175. Available online: http://www.iplant.cn/frps/cname/ (accessed on 16 April 2023).
- Cheng, Y.J.; Vicente, M.C.; Meng, H.J.; Guo, W.W.; Tao, N.G.; Deng, X.X. A set of primers for analyzing chloroplast DNA diversity in Citrus and related genera. Tree Physiol. 2005, 25, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Yahata, M.; Komatsu, H.; Kunitake, H. Phylogeny and classification of Fortunella (Aurantioideae) inferred from DNA polymorphisms. Bul. Fac. Agr. Univ. Miyazaki 2010, 56, 103–110. [Google Scholar]
- Wang, T.; Chen, L.L.; Shu, H.J.; You, F.L.; Xiao, L.; Li, J.; Ren, J.; Wanga, V.O.; Mutie, F.; Cai, X.Z.; et al. Fortunella venosa (Champ. ex Benth.) CC Huang and F. hindsii (Champ. ex Benth.) Swingle as independent species: Evidence from morphology and molecular systematics and taxonomic revision of Fortunella (Rutaceae). Front. Plant Sci 2022, 13, 867659. [Google Scholar] [CrossRef]
- Zhu, C.Q.; Chen, P.; Ye, J.L.; Li, H.; Huang, Y.; Yang, X.M.; Chen, C.W.; Zhang, C.L.; Xu, Y.T.; Wang, X.L.; et al. New insights into the phylogeny and speciation of kumquat (Fortunella spp.) based on chloroplast SNP, nuclear SSR and whole-genome sequencing. Front. Agr. Sci. Eng 2022, 9, 627–641. [Google Scholar] [CrossRef]
- Zhang, L.F.; He, J.; Feng, Y.; Liu, L.; Guo, Q.G.; Liang, G.L. Genetic relationship of kumquat and its related genera by SSR analysis. J. Fruit Sci. 2006, 23, 335–338. [Google Scholar]
- Zhang, Z.H.; Zhang, A.S.; Gao, D.T.; Wei, Z.F. Genetic diversity analysis of Main Kyoho grapevine series cultivars based on SRAP markers and rapid identification by MCID method. J. Northeast Agric. Sci. 2022, 4, 38–42. [Google Scholar]
- Zhou, L.X.; Yarra, R.; Cao, H.X.; Zhao, Z.H. Sequence-related amplified polymorphism (SRAP) markers based genetic diversity and population structure analysis of oil palm (Elaeis guineensis Jacq.). Trop. Plant Biol. 2021, 14, 63–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, G.D.; Mo, Y.L.; Luo, S.X.; Zhao, Y.; Tang, Y.J.; Lu, Z.S.; Shan, B.; Rong, T. Genetic diversity of mango seed was analyzed using CDDP and SRAP markers. South China Fruits 2022, 2, 57–63. [Google Scholar]
- Sun, L.L.; Peng, L.N.; Li, Z.; Hou, R.N.; Mou, Y.H. Construction of Genetic Linkage Map of Plum (Prunus salicina L.) with ISSR and SRAP Markers. Guangdong Agric. Sci. 2022, 49, 40–48. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, W.; Zhang, X.Y.; Zhou, M.; Jiang, Q.Q.; Deng, Z.N.; Li, D.Z. Identification of the hybrid progeny of Shatian pomelo × citron by embryo rescue technique and its SRAP detection. J. Fruit Sci. 2013, 30, 386–389. [Google Scholar]
- Zhang, L.H.; Han, H.Z.; Wang, X.L.; Li, S.H.; Wang, F.; Dong, R.; Liu, Y. Screening of molecular markers for SRAP of Cinnamomum camphora . Anhui Agric. Sci. Bull. 2019, 25, 25–27, 57. [Google Scholar]
- Xu, J.; Tan, L.M.; Fu, H.Y.; Zhu, Z.M.; Long, L.B.; Hu, Z.; Ma, X.F.; Deng, Z.N. Genetic diversity analysis of 14 citron genotypes based on molecular markers. Fenzi Zhiwu Yuzhong (Molecular Plant Breeding) 2021, 19, 4726–4737. [Google Scholar]
- Jaccard, P. Nouvelles recherches sur la distribution florale. Bull. Soc. Vaud. Sci. Nat 1908, 44, 223–270. [Google Scholar]
- Pritchard, J.K.; Stephens, M.J.; Donnelly, P.J. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Earl, D.A.; Vonholdt, B.M. Structure harvester: A website and program for visualizing structure output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron 2001, 4, 9. [Google Scholar]
- Al-Ghamedi, K.; Alaraidh, I.; Afzal, M.; Mahdhi, M.; Al-Faifi, Z.; Oteef, M.D.Y.; Tounekti, T.; Alghamdi, S.S.; Khemira, H. Assessment of genetic diversity of local coffee populations in southwestern Saudi Arabia using SRAP markers. Agronomy. 2023, 13, 302. [Google Scholar] [CrossRef]
- Chown, S.L.; Hodgins, K.A.; Griffin, P.C.; Oakeshott, J.G.; Byrne, M.; Hoffmann, A.A. Biological invasions, climate change, and genomics. In Crop Breeding: Bioinformatics and Preparing for Climate Change; Santosh, K., Ed.; Apple Academic Press: Waretown, NJ, USA, 2016; pp. 37–91. [Google Scholar]
- Al-Murish, T.M.; Elshafei, A.A.; Al-Doss, A.A.; Barakat, M.N. Genetic diversity of coffee (Coffea arabica L.) in Yemen via SRAP, TRAP and SSR markers. Food Agric. Env. 2013, 11, 411–416. [Google Scholar]
- Hasan, N.; Choudhary, S.; Naaz, N.; Sharma, N.; Laskar, R.A. Recent advancements in molecular marker-assisted selection and applications in plant breeding programs. J. Genet. Eng. Biotechnol 2021, 19, 128. [Google Scholar] [CrossRef]
- Nadeem, M.A.; Nawaz, M.A.; Shahid, M.Q.; Doğan, Y.; Comertpay, G.; Yıldız, M.; Hatipoğlu, R.; Ahmad, F.; Alsaleh, A.; Labhane, N. DNA molecular markers in plant breeding: Current status and recent advancements in genomic selection and genome editing. Biotechnol. Biotechnol. Equip. 2018, 32, 261–285. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, Y.J.; Li, Y.; Liu, X.L.; Guo, Y.Q.; Xia, H.; Liang, D. Genetic diversity analysis of kiwifruit germplasm based on SRAP and SCoT markers. J. Fruit Sci. 2021, 38, 2059–2071. [Google Scholar]
- Hu, F.C.; Wu, X.B.; Chen, Z.; Wu, F.Z.; Zhou, W.J.; Feng, X.J.; Fan, H.Y.; Zhou, R.Y.; Wang, X.H. Genetic diversity analysis of litchi germplasm resources based on SRAP molecular markers. J. Trop. Crops 2021, 42, 920–926. [Google Scholar]
- Li, H.F.; Ran, K.; Wang, T. Construction of fingerprint of apple resources in Shandong province by using SRAP markers. J. Shenyang Agric. Univ. 2020, 51, 470–475. [Google Scholar]
- Zhang, A.S.; Si, Q.L.; Qi, X.J.; Zhang, Z.H. Genetic diversity analysis and fingerprint construction of germplasm resources of kiwifruit. Jiangsu Agric. J. 2018, 34, 138–144. [Google Scholar]
- Shang, X.X.; Zhang, A.S.; Liu, Y.; Gao, D.T. Genetic diversity analysis and fingerprint construction of grapevine germplasm resources based on SRAP. Mol. Plant Breed. 2020, 18, 1916–1922. [Google Scholar]



| Code | Abbreviation | Genotype Name | Scientific Name | Possible Origin |
|---|---|---|---|---|
| 1 | NB jindan | Ningbo jindan | F. crassifolia | Ningbo, Zhejiang |
| 2 | Daguojindou | Daguojindou | F. hindsii | Citrus Research Institute, SWU/CAAS |
| 3 | Jinganzazhong | Guangxi natural kumquat hybrid | Citrus × Fortunella | Hezhou, Guangxi |
| 4 | WZ luofu | Wenzhou luofu | F. margarita | Wenzhou, Zhejiang |
| 5 | NB luofu | Ningbo luofu | F. margarita | Ningbo, Zhejiang |
| 6 | WZ jingdan | Wenzhou jingdan | F. crassifolia | Wenzhou, Zhejiang |
| 7 | Sijiju | Sijiju | Citrus × Fortunella | Citrus Research Institute, SWU/CAAS |
| 8 | Wenzhouju | Wenzhouju (kumquat hybrid) | Citrus × Fortunella | Wenzhou, Zhejiang |
| 9 | Shouxingju | Shouxingju (kumquat hybrid) | Citrus × Fortunella | Citrus Research Institute, SWU/CAAS |
| 10 | Dajindou | Dajindou | F. hindsii | Citrus Research Institute, SWU/CAAS |
| 11 | NB luowen | Ningbo luowen | F. japonica | Ningbo, Zhejiang |
| 12 | RA jingan | Rongan jingan | F. crassifolia | Liuzhou, Guangxi |
| 13 | FY jingan | Fuyuan jingan | F. crassifolia | Liuzhou, Guangxi |
| 14 | CM jingan | Cuimi jingan | F. crassifolia | Liuzhou, Guangxi |
| 15 | Guijingan1 | Guijingan No.1 | F. crassifolia | Yangshuo, Guangxi |
| 16 | Guijingan2 | Guijingan No.2 | F. crassifolia | Yangshuo, Guangxi |
| 17 | YS jingan | Yangshuo jingan | F. crassifolia | Yangshuo, Guangxi |
| 18 | F15-1 | F15-1 | F. crassifolia | Liuzhou, Guangxi |
| 19 | Shanjingan | Hunan Shanjingan | F. hindsii | Changsha, Hunan |
| 20 | LY jingan | Liuyang jingan | F. crassifolia | Changsha, Hunan |
| 21 | HP jingan | Huapi jingan | F. crassifolia | Liuzhou, Guangxi |
| 22 | FC-1 | Guangxi wild kumquat FC-1 | Fortunella sp. | Fangchenggang, Guangxi |
| 23 | FC-2 | Guangxi wild kumquat FC-2 | Fortunella sp. | Fangchenggang, Guangxi |
| 24 | FC-3 | Guangxi wild kumquat FC-3 | Fortunella sp. | Fangchenggang, Guangxi |
| 25 | FC-4 | Guangxi wild kumquat FC-4 | Fortunella sp. | Fangchenggang, Guangxi |
| 26 | FC-5 | Guangxi wild kumquat FC-5 | Fortunella sp. | Fangchenggang, Guangxi |
| Forward Primer | Reverse Primer | ||
|---|---|---|---|
| ME Primer Code | Primer Sequence (5′-3′) | EM Primer Code | Sequence (5′-3′) |
| ME1 | TGAGTCCAAACCGGAAA | EM1 | GACTGCGTACGAATTAAC |
| ME2 | TGAGTCCAAACCGGAAC | EM2 | GACTGCGTACGAATTAAT |
| ME3 | TGAGTCCAAACCGGAAG | EM3 | GACTGCGTACGAATTACG |
| ME4 | TGAGTCCAAACCGGAAT | EM4 | GACTGCGTACGAATTAGC |
| ME5 | TGAGTCCAAACCGGACA | EM5 | GACTGCGTACGAATTATG |
| ME6 | TGAGTCCAAACCGGACC | EM6 | GACTGCGTACGAATTCAA |
| ME7 | TGAGTCCAAACCGGACG | EM7 | GACTGCGTACGAATTCAC |
| ME8 | TGAGTCCAAACCGGACT | EM8 | GACTGCGTACGAATTCAG |
| ME9 | TGAGTCCAAACCGGAGA | EM9 | GACTGCGTACGAATTCAT |
| ME10 | TGAGTCCAAACCGGAGC | EM10 | GACTGCGTACGAATTCCA |
| ME11 | TGAGTCCAAACCGGAGG | EM11 | GACTGCGTACGAATTCGA |
| ME12 | TGAGTCCAAACCGGATA | EM12 | GACTGCGTACGAATTCGG |
| ME13 | TGAGTCCAAACCGGTAA | EM13 | GACTGCGTACGAATTCTA |
| ME14 | TGAGTCCAAACCGGTAG | EM14 | GACTGCGTACGAATTCTC |
| ME15 | TGAGTCCAAACCGGTCA | EM15 | GACTGCGTACGAATTCTG |
| ME16 | TGAGTCCAAACCGGTCC | EM16 | GACTGCGTACGAATTCTT |
| ME17 | TGAGTCCAAACCGGTGC | EM17 | GACTGCGTACGAATTGAT |
| ME18 | TGAGTCCAAACCGGTGT | EM18 | GACTGCGTACGAATTGCA |
| ME19 | TGAGTCCAAACCGGTTA | EM19 | GACTGCGTACGAATTGGT |
| ME20 | TGAGTCCAAACCGGTTG | EM20 | GACTGCGTACGAATTGTC |
| EM21 | GACTGCGTACGAATTTAG | ||
| EM22 | GACTGCGTACGAATTTCG | ||
| EM23 | GACTGCGTACGAATTTGA | ||
| EM24 | GACTGCGTACGAATTTGC | ||
| No. | Primer Pair Code | Amplified Bands | Polymorphic Bands | Polymorphic Rate (%) |
|---|---|---|---|---|
| 1 | Me1Em15 | 6 | 4 | 66.67 |
| 2 | Me1Em22 | 4 | 4 | 100 |
| 3 | Me1Em23 | 6 | 6 | 100 |
| 4 | Me2Em17 | 6 | 6 | 100 |
| 5 | Me9Em23 | 7 | 4 | 57.14 |
| 6 | Me2Em21 | 6 | 5 | 83.33 |
| 7 | Me10Em7 | 10 | 8 | 80 |
| 8 | Me4Em7 | 1 | 1 | 100 |
| 9 | Me4Em12 | 10 | 8 | 80 |
| 10 | Me3Em17 | 4 | 4 | 100 |
| 11 | Me4Em17 | 4 | 4 | 100 |
| 12 | Me11Em21 | 2 | 2 | 100 |
| 13 | Me10Em13 | 4 | 4 | 100 |
| 14 | Me14Em12 | 6 | 6 | 100 |
| 15 | Me16Em19 | 4 | 2 | 50 |
| 16 | Me7Em4 | 10 | 8 | 80 |
| 17 | Me20Em2 | 7 | 7 | 100 |
| 18 | Me17Em2 | 2 | 2 | 100 |
| 19 | Me18Em22 | 5 | 5 | 100 |
| Sum/Average | 104/5.47 | 90/4.74 | 86.54 | |
| Axis | Eigenvalue | Cumulative Eigenvalue | Percent (%) | Cumulative (%) |
|---|---|---|---|---|
| 1 | 0.53 | 0.53 | 34.80 | 34.80 |
| 2 | 0.29 | 0.83 | 19.25 | 54.05 |
| 3 | 0.19 | 1.02 | 12.74 | 66.78 |
| 4 | 0.17 | 1.19 | 10.90 | 77.68 |
| 5 | 0.09 | 1.28 | 5.67 | 83.35 |
| 6 | 0.08 | 1.36 | 5.38 | 88.72 |
| 7 | 0.03 | 1.39 | 2.10 | 90.82 |
| 8 | 0.02 | 1.41 | 1.61 | 92.43 |
| 9 | 0.02 | 1.43 | 1.34 | 93.76 |
| 10 | 0.02 | 1.45 | 1.01 | 94.78 |
| 11 | 0.01 | 1.46 | 0.78 | 95.56 |
| 12 | 0.01 | 1.47 | 0.52 | 96.08 |
| 13 | 0.00 | 1.47 | 0.24 | 96.32 |
| 14 | 0.00 | 1.48 | 0.11 | 96.42 |
| 15 | 0.00 | 1.48 | 0.00 | 96.43 |
| 16 | 0.00 | 1.48 | 0.00 | 96.43 |
| No. | Germplasm | 1. NB jindan | 2. Daguojindou | 3. Jinganzazhong | 4. WZ luofu | 5. NB luofu | 6. WZ jingdan | 7. Sijiju | 8. Wenzhouju | 9. Shouxingju | 10. Dajindou | 11. NB luowen | 12. RA jingan | 13. FY jingan | 14. CM jingan | 15. Guijingan1 | 16. Guijingan2 | 17. YS jingan | 18. F15-1 | 19. Shanjingan | 20. LY jingan | 21. HP jingan | 22. FC14-1 | 23. FC14-2 | 24. FC14-3 | 25. FC14-4 | 26. FC14-5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NB jindan | 1.000 | |||||||||||||||||||||||||
| 2 | Daguojindou | 0.634 | 1.000 | ||||||||||||||||||||||||
| 3 | Jinganzazhong | 0.634 | 0.604 | 1.00 | |||||||||||||||||||||||
| 4 | WZ luofu | 0.941 | 0.634 | 0.594 | 1.000 | ||||||||||||||||||||||
| 5 | NB luofu | 0.812 | 0.6436 | 0.584 | 0.851 | 1.000 | |||||||||||||||||||||
| 6 | WZ jingdan | 0.941 | 0.634 | 0.653 | 0.921 | 0.812 | 1.000 | ||||||||||||||||||||
| 7 | Sijiju | 0.762 | 0.673 | 0.634 | 0.762 | 0.851 | 0.782 | 1.000 | |||||||||||||||||||
| 8 | Wenzhouju | 0.703 | 0.594 | 0.584 | 0.703 | 0.772 | 0.703 | 0.871 | 1.000 | ||||||||||||||||||
| 9 | Shouxingju | 0.822 | 0.673 | 0.673 | 0.782 | 0.831 | 0.822 | 0.941 | 0.851 | 1.000 | |||||||||||||||||
| 10 | Dajindou | 0.663 | 0.931 | 0.594 | 0.644 | 0.673 | 0.663 | 0.663 | 0.584 | 0.703 | 1.000 | ||||||||||||||||
| 11 | NB luowen | 0.911 | 0.663 | 0.644 | 0.911 | 0.822 | 0.911 | 0.772 | 0.723 | 0.832 | 0.693 | 1.000 | |||||||||||||||
| 12 | RA jingan | 0.960 | 0.653 | 0.653 | 0.941 | 0.812 | 0.980 | 0.762 | 0.703 | 0.822 | 0.683 | 0.931 | 1.000 | ||||||||||||||
| 13 | FY jingan | 0.950 | 0.663 | 0.663 | 0.931 | 0.802 | 0.970 | 0.752 | 0.693 | 0.812 | 0.693 | 0.921 | 0.990 | 1.000 | |||||||||||||
| 14 | CM jingan | 0.941 | 0.673 | 0.653 | 0.921 | 0.792 | 0.960 | 0.743 | 0.683 | 0.802 | 0.703 | 0.931 | 0.980 | 0.970 | 1.000 | ||||||||||||
| 15 | Guijingan1 | 0.950 | 0.644 | 0.644 | 0.950 | 0.822 | 0.970 | 0.772 | 0.693 | 0.812 | 0.673 | 0.921 | 0.990 | 0.980 | 0.970 | 1.000 | |||||||||||
| 16 | Guijingan2 | 0.970 | 0.644 | 0.663 | 0.931 | 0.802 | 0.970 | 0.772 | 0.712 | 0.832 | 0.673 | 0.921 | 0.990 | 0.980 | 0.970 | 0.980 | 1.000 | ||||||||||
| 17 | YS jingan | 0.960 | 0.653 | 0.653 | 0.941 | 0.792 | 0.960 | 0.782 | 0.723 | 0.822 | 0.663 | 0.911 | 0.980 | 0.970 | 0.960 | 0.970 | 0.990 | 1.000 | |||||||||
| 18 | F15-1 | 0.931 | 0.683 | 0.644 | 0.931 | 0.782 | 0.950 | 0.752 | 0.693 | 0.792 | 0.693 | 0.921 | 0.970 | 0.960 | 0.990 | 0.960 | 0.960 | 0.970 | 1.000 | ||||||||
| 19 | Shanjingan | 0.644 | 0.911 | 0.594 | 0.644 | 0.653 | 0.624 | 0.683 | 0.614 | 0.683 | 0.881 | 0.653 | 0.644 | 0.653 | 0.663 | 0.634 | 0.653 | 0.663 | 0.673 | 1.000 | |||||||
| 20 | LY jingan | 0.941 | 0.634 | 0.634 | 0.921 | 0.772 | 0.901 | 0.743 | 0.703 | 0.782 | 0.644 | 0.871 | 0.921 | 0.911 | 0.901 | 0.911 | 0.931 | 0.941 | 0.911 | 0.644 | 1.000 | ||||||
| 21 | HP jingan | 0.960 | 0.653 | 0.653 | 0.921 | 0.792 | 0.941 | 0.743 | 0.693 | 0.802 | 0.683 | 0.931 | 0.960 | 0.950 | 0.980 | 0.950 | 0.970 | 0.960 | 0.970 | 0.663 | 0.921 | 1.000 | |||||
| 22 | FC14-1 | 0.683 | 0.634 | 0.574 | 0.644 | 0.634 | 0.683 | 0.584 | 0.525 | 0.644 | 0.663 | 0.693 | 0.703 | 0.713 | 0.703 | 0.693 | 0.713 | 0.703 | 0.693 | 0.644 | 0.6434 | 0.703 | 1.000 | ||||
| 23 | FC14-2 | 0.703 | 0.713 | 0.554 | 0.663 | 0.693 | 0.683 | 0.683 | 0.614 | 0.723 | 0.743 | 0.713 | 0.703 | 0.713 | 0.703 | 0.693 | 0.713 | 0.703 | 0.693 | 0.723 | 0.663 | 0.703 | 0.822 | 1.000 | |||
| 24 | FC14-3 | 0.693 | 0.644 | 0.683 | 0.673 | 0.743 | 0.713 | 0.653 | 0.604 | 0.693 | 0.653 | 0.723 | 0.713 | 0.723 | 0.713 | 0.703 | 0.703 | 0.693 | 0.703 | 0.634 | 0.673 | 0.713 | 0.713 | 0.772 | 1.000 | ||
| 25 | FC14-4 | 0.762 | 0.693 | 0.614 | 0.723 | 0.733 | 0.723 | 0.663 | 0.614 | 0.683 | 0.723 | 0.733 | 0.743 | 0.733 | 0.743 | 0.733 | 0.752 | 0.743 | 0.733 | 0.703 | 0.762 | 0.762 | 0.802 | 0.822 | 0.772 | 1.000 | |
| 26 | FC14-5 | 0.683 | 0.653 | 0.594 | 0.663 | 0.673 | 0.644 | 0.604 | 0.545 | 0.624 | 0.683 | 0.653 | 0.663 | 0.673 | 0.663 | 0.653 | 0.673 | 0.683 | 0.673 | 0.663 | 0.703 | 0.683 | 0.762 | 0.782 | 0.733 | 0.861 | 1.000 |
| Code | Genotypes | Unique Specific Markers |
|---|---|---|
| 1 | NB jindan | M1E15(1800−), M3E17(250−) |
| 2 | Daguojindou | M1E23(400−) |
| 3 | Jinganzazhong | M1E22(250+), M4E17(500+), M9E23(700−), M10E13(200+) |
| 4 | WZ luofu | None |
| 5 | NB luofu | M2E21(400−), M3E17(450+), M11E21(250+) |
| 6 | WZ jingdan | M2E21(450+), M16E9(350−) |
| 7 | Sijiju | M4E12(1300−) |
| 8 | Wenzhouju | M17E2(530−) |
| 9 | Shouxingju | None |
| 10 | Dajindou | M3E17(100+), M4E17(250−) |
| 11 | NB luowen | None |
| 12 | RA jingan | None |
| 13 | FY jingan | None |
| 14 | CM jingan | None |
| 15 | Guijingan1 | None |
| 16 | Guijingan2 | None |
| 17 | YS jingan | None |
| 18 | F15-1 | None |
| 19 | Shanjingan | M1E22(300−) |
| 20 | LY jingan | M7E4(500+) |
| 21 | HP jingan | None |
| 22 | FC-1 | M2E17(500+), M2E17(1000+) |
| 23 | FC-2 | M4E7(500−) |
| 24 | FC-3 | None |
| 25 | FC-4 | M4E17(300+) |
| 26 | FC-5 | M14E12(1300+) |
| Genotypes | Common Specific Markers | Combination of Unique Markers |
|---|---|---|
| FC-3 | M3E17(180+) FC-1; FC-4; FC-5 | FC-1 M2E7(500+); FC-4 M4E17(300+); FC-5 M14E12(1300+) |
| Shouxingju | M18E22(300+) Wenzhouju; Sijiju; Jinganzazhong | Wenzhouju M17E2(530−); Sijiju M4E12(1300−); JinganzazhongM1E22(250+) |
| NB luowen | M10E7(850−) WZ Luofu; NB luofu; Wenzhouju | WZ Luofu M10E7(320−); NB luofu M2E21(400−), M3E17(450+), M11E21(250+); Wenzhouju M17E2(530−) |
| WZ Luofu | M10E7(320−) NB luofu; Wenzhouju; Shanjingan | NB luofu M2E21(400−), M3E17(450+), M11E21(250+); Wenzhouju M17E2(530−); Shanjingan M1E22(300−) |
| Group Markers | Shared Genotypes with | Unique Markers for Discrimination |
|---|---|---|
| M1E23(800−) | NB jindan, WZ luofu, WZ jingdan LY jingan | NB jindan M1E15(1800−), M3E17(250−) WZ luofu M10E7(320−) WZ jingdan M2E21(450+), M16E9(350−) LY jingan M7E4(500+) |
| M7E4(1050+) | NB jindan NB luofu WZ jingdan LY jingan | NB jindan M1E15(1800−), M3E17(250−); NB luofu M2E21(400−), M3E17(450+), M11E21(250+) WZ jingdan M2E21(450+), M16E9(350−) LY jingan M7E4(500+) |
| Marker Types | Genotypes | Markers for Discrimination |
|---|---|---|
| Single marker | Guijingan1 | M1E22(740−) |
| HP jingan | M10E7(710−) | |
| FY jingan | M3E17(90−) | |
| Bi/Tri- markers | CM jingan + HP jingan | M2E21(480−) |
| YS jingan + F15-1 | M14E12(760−) | |
| Guijingan2 + YS jingan + HP jingan | M14E12(700−) | |
| No specific marker | RA jingan |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Ding, P.; Ye, R.; Li, Y.; Ou, S.; Gentile, A.; Ma, X.; Deng, Z. Genetic Diversity Analysis of Guangxi Kumquat (Fortunella Swing) Germplasm Using SRAP Markers. Horticulturae 2023, 9, 689. https://doi.org/10.3390/horticulturae9060689
Liu B, Ding P, Ye R, Li Y, Ou S, Gentile A, Ma X, Deng Z. Genetic Diversity Analysis of Guangxi Kumquat (Fortunella Swing) Germplasm Using SRAP Markers. Horticulturae. 2023; 9(6):689. https://doi.org/10.3390/horticulturae9060689
Chicago/Turabian StyleLiu, Binghao, Ping Ding, Rongchun Ye, Yi Li, Shanhan Ou, Alessandra Gentile, Xianfeng Ma, and Ziniu Deng. 2023. "Genetic Diversity Analysis of Guangxi Kumquat (Fortunella Swing) Germplasm Using SRAP Markers" Horticulturae 9, no. 6: 689. https://doi.org/10.3390/horticulturae9060689
APA StyleLiu, B., Ding, P., Ye, R., Li, Y., Ou, S., Gentile, A., Ma, X., & Deng, Z. (2023). Genetic Diversity Analysis of Guangxi Kumquat (Fortunella Swing) Germplasm Using SRAP Markers. Horticulturae, 9(6), 689. https://doi.org/10.3390/horticulturae9060689




