Abstract
Sustainable nutrient management approaches could improve production and quality without affecting soil health. We conducted a field experiment to investigate the interactive effects of mulching (no mulch, plastic mulch, and straw mulch) and nanobiochar (NBC) foliar application on biomass, nutrient contents, and metabolites in the leaf and curd of cauliflower (Brassica oleracea var. botrytis). After 20 days of transplantation, NBC (0.1% w/v) was applied as a foliar spray for four consecutive weeks (one spray per week). At the curd initiation stage, changes in chlorophyll, carotenoids, and metabolite concentrations in leaves and curd were determined. The application of nanobiochar significantly enhanced the curd weight and improved curd morphology. Yield traits including curd weight, curd diameter, basal diameter, and stalk length were increased by 30, 13, 16, and 20% by NBC application compared to control. Plastic mulching also increased the aboveground biomass by 32% when compared to no mulching. Moreover, plastic mulching and nanobiochar prominently enhanced root dry weight, curd weight, rough solidity index, total soluble sugar in leaf and curd, calcium in curd, and potassium in leaf and curd of cauliflower. Overall, this study revealed the potential of the foliar application of NBC in promoting the biomass and nutritional properties of cauliflower.
1. Introduction
Excessive application of chemical fertilizers is an ineffective practice that alters plant nutrient use efficiency [1,2]. Additionally, a large fraction of chemical fertilizers that are not taken up by the plants enter the water table and causes environmental pollution [3,4]. The release of nitrous oxide is another negative aspect to consider that is a major contributor to climate change [5]. Alternatively, increasing crop production while maintaining environmental security could only be possible by promoting sustainable agroecological practices [4,6,7].
Mulching is a sustainable practice to minimize soil salinity, weed germination, and soil erosion and to increase soil organic matter and soil moisture [8,9]. Various mulching materials are normally derived from organic agricultural and industrial waste and animal residues [10,11], while for inorganic mulching, polyethylene plastic film is generally used [12,13]. It is pertinent to mention here that both water and heat exchange between the soil and air are affected by mulching practices which reduce plant water requirements while simultaneously promoting soil biological activities [14,15]. In short, the use of mulch could potentially benefit plant growth and yield [16].
Carbon-based materials such as biochar (BC) have gained much attention as they contain condensed aromatic carbon, which is pre-dominantly resistant to chemical, microbial oxidation and decomposition [17,18] and could promote carbon sequestration [19,20,21]. The estimated half-life of biochar can range between hundreds to thousands of years; therefore, its addition to soil slows down the release of carbon from the soil to the environment [22]. Biochar is formed by the thermodynamic conversion of organic waste material in the limited presence of oxygen (pyrolysis). Soil amendment with BCs increases soil fertility and reduces the mobility of toxic heavy metals from soil [23,24]. Some reports also suggested that it could ameliorate both soil-borne and air-borne plant diseases [25,26].
Researchers have investigated the effects of various water-extractable and nano forms of biochar on plant growth and yield attributes. In this context, Eskandari et al. [27] reported that extractable biochar has a small amount of dissolved organic carbon, which can be used as a bioactive agent to promote plant growth. Liu et al. [28] compared different forms of biochar and reported that the water-soluble biochar extract increased maize growth compared with biochar ash and washed biochar residue. Kumar et al. [29] reported that the aqueous extract of biochar contains nanosized particles of biochar that led to an increase in biomass and chlorophyll content of lettuce by over 50%. Similarly, a composite of biochar with fertilizers caused a significant increase in yield at a lower application rate than the chemical fertilizers alone [30,31,32]. Above all, the application of biochar-derived nano-forms could be an efficient way to promote crop growth [33,34,35].
Nanobiochar (NBC) generally exhibits a particle size of less than 100 nm, and these can be synthesized from bulk biochar (BC) by various top-down physical methods such as ball milling [18,36]. The researchers reported the positive effects of various other carbon nanomaterials on the soil as well as plant growth and productivity [28,37,38,39]. Only a few studies have been conducted using NBC as a foliar application and soil amendment and have reported positive effects on plant production and physiological traits [35,40,41]. However, the interactive effect of foliar application of nanobiochar and mulching has not been explored yet on the growth and nutrient content of plants. In our previous experiments, NBC application to carrot plants caused enhancements in leaf pigments and improved the concentration of soluble sugars, amino acids, and nutrients in edible plant parts [35], but the higher concentration of applied NBC enhanced vegetative growth rather than the reproductive growth and storage root. Based on our previous study, we hypothesized that the foliar application of NBC before and during curd initiation could improve cauliflower production by affecting the nutrient status, curd weight, and curd architecture. Keeping this in view, we investigated whether the foliar application of NBC and mulching could increase the growth and yield of cauliflower by providing nutrients and adequate water supply while maintaining soil fertility. Based on our findings, we report positive growth promoting interactive effects of mulching and NBC application on cauliflower production.
2. Materials and Methods
2.1. Experiment Details
A field experiment was performed during September–December 2021 at the Botanical Garden of the University of Lahore (31°23′22.4484″ N, 74°14′27.0414″ E), Pakistan, to study the interactive effects of mulching and nanobiochar (NBC) on the growth of cauliflower (Brassica oleracea L. var. botrytis). The field consisted of 3 main plots, and treatments were laid out in a randomized complete block design (RCBD). After the tillage, three blocks of 20 m2 (5 × 4 m) were prepared, and in each block, six raised beds (0.45 m width and 0.25 m height) were prepared. Three types of mulching [no mulch (NM), plastic mulch (PM), and wheat straw mulch (SM) were applied to two beds each in all blocks (Figure 1). For plastic mulching, black plastic film (0.01 mm thick) with holes was spread on the beds. Then 10 d old cauliflower seedlings were transplanted to both sides of each bed by maintaining a 0.3 m plant-to-plant distance. For straw mulching (SM), a 40–50 mm thick layer of wheat straw was evenly spread after 1 week of transplanting seedlings. The soil was silt-loam with a pH 7.6, EC1:1 0.85 dS m−1, 0.65% organic matter, 0.06% total nitrogen, 5.61 mg kg−1 available phosphorus, and 151 mg kg−1 extractable potassium. The maximum temperature at the site was 35 °C in September, and the minimum was 6 °C in December 2021.
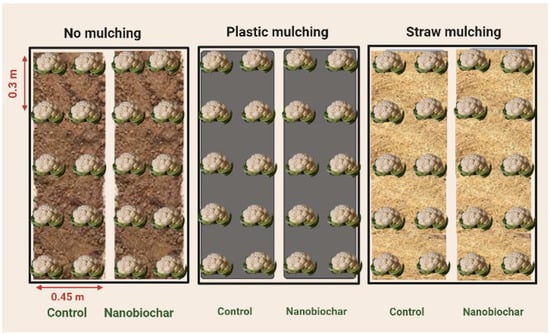
Figure 1.
Schematic diagram representing three mulching treatments and foliar application of nanobiochar in one block.
2.2. Nanobiochar Foliar Application
The nanobiochar was applied as a foliar spray at control (0% NBC) and 0.1% NBC concentration [35]. The first spray treatment was performed after 20 d of transplantation and was repeated every 7 days for a total of 4 weeks. A total of 100 mL of NBC (0.1%) was applied to each plant, and Tween-20 (polyoxyethylene sorbitol ester, C26H50O10) at 0.1% concentration was added to the spray treatments as a surfactant (wetting agent) to increase the adsorption of liquid on to the leaf surfaces. Control plants were sprayed with de-ionized water containing the same amount of surfactant.
2.3. Nanobiochar Characterization
The nanobiochar manufactured from the plant biomass by Shaanxi Hengtian Biological Agriculture Co., Ltd., Xi’an, China, was used in this study. The pH and electrical conductivity (EC1:1) of nanobiochar were determined using pH and EC meter (AD8000 pH/mV/E.C. meter, Adwa Instruments Inc., Szeged, Hungary) (Table S1). The elemental concentration of C, H, O, N, and S was determined by using the elemental analyzer (Elementar, Vario micro cube, Langenselbold, Germany). Other elements were analyzed with ICP-OES (Agilent Technologies 5110-vdv) after wet-acid digestion using the protocol mentioned by USEPA 3050b-1996 [42].
2.4. Growth Parameters
Different growth parameters such as leaf area, shoot length, root length, shoot fresh weight, root fresh weight, root dry weight, and the number of leaves per plant were recorded at the maturity stage of cauliflower plants (harvested 65 days after transplanting).
2.5. Curd Architecture and Yield
At harvest, curd weight and its architectural traits were recorded. The basal diameter was recorded by measuring the mean value from three random directions of curd [43]. The stalk angle was determined by using the ratio of stalk length to basal diameter, while the curd solidity index was calculated by using the following formula [44]:
where Wt is the weight of the curd and bd is the basal diameter.
2.6. Biochemical Traits
2.6.1. Pigments in the Leaves and Curd
Fresh leaf and curd material (100 mg) was extracted with 80% acetone (5 mL), and the OD of the extracts was recorded at 663, 645, and 480 nm using a UV/Vis spectrophotometer (Halo SB-10, Dynamica Scientific Ltd., Livingston, UK) [45]. Chlorophyll contents (mg g−1 fresh weight) were calculated as follows:
where A represents the absorbance at the corresponding wavelength (663 and 645 nm).
Carotenoid content (mg g−1 fresh weight) was calculated according to Davies [46] as:
where Em = 2500.
2.6.2. Primary Metabolites Determination
Some key metabolites (total free amino acids, soluble proteins, sugars, flavonoids, and phenolic content) were determined from leaves and curd. Fresh samples of leaves and curd (100 mg) were homogenized in 2 mL of 0.2 M potassium-phosphate buffer solution (pH 7.4), followed by centrifugation at 10,000 rpm for 10 min. The supernatant was used for the analyses of various metabolites.
- a.
- Total Free Amino Acids
Amino acid contents were determined by mixing the plant extracts (1 mL) with 1 mL ninhydrin solution (2%), followed by the addition of 1 mL pyridine solution (10%). The reaction mixture was vortexed, followed by incubation in a water bath at 97 °C for 30 min. The reaction was stopped by placing the test tubes in cold water, and the volume of the reaction mixture was diluted to 10 mL, and the OD was recorded at 570 nm [47]. Free amino acid contents (mg g−1 fresh weight) were calculated using a standard curve prepared from glycine.
- b.
- Total soluble proteins
Lowry’s method [48] was used to determine the protein content in the sample extracts. A solution of 100 mL (2 g Na2CO3, 0.2 g NaOH, and 1 g sodium potassium tartrate) was mixed with 1 mL of CuSO4.5H2O (0.5g/100 mL). Afterward, 1 mL of the solution mixture was added to the extract (1 mL) and incubated at room temperature for 30 min. The Folin-Ciocalteu’s phenol reagent of 0.5 mL (1:1 diluted) was mixed in the solution mixture and kept at room temperature for 30 min, and the OD was recorded at 620 nm in a spectrophotometer. The standard curve prepared from bovine serum albumin was used to estimate protein contents (mg g−1 fresh weight).
- c.
- Total sugars and reducing and non-reducing sugars
Total sugar contents were determined according to Riazi et al. [49]. Leaf extracts (1 mL) were mixed with 3 mL of freshly prepared anthrone reagent (0.2% anthrone in 0.1 mL diluted 65% H2SO4). The solution mixture was heated at 97 °C in a water bath for 10 min, and the OD of the reaction mixture was recorded at 625 nm by spectrophotometer. The concentrations of soluble sugars (mg g−1 fresh weight) were calculated using a standard curve prepared from glucose. Reducing sugars were estimated using 3,5-dinitro salicylic acid (DNSA) reagent according to the procedure as described by Miller [50] with some modifications [51]. Firstly, the DNSA reagent was prepared by dissolving DNSA (1 g) and sodium-potassium tartaric acid (30 g) in 0.5 N NaOH (80 mL) at 45 °C, cooled to room temperature, and diluted to 100 mL with the help of distilled water. Then, plant extract (1 mL) was mixed with DNSA reagent (2 mL) in a test tube and heated at 95 °C for 5 min, cooled, and the final volume was made 10 mL by adding distilled water. Finally, the OD was recorded at 540 nm using a UV-VIS spectrophotometer (Halo SB-10, Dynamica Scientific Ltd., UK). The calibration curve of D-glucose was used to calculate reducing sugar contents (mg D-glucose equivalent g−1 fresh weight). Non-reducing sugars (mg g−1 fresh weight) were determined as the difference between total and reducing sugars.
- d.
- Phenolics
Total phenolics were determined by following the method of Julkunen-Tiitto [52]. For this purpose, 1 mL of Folin-Ciocalteu’s reagent (1:10 diluted) was mixed with 1 mL extract, followed by the addition of 2 mL Na2CO3 (7.5% w/v). The reaction mixture was vortexed and incubated at room temperature for 30 min, and OD was recorded at 765 nm using a UV/Vis spectrophotometer (Halo SB-10, Dynamica Scientific Ltd., UK). Total phenolics (mg gallic acid equivalent g−1 fresh weight) were calculated using a standard curve prepared from catechol.
- e.
- Flavonoids
Pękal and Pyrzynska’s [53] method was used to estimate flavonoid content using the aluminum complexation reaction. Plant extracts (1 mL) were mixed with 0.3 mL of 5% NaNO2 followed by the addition of 0.5 mL of 2% AlCl3. Finally, 2 mL of 4% NaOH was added, and the reaction mixture was vortexed and incubated at room temperature for 10 min. The OD was recorded at 510 nm using a spectrophotometer, and flavonoid contents (mg catechin per g−1 fresh weight) were calculated using a standard curve prepared from catechin (50–500 µM).
2.7. Nutrients Analysis
Finely ground leaf and curd samples (0.5 g) were incubated overnight in 5 mL concentrated H2SO4 and then digested at 300 °C using hydrogen peroxide [54]. The final colorless solution was filtered through Whatman No. 41 filter paper and a final volume of 50 mL and used for the analyses of mineral contents. Additionally, total nitrogen (% dry weight) was determined by micro-Kjeldahl’s method [55]. The digested extract (5 mL) was placed with 40% NaOH (5 mL) in the Kjeldahl ammonia distillation unit, and then, 5 mL of boric acid with a few mixed indicator drops was added when the distillate reached 40 mL. Then titrated with 0.01 N H2SO4 until the solution turned a pink color. The phosphorus content (µg g−1 dry weight) was estimated using a 5 mL digested sample after its reaction with 5 mL of Barton’s reagent [56]. The final volume of the reaction mixture was made up to 50 mL, and the OD was recorded at 470 nm. Potassium and calcium concentrations (µg g−1 dry weight) were determined using a flame photometer (Model Jenway, PFP7, UK).
2.8. Statistical Analyses
The numerical values obtained through experiments were analyzed by analysis of variance (ANOVA) through Statistix 8.1. The significant differences among the treatments were evaluated by the Least Significant Difference (LSD) test.
3. Results
3.1. Morphological Indices
The plant height and shoot fresh weight of cauliflower were significantly influenced by different mulching (Table 1). The plant height was maximum with no mulching, which was significantly similar to plastic mulching and higher than straw mulching. The aboveground fresh biomass was maximized with plastic mulching and was 32% more than with no mulching. The effect of nanobiochar (NBC) application was non-significant on the plant height, root length, aboveground biomass, and fresh and dry weight of the root. The root dry weight is the only factor significantly influenced by the interactive effect of mulching and NBC, and the maximum was recorded for plastic-mulched NBC-treated plants.

Table 1.
The morphological indices of Brassica oleraceae var. botrytis measured in response to mulching and foliar application of nanobiochar.
3.2. Curd Weight and Architecture
The weight and diameter of curd and architectural traits of curd, i.e., basal diameter, stalk length, stalk angle, and rough solidity index (RSI), were not significantly different under different mulching (Table 2; Figure S1). The application of NBC significantly enhanced the curd weight, curd diameter, basal diameter, and stalk length of cauliflower by 30, 13, 16, and 20%, respectively. The curd weight and RSI were significantly affected by the interactive effect of mulching and NBC. The maximum curd weight was recorded at plastic mulch with NBC, and RSI was found at straw mulching, followed by plastic mulch with NBC. The straw mulching and plastic mulching with NBC enhanced the RSI by 70% and 60%, respectively, compared to no mulching without NBC (control).

Table 2.
The growth indices of curd of Brassica oleraceae var. botrytis measured in response to mulching and foliar application of nanobiochar.
3.3. Pigments
The chlorophyll a and b content and chlorophyll a/b ratio were not affected by NBC and mulching. The total chlorophyll content was increased by 21% by NBC compared to no biochar under no mulching (Figure 2). The carotenoid content was not affected by mulching, although the NBC application caused a 93% and 33% increase in carotenoid content to no biochar under no mulching and straw mulching, respectively.
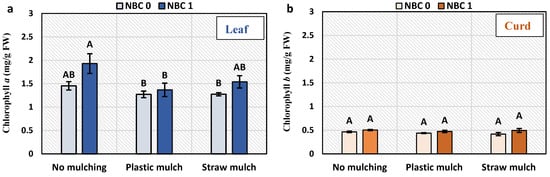
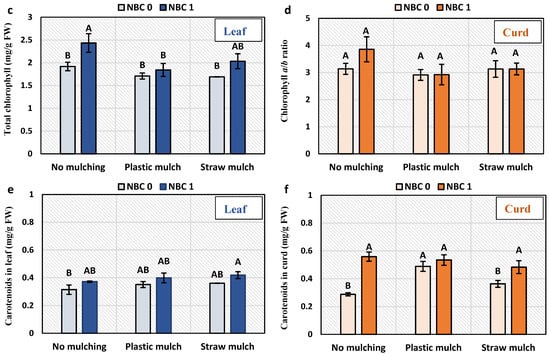
Figure 2.
Chlorophyll and carotenoids content in leaves carotenoid in leaf and curd of Brassica oleracea var. botrytis. (a) Chlorophyll a; (b) chlorophyll b; (c) total chlorophyll; (d) chlorophyll a/b ratio; and (e) carotenoids in leaf; (f) carotenoids in curd. The different alphabets on the bars indicate significant differences among treatments using LSD at p ≤ 0.05.
3.4. Total Sugar, Reducing and Non-Reducing Sugars in Leaf and Curd
Total soluble sugar in the leaf and curd and reducing sugars in the leaf were significantly increased by the NBC application (Figure 3). The total soluble sugar in curd was increased by NBC by 36%, 20.5%, and 23% under no mulching, plastic mulching, and straw mulching conditions, respectively, than under no spray. The total soluble sugar of the leaf was increased by NBC by 48% under plastic mulching and 42% under straw mulching, as compared to no biochar. Reducing sugars in curd and non-reducing sugars in leaf and curd were not affected by NBC. The only significant effect of NBC on reducing sugar in the leaf was a 38% increase under straw mulching than no biochar. The effect of mulching was only significant for leaf total soluble sugars of NBC applied plants, which were 24% and 29% higher under plastic mulching than under no mulching and straw mulching.
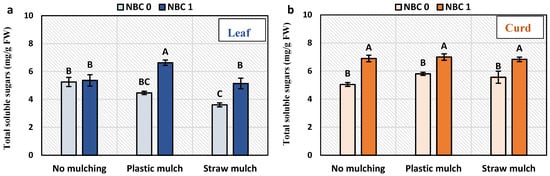
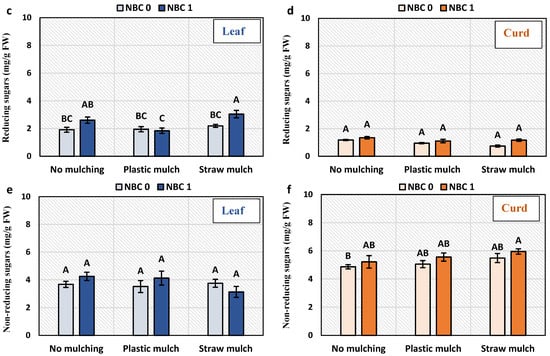
Figure 3.
Sugar contents in leaf and curd of Brassica oleracea var. botrytis. (a,b) Total soluble sugars in leaf and curd; (c,d) reducing sugars; (e,f) non-reducing sugars. The Y-axis bars are kept constant to show relative changes in leaf and curd sugar fractions. The different alphabets on the bars indicate significant differences among treatments using LSD at p ≤ 0.05.
3.5. Amino Acids, Proteins, Flavonoids, and Phenolic Content in Leaf and Curd
Total free amino acids content in the leaf was significantly enhanced by NBC under plastic mulching, while no significant effect was observed under no mulch and straw mulch (Figure 4). Overall, total free amino acid content was highest for straw mulch and NBC and lowest for plastic mulch without NBC. Total soluble protein was significantly increased in leaves by NBC application under no mulch, while not significantly affected under plastic mulch and straw mulch in leaves and curd. Phenolic content in the leaf was significantly increased by NBC under plastic mulching. Total free amino acid, total soluble protein, and phenolics in curd remained unaffected under mulching and NBC. Flavonoids in the leaf were significantly enhanced by NBC by 140% and 85% in no mulching and straw mulching conditions as compared to no biochar.
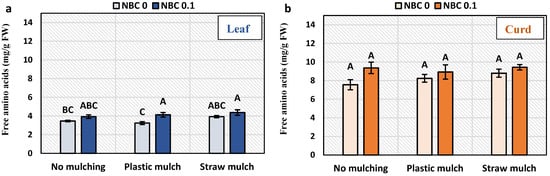
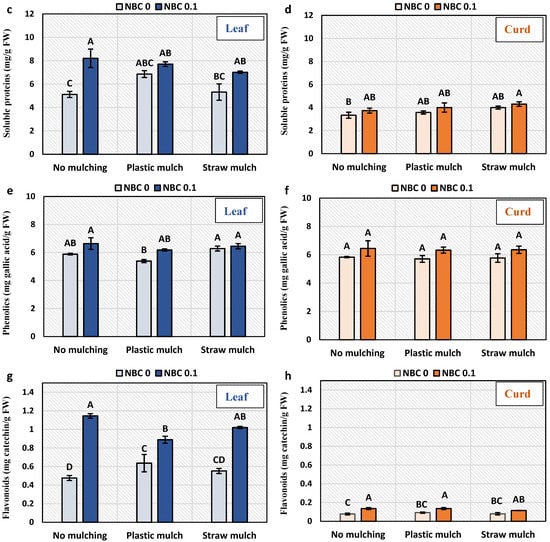
Figure 4.
Metabolites in leaf and curd of Brassica oleracea var. botrytis. (a,b) Total free amino acids; (c,d) soluble proteins; (e,f) phenolics; (g,h) flavonoids in leaf and curd. The Y-axis bars are kept constant to show relative changes in leaf and curd sugar fractions. The different alphabets on the bars indicate significant differences among treatments using LSD at p ≤ 0.05.
3.6. Nutrient Contents (N, P, K, and Ca) in Leaf and Curd
The foliar application of NBC enhanced the N content in the leaf by 77, 51, and 26% in no mulching, plastic mulching, and straw mulching, respectively (Figure 5). The effect of mulching was non-significant on leaf N. While the N in curd was not significantly affected by biochar and mulching. The NBC had no significant effect on the P content of the leaf and curd. Potassium in the leaf was significantly enhanced by the NBC application.
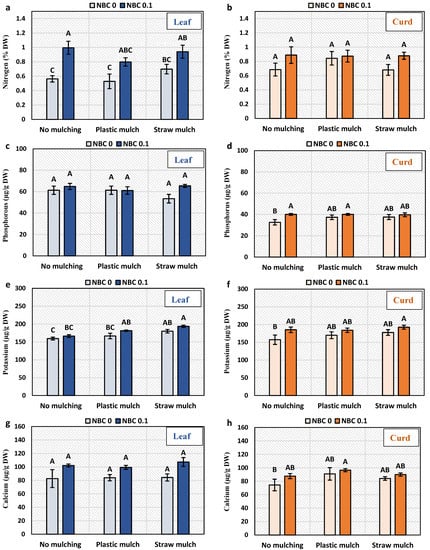
Figure 5.
Nutrient contents in leaves and curd of Brassica oleracea var. botrytis. (a,b) Nitrogen, (c,d) phosphorus, (e,f) potassium, and (g,h) calcium content. The Y-axis bars are kept constant to present real-time changes in relative ionic constituents in leaf and curd. The different alphabets on the bars indicate significant differences among treatments using LSD at p ≤ 0.05.
4. Discussion
4.1. Nanobiochar and Mulching Promote Root Growth and Crop WUE
Nanobiochar prepared from various lignocellulosic wastes could serve as an important bio-stimulant and can improve crop growth and production due to the presence of nutrients, growth-promoting substances, and its ability to regulate plant developmental processes [35]. We applied NBC as a foliar spray to cauliflower plant growth with and without mulching practice. NBC did not significantly enhance the plant height, root length, and aboveground weight, but the curd weight, curd diameter, and stalk length were significantly enhanced as compared to the control. Our previous study indicated that the higher application rate of NBC started at the initial growth stages and enhanced more aboveground than the belowground part of the carrot [35]. Based on our previous study, in this study, we applied NBC to cauliflower before the start of reproductive growth (before bolting) to enhance production. Results showed that the NBC induced more growth of productive parts (i.e., curd growth) while not much influencing the vegetative tissues. This might be because of foliar spray of NBC was started near bolting, and further spray at curd initiation promoted curd growth rather than the vegetative early growth.
Our findings depicted shorter plant height in response to plastic and straw mulches. However, plastic mulching improved the aboveground biomass and root growth traits of cauliflower plants. The interactive effect of NBC and plastic mulching maximally improved the root dry weight and curd weight. A better root structure is crucial for plant productivity [57]. It has been reported that plastic mulching is very effective in improving crop water use efficiency [13,58,59] and regulating soil temperature [60], which improved root growth. NBC has a valuable concentration of essential nutrients such as Ca, K, Fe, N, and P (Table S1). Our results also showed NBC enhanced the nutrient status as indicated by higher K and Ca content (Figure 5). Thus, better root growth contributes to improvements in mineral uptake and photosynthesis.
4.2. Organo-Mineral Particles in NBC Improve Photosynthetic Activity
In our study, the NBC foliar spray caused an increase in the leaf chlorophyll fraction, leading to enhancements in total chlorophyll. By contrast, the effect of mulching remained non-significant on photosynthetic pigments. This can be explained based on NBC’s chemical composition, as it contains organo-mineral particles and agglomerates [29,61]. Similar to NBC, the BCs application to peanut plants improved chlorophyll fluorescence and photosynthesis parameters [62]. Similarly, Kumar et al. [29] reported a significant increase in chlorophyll content in lettuce when exposed to foliar application of the aqueous extract of BC. The NBC-mediated improvement in cauliflower leaves can be due to the presence of N and other minerals such as Fe and K content of NBC, which directly influence leaf chlorophyll biosynthesis. Furthermore, previous reports have shown the effectiveness of the foliar application of nanocarbon and other nanomaterials as they could be easily taken up through the stomata and provide instant nutrients [29,63].
4.3. NBC Mediated Positive Regulation of Plant Metabolism and Photo-Assimilate Transport
Apart from that, carotenoids in curd under no mulching and straw mulching were increased by NBC. It has been reported that the Or gene is responsible for the high accumulation of β-carotene and carotenoids in cauliflower and confers resistance [64]. In plants, carotenoids in usually synthesized in plastids via the 1-deoxy-D-xylulose-5-phosphate pathway rather than the mevalonic acid pathway used for cholesterol biosynthesis in animals [65]. It is still not established how NBC is linked to the regulation of Or genes, which urges exploration. However, the presence of growth stimulatory chemicals can alter plant secondary metabolism, most particularly the mevalonic acid pathway that is connected to carotenoid biosynthesis. However, no direct evidence has been reported so far in the literature.
We also investigated carbohydrate partitioning in the cauliflower leaves and curd. Reduction in leaf sugars compared with control was evident in leaves, but an opposite trend was recorded in the case of curd, and mulching increased sugars in cauliflower curd. Mulching improves root water use efficiency and can lead to photoassimilates transport into the curd from the leaves. This could be the reason for the lower sugar fraction in the leaves compared to the cauliflower curd. By contrast, the foliar application of NBC improved leaf total soluble sugars under both plastic and straw mulching practices. In agreement, an increase in the total soluble sugars, sucrose, and starch was evident in response to BC application [66]. Therefore, we explain NBC-mediated improvements in the soluble sugars in curd based on the improvements in leaf chlorophyll status and nutrient fraction, particularly nitrogen.
Apart from these findings, an increase in the synthesis of reducing sugars in cauliflower leaves was recorded by NBC under straw mulching. While non-reducing sugars remain unaffected by mulching and NBC. We also investigated changes in primary and secondary metabolite concentrations in leaf and cauliflower curd. The foliar application of NBC increased leaf amino acids under plastic mulching, while total amino acids in curd remained unchanged. Likewise, NBC improved total soluble proteins in the leaf under no mulching and plastic mulching, and straw mulching. However, total soluble proteins in curd remained unchanged. Furthermore, NBC improved flavonoid concentration in leaf and curd. Plastic mulching improved the flavonoid content in leaves. In addition, the concentration of phenolics and flavonoids in curd remains unchanged. The NBC-mediated improvements in leaf sugars, phenolics, flavonoids, amino acids, and soluble proteins can be explained based on the presence of bioactive substances including phenolic acids, aldehydes, alcohols, karrikins, and polyphenols [67,68,69].
4.4. Positive Regulation of Plant Mineral Nutrition
Our findings depict that the foliar treatment with NBC improved leaf N fraction as well as in combination with straw mulching. Both leaf and curd nitrogen fractions were improved by foliar NBC treatments. The leaf N ranged from 0.5–0.7 mg/g DW in control while 0.8–1.0 mg/g DW in response to NBC treatments. Moreover, the curd N ranged from 0.68–0.84 mg/g DW in control and 0.87–0.88 mg/g DW in NBC, respectively. Since the N concentration in the NBC spray treatments was minute, we, therefore, predict that the enhancements in the plant N status were due to improvements in plant water and nutrient uptake (instead of NBC acting as a source of N). By contrast, the leaf and curd phosphorus remain unaffected by NBC and mulching, and leaf P ranged up to 60 ug/g DW, while the phosphorus fraction ranged from 30 to 40 µg/g DW in the cauliflower curd. Similarly, the leaf K ranged from 160 to 190 ug/g DW and was slightly increased in response to NBC and straw mulching. The highest leaf K was recorded in response to NBC and straw mulching. A similar trend was recorded in curd in response to NBC and straw mulching. In terms of Ca2+ concentrations in leaf and curd, it ranged up to 110 ug/g DW and higher Ca values were recorded in response to mulching and NBC (although unchanged). Primarily, NBC is rich in multiple nutrients, which are mainly dependent on feedstock materials as well [18]. Nonetheless, these can serve as a direct source of some macronutrients such as N, P, and K. The FTIR, EDX, and ICP-OES analyses of the NBC confirmed the presence of significant amounts of N, K, P, Ca, Mg, and some beneficial elements such as Si in the NBC we applied to the cauliflower plants (Table S1).
4.5. Enhancement in Yield and Architectural Traits through Synergistic Effects of NBC and Mulching
Above all, the yield of cauliflower plants increased prominently in response to the NBC application. The interactive effects of NBC in combination with mulching were maximum in promoting curd growth and architecture (Table 1 and Table 2). Additionally, NBC improved curd weight, curd diameter, basal diameter, stalk length, stalk angle, and RSI values. The curd of cauliflower is the edible part of this vegetable made from floral primordia. Curd architecture is one of the most economically essential characteristics affecting cauliflower curd shape. We determined the curd architecture by measuring four factors, i.e., basal diameter, stalk length, stalk angle, and curd solidity index [44]. Curd solidity depends on the inside architecture of cauliflower curd. A short stalk length and higher stalk angle make curd compact with a higher RSI, while a higher stalk length and less stalk angle make loose curd with a low RSI. The effect of mulching and NBC alone was non-significant, but their interactive effectiveness was significant, and the highest RSI was recorded at straw mulching, followed by plastic mulching along with 0.1% NBC. The cauliflower without mulching and NBC had the lowest RS showing deformed and loose curd. Overall, these results can be explained based on NBC-mediated improvements in physio-biochemical traits and the presence of growth-promoting substances. Curd architecture is mainly determined by polygenetic factors, although also influenced by environmental conditions such as nutrient availability [70]. Metwaly [71] reported that the P and K influenced the curd morphological traits and curd compactness. The improved yield and curd architecture by mulching and NBC are due to the improved nutrient availability and soil condition.
5. Conclusions
Based on our findings, we recommend the use of NBC as a foliar spray owing to its beneficial effects on crop yield and improving the N and K content, soluble protein, flavonoids in leaves, carotenoids, total soluble sugar, and P in curd. Furthermore, the interactive use of plastic mulching and NBC could significantly enhance production and quality. However, before large-scale applications of NBC, the potential negative impacts of foliar-applied NBC in the form of residual carbon aerosols should be carefully studied.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9060687/s1, Figure S1: The (a) ventral and (b) dorsal view of the curd of Brassica oleracea var. botrytis at the end of vegetative growth in response to mulching and nanobiochar; Table S1: The pH, EC, and elemental composition of nanobiochar.
Author Contributions
Conceptualization, S.A. and M.A.; field experimentation, K.U., M.Z. and I.H.; formal analysis, G.-e.-K., K.U. and I.H.; writing—original draft preparation, G.-e.-K. and M.Z.; writing—review and editing, S.A., F.S. and N.X.; supervision, S.A. and F.S.; project administration, M.A.; funding acquisition, N.X. All the authors have read the final manuscript and approved its submission in its current form to Environmental Research. All authors have read and agreed to the published version of the manuscript.
Funding
This research project is supported by a Doctoral research start-up funding project of Shanxi Datong University (2018-B-30), Shanxi Scholarship Council of China (2021-145), and Shanxi basic research program (20210302124068).
Data Availability Statement
The authors agree to share the data files and relevant material.
Acknowledgments
The authors acknowledge the support of the University of Lahore and NAVTTC Pakistan through the Agricultural Farm Manager Training Program for providing all the resources to execute the research project.
Conflicts of Interest
The authors declare that they have no competing financial interest of any sort.
References
- Bhatt, M.K.; Labanya, R.; Joshi, H.C. Influence of long-term chemical fertilizers and organic manures on soil fertility—A review. Univers. J. Agric. Res. 2019, 7, 177–188. [Google Scholar] [CrossRef]
- Iqbal, A.; He, L.; Khan, A.; Wei, S.; Akhtar, K.; Ali, I.; Ullah, S.; Munsif, F.; Zhao, Q.; Jiang, L. Organic manure coupled with inorganic fertilizer: An approach for the sustainable production of rice by improving soil properties and nitrogen use efficiency. Agronomy 2019, 9, 651. [Google Scholar] [CrossRef]
- Chandini; Kumar, R.; Kumar, R.; Prakash, O. The impact of chemical fertilizers on our environment and ecosystem. In Research Trends in Environmental Sciences, 2nd ed.; Sharma, P., Ed.; Akinik Publications: Delhi, India, 2019; p. 69. [Google Scholar]
- Shah, F.; Wu, W. Soil and crop management strategies to ensure higher crop productivity within sustainable environments. Sustainability 2019, 11, 1485. [Google Scholar]
- Simpson, D.; Arneth, A.; Mills, G.; Solberg, S.; Uddling, J. Ozone—The persistent menace: Interactions with the N cycle and climate change. Current Opin. Environ. Sustain. 2014, 9, 9–19. [Google Scholar]
- FAO. Save and Grow: A Policymaker’s Guide to the Sustainable Intensification of Smallholder Crop Production; Food and Agricultural Organization of the United Nations: Rome, Italy, 2011; Available online: http://www.fao.org/docrep/014/i2215e/i2215e.pdf (accessed on 5 May 2023).
- Xie, L.; Li, L.; Xie, J.; Wang, J.; Anwar, S.; Du, C.; Zhou, Y. Substituting inorganic fertilizers with organic amendment reduced nitrous oxide emissions by affecting nitrifiers’ microbial community. Land 2022, 11, 1702. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Wang, J.; Pang, H.; Li, Y. Buried straw layer plus plastic mulching reduces soil salinity and increases sunflower yield in saline soils. Soil. Till Res. 2016, 155, 363–370. [Google Scholar]
- Abd El-Wahed, M.H.; Baker, G.A.; Ali, M.M.; Abd El-Fattah, F.A. Effect of drip deficit irrigation and soil mulching on growth of common bean plant, water use efficiency and soil salinity. Sci. Hort. 2017, 225, 235–242. [Google Scholar]
- Sun, H.; Shao, L.; Liu, X.; Miao, W.; Chen, S.; Zhang, X. Determination of water consumption and the water-saving potential of three mulching methods in a jujube orchard. Eur. J. Agron. 2012, 43, 87–95. [Google Scholar]
- Kader, M.A.; Senge, M.; Mojid, M.A.; Ito, K. Recent advances in mulching materials and methods for modifying soil environment. Soil. Till 2017, 168, 155–166. [Google Scholar]
- Xie, J.; Wang, L.; Li, L.; Anwar, S.; Luo, Z.; Zechariah, E.; Kwami Fudjoe, S. Yield, economic benefit, soil water balance, and water use efficiency of intercropped maize/potato in responses to mulching practices on the semiarid loess plateau. Agriculture 2021, 11, 1100. [Google Scholar]
- Wang, L.; Li, L.; Xie, J.; Luo, Z.; Anwar, S.; Zechariah, E.; Fudjoe, S.K.; Palta, J.A.; Chen, Y. Does plastic mulching reduce water footprint in field crops in China? A meta-analysis. Agric. Water Manag. 2022, 260, 107293. [Google Scholar]
- Chen, W.; Jin, M.; Ferré, T.P.; Liu, Y.; Xian, Y.; Shan, T.; Ping, X. Spatial distribution of soil moisture, soil salinity, and root density beneath a cotton field under mulched drip irrigation with brackish and fresh water. Field Crops Res. 2018, 215, 207–221. [Google Scholar]
- Romanova, S.M.; Ponomarenko, O.I.; Matveyeva, I.V.; Beisembayeva, L.K.; Kazangapova, N.B.; Tukenova, Z.A. Evaluation of mulching technology application for cultivation of agricultural crops. J. Chem. Technol. Metall. 2019, 54, 514–521. [Google Scholar]
- Zhang, F.; Zhang, W.; Qi, J.; Li, F.M. A regional evaluation of plastic film mulching for improving crop yields on the Loess Plateau of China. Agric. Forest Meteorol. 2018, 248, 458–468. [Google Scholar]
- Xu, G.; Lv, Y.; Sun, J.; Shao, H.; Wei, L. Recent advances in biochar applications in agricultural soils: Benefits and environmental implications. Clean Soil. Air Water 2012, 40, 1093–1098. [Google Scholar]
- Shafiq, F.; Anwar, S.; Zhang, L.; Ashraf, M. Nano-biochar: Properties and prospects for sustainable agriculture. Land Degrad. Dev. 2023, 34, 2445–2463. [Google Scholar] [CrossRef]
- Saxena, J.; Rawat, J.; Kumar, R. Conversion of biomass waste into biochar and the effect on mung bean crop production. Clean–Soil Air Water 2017, 45, 1501020. [Google Scholar]
- Ashiq, A.; Adassooriya, N.M.; Sarkar, B.; Rajapaksha, A.U.; Ok, Y.S.; Vithanage, M. Municipal solid waste biochar-bentonite composite for the removal of antibiotic ciprofloxacin from aqueous media. J. Environ. Manag. 2019, 236, 428–435. [Google Scholar]
- Kumar, A.; Bhattacharya, T. Biochar: A sustainable solution. Environ. Dev. Sustain. 2021, 23, 6642–6680. [Google Scholar]
- Zimmerman, A.R. Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ. Sci. Tech. 2010, 44, 1295–1301. [Google Scholar] [CrossRef]
- Tripathi, S.; Sonkar, S.K.; Sarkar, S. Growth stimulation of gram (Cicer arietinum) plant by water soluble carbon nanotubes. Nanoscale 2011, 3, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, M.; Verma, N.; Khan, S. Carbon nanofibers as a micronutrient carrier in plants: Efficient translocation and controlled release of Cu nanoparticles. Environ. Sci. Nano 2017, 4, 138–148. [Google Scholar]
- Rasool, M.; Akhter, A.; Soja, G.; Haider, M.S. Role of biochar, compost and plant growth promoting rhizobacteria in the management of tomato early blight disease. Sci. Rep. 2021, 11, 6092. [Google Scholar] [PubMed]
- Mehari, Z.H.; Elad, Y.; Rav-David, D.; Graber, E.R.; Meller Harel, Y. Induced systemic resistance in tomato (Solanum lycopersicum) against Botrytis cinerea by biochar amendment involves jasmonic acid signaling. Plant Soil 2015, 395, 31–44. [Google Scholar]
- Eskandari, S.; Mohammadi, A.; Sandberg, M.; Eckstein, R.L.; Hedberg, K.; Granström, K. Hydrochar-amended substrates for production of containerized pine tree seedlings under different fertilization regimes. Agronomy 2019, 9, 350. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Feng, Y.; Qiao, J.; Zhao, H.; Xie, J.; Fang, Y.; Shen, S.; Liang, S. The effectiveness of nanobiochar for reducing phytotoxicity and improving soil remediation in cadmium-contaminated soil. Sci. Rep. 2020, 10, 858. [Google Scholar]
- Kumar, A.; Joseph, S.; Graber, E.R.; Taherymoosavi, S.; Mitchell, D.R.; Munroe, P.; Tsechansky, L.; Lerdahl, O.; Aker, W.; Sæbø, M. Fertilizing behavior of extract of organomineral-activated biochar: Low-dose foliar application for promoting lettuce growth. Chem. Biol. Tech. Agric. 2021, 8, 21. [Google Scholar]
- Schmidt, H.P.; Pandit, B.H.; Cornelissen, G.; Kammann, C.I. Biochar-based fertilization with liquid nutrient enrichment: 21 field trials covering 13 crop species in Nepal. Land Deg. Develop. 2017, 28, 2324–2342. [Google Scholar]
- Um-e-Laila; Hussain, A.; Nazir, A.; Shafiq, M. Potential application of biochar composite derived from rice straw and animal bones to improve plant growth. Sustainability 2021, 13, 11104. [Google Scholar]
- Das, S.K.; Ghosh, G.K. Hydrogel-biochar composite for agricultural applications and controlled release fertilizer: A step towards pollution free environment. Energy 2022, 242, 122977. [Google Scholar]
- Dimkpa, C.O.; White, J.C.; Elmer, W.H.; Gardea-Torresdey, J. Nanoparticle and ionic Zn promote nutrient loading of sorghum grain under low NPK fertilization. J. Agric. Food Chem. 2017, 65, 8552–8559. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Chen, X.; Wang, Q.; Wei, W.; Zhang, T. Effects of nano carbon on soil erosion and nutrient loss in a semi-arid loess region of Northwestern China. Int. J. Agric. Biol. Eng. 2018, 11, 138–145. [Google Scholar] [CrossRef]
- Khaliq, H.; Anwar, S.; Shafiq, F.; Ashraf, M.; Zhang, L.; Haider, I.; Khan, S. Interactive effects of soil and foliar-applied nanobiochar on growth, metabolites, and nutrient composition in Daucus carota. J. Plant Growth Reg. 2022, 42, 3715–3729. [Google Scholar]
- Lyu, H.; Gao, B.; He, F.; Zimmerman, A.R.; Ding, C.; Tang, J.; Crittenden, J.C. Experimental and modeling investigations of ball-milled biochar for the removal of aqueous methylene blue. Chem. Eng. J. 2018, 335, 110–119. [Google Scholar]
- Saxena, M.; Maity, S.; Sarkar, S. Carbon nanoparticles in ‘biochar’ boost wheat (Triticum aestivum) plant growth. RSC Adv. 2014, 4, 39948–39954. [Google Scholar] [CrossRef]
- Gámiz, B.; Cox, L.; Hermosín, M.C.; Spokas, K.; Celis, R. Assessing the effect of organoclays and biochar on the fate of abscisic acid in soil. J. Agric. Food Chem. 2017, 65, 29–38. [Google Scholar] [CrossRef]
- Shafiq, F.; Iqbal, M.; Raza, S.H.; Akram, N.A.; Ashraf, M. Fullerenol [60] Nano-cages for protection of crops against oxidative stress: A critical review. J. Plant Growth Reg. 2022, 42, 1267–1290. [Google Scholar]
- Yang, Y.; Zhou, B.; Hu, Z.; Lin, H. The effects of nano-biochar on maize growth in northern Shaanxi Province on the Loess Plateau. Appl. Ecol. Environ. Res. 2020, 18, 2863–2877. [Google Scholar]
- Ramzan, M.; Zia, A.; Naz, G.; Shahid, M.; Shah, A.A.; Farid, G. Effect of nanobiochar (nBC) on morpho-physio-biochemical responses of black cumin (Nigella sativa L.) in Cr-spiked soil. Plant. Physiol. Biochem. 2023, 196, 859–867. [Google Scholar] [PubMed]
- US EPA. Method 3050B: Acid Digestion of Sediments, Sludges, and Soils. Revision 2; US EPA: Washington, DC, USA, 1996.
- Lan, T.H.; Paterson, A.H. Comparative mapping of quantitative trait loci sculpting the curd of Brassica oleracea. Genetics 2000, 155, 1927–1954. [Google Scholar]
- Zhao, Z.; Gu, H.; Wang, J.; Sheng, X.; Yu, H. Development and comparison of quantitative methods to evaluate the curd solidity of cauliflower. J. Food Eng. 2013, 119, 477–482. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant. Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Davies, B.H. Carotenoids. In Chemistry and Biochemistry of Plant Pigments; Goodwin, T.W., Ed.; Academic Press: London, UK, 1976; pp. 38–155. [Google Scholar]
- Hamilton, P.B.; Van Slyke, D.D.; Lemish, S. The gasometric determination of free amino acids in blood filtrates by the ninhydrin-carbon dioxide method. J. Biol. Chem. 1943, 150, 231–250. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Riazi, A.; Matsuda, K.; Arslan, A. Water-stress induced changes in concentrations of proline and other solutes in growing regions of young barley leaves. J. Exp. Bot. 1985, 36, 1716–1725. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 420–428. [Google Scholar] [CrossRef]
- Krivorotova, T.; Sereikaite, J. Determination of fructan exohydrolase activity in the crude extracts of plants. Electron. J. Biotechnol. 2014, 17, 329–333. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R. Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Method. 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Wolf, B.A. comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun. Soil. Sci. Plant Anal. 1982, 13, 1035–1059. [Google Scholar] [CrossRef]
- Bremner, J.M.; Edwards, A.P. Determination and isotope-ratio analysis of different forms of nitrogen in soils: I. Apparatus and procedure for distillation and determination of ammonium. Soil. Sci. Soc. Am. J. 1965, 29, 504–507. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Constable and Co. Ltd.: London, UK, 1962; p. 497. [Google Scholar]
- Ristova, D.; Busch, W. Natural variation of root traits: From development to nutrient uptake. Plant Physiol. 2014, 166, 518–527. [Google Scholar] [CrossRef]
- Kader, M.A.; Senge, M.; Mojid, M.A.; Nakamura, K. Mulching type-induced soil moisture and temperature regimes and water use efficiency of soybean under rain-fed condition in central Japan. Int. Soil Water Conserv. Res. 2017, 5, 302–308. [Google Scholar] [CrossRef]
- Luo, L.; Hui, X.; He, G.; Wang, S.; Wang, Z.; Siddique, K.H. Benefits and limitations to plastic mulching and nitrogen fertilization on grain yield and sulfur nutrition: Multi-site field trials in the semiarid area of China. Front. Plant Sci. 2022, 13, 799093. [Google Scholar] [CrossRef]
- Ibarra-Jiménez, L.; Zermeño-González, A.; Munguia-Lopez, J.; Rosario Quezada-Martín, M.A.; De La Rosa-Ibarra, M. Photosynthesis, soil temperature and yield of cucumber as affected by colored plastic mulch. Acta Agric. Scand. Sect. B–Soil Plant Sci. 2008, 58, 372–378. [Google Scholar] [CrossRef]
- Hernandez-Soriano, M.C.; Kerré, B.; Kopittke, P.M.; Horemans, B.; Smolders, E. Biochar affects carbon composition and stability in soil: A combined spectroscopy microscopy study. Sci. Rep. 2016, 6, 25127. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, J.; Wang, Y.; Yang, Q.; Chen, T.; Chen, Y.; Chi, D.; Xia, G.; Siddique, K.H.M.; Wang, T. Photosynthesis, chlorophyll fluorescence, and yield of peanut in response to biochar application. Front. Plant Sci. 2021, 12, 650432. [Google Scholar] [CrossRef]
- Su, Y.; Ashworth, V.; Kim, C.; Adeleye, A.S.; Rolshausen, P.; Roper, C.; White, J.; Jassby, D. Delivery, uptake, fate, and transport of engineered nanoparticles in plants: A critical review and data analysis. Environ. Sci: Nano 2019, 6, 2311–2331. [Google Scholar]
- Lopez, A.B.; Van Eck, J.; Conlin, B.J.; Paolillo, D.J.; O’Neill, J.; Li, L. Effect of the cauliflower Or transgene on carotenoid accumulation and chromoplast formation in transgenic potato tubers. J. Exp. Bot. 2008, 59, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, N.J.; Clinton, S.K.; Erdman, J.W., Jr. Nutritional aspects of phytoene and phytofluene, carotenoid precursors to lycopene. Adv. Nutr. 2011, 2, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.H.U.; Kong, L.J.; Shan, Y.Z.; Yao, X.D.; Zhang, H.J.; Xie, F.T.; Xue, A.O. Effect of biochar on grain yield and leaf photosynthetic physiology of soybean cultivars with different phosphorus efficiencies. J. Integr. Agric. 2019, 18, 2242–2254. [Google Scholar]
- Graber, E.R.; Harel, Y.M.; Kolton, M.; Cytryn, E.; Silber, A.; David, D.R.; Tsechhansky, L.; Borenshtein, M.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Reynolds, A.; Joseph, S.D.; Verheyen, T.V.; Chinu, K.; Taherymoosavi, S.; Munroe, P.R.; Donne, S.; Pace, B.; van Zwieten, L.; Marjo, C.E.; et al. Effect of clay and iron sulphate on volatile and water extractable organic compounds in bamboo biochars. J. Anal. Appl. Pyrolysis 2018, 133, 22–29. [Google Scholar] [CrossRef]
- Taherymoosavi, S.; Joseph, S.; Pace, B.; Munroe, P. A comparison between the characteristics of single-and mixed feedstock biochars generated from wheat straw and basalt. J. Anal. Appl. Pyrolysis 2018, 129, 123–133. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Sheng, X.G.; Yu, H.F.; Wang, J.S.; Shen, Y.S.; Gu, H.H. Identification of QTLs associated with curd architecture in cauliflower. BMC Plant. Biol. 2020, 20, 177. [Google Scholar] [CrossRef] [PubMed]
- Metwaly, E.E. Influence of phosphorus and potassium on growth and yield of cauliflower. J. Plant Prod. 2017, 8, 329–334. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).