Application of Cinnamaldehyde Solid Lipid Nanoparticles in Strawberry Preservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Strawberry
2.2. Preparation of SLN-CA
2.3. Handling Strawberries and Preservation
2.4. Decay Index of Strawberry Spoilage
2.5. Quality Evaluation
2.5.1. Weight Loss
2.5.2. Firmness
2.5.3. Colour Variation and Visual Appearance
2.6. Biochemical Index
2.6.1. Preparation of Strawberry Homogenate
2.6.2. Total Soluble Solids (TSS)
2.6.3. Titratable Acid (TA)
2.6.4. SOD Activity
2.6.5. MDA
2.6.6. Vitamin C (Vc)
2.6.7. CAT
2.7. Sensory Evaluation
3. Results and Discussion
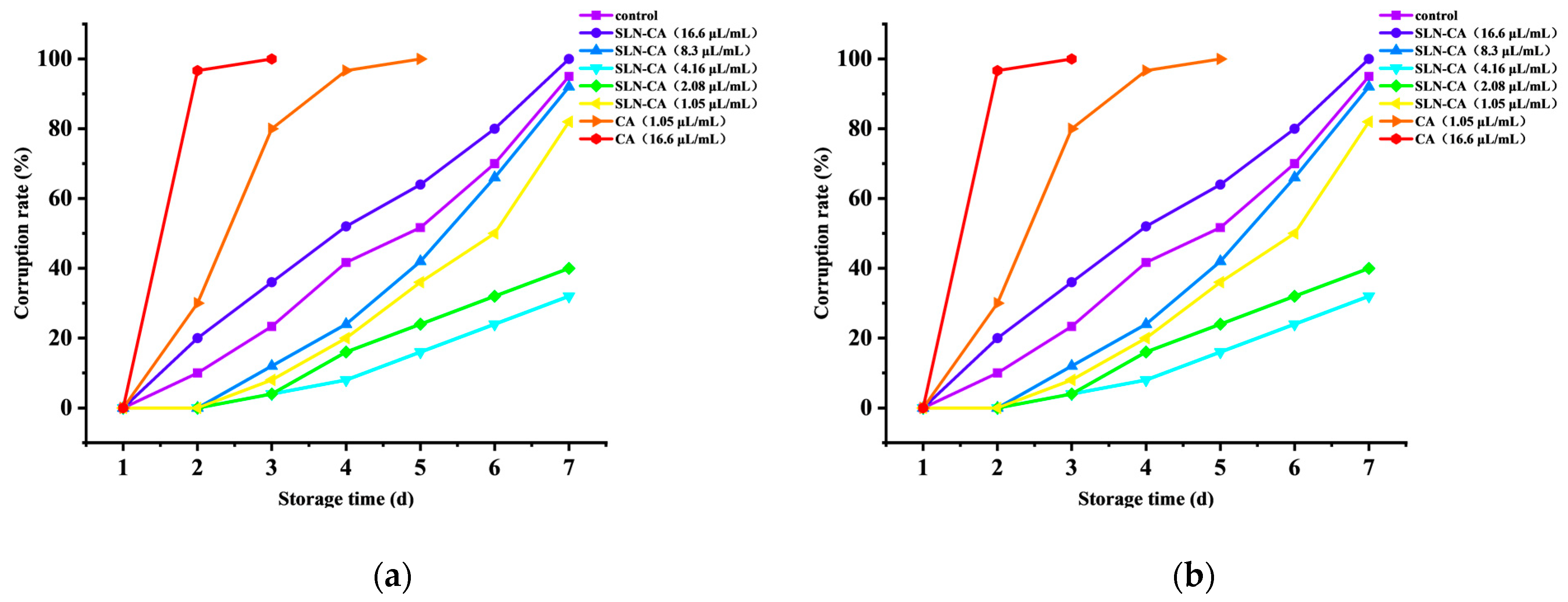
3.1. Decay Index of Strawberry Spoilage
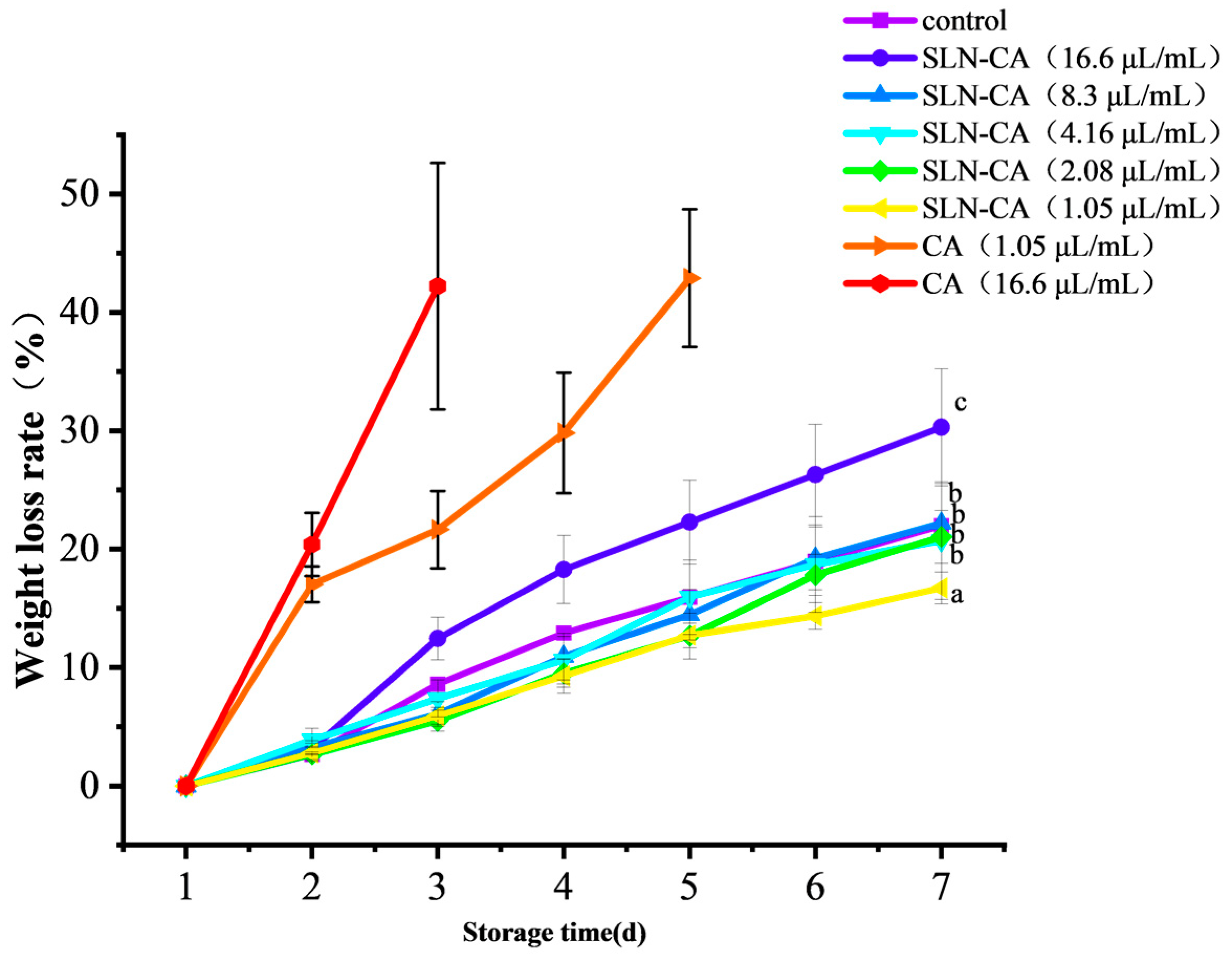
3.2. Weight Loss
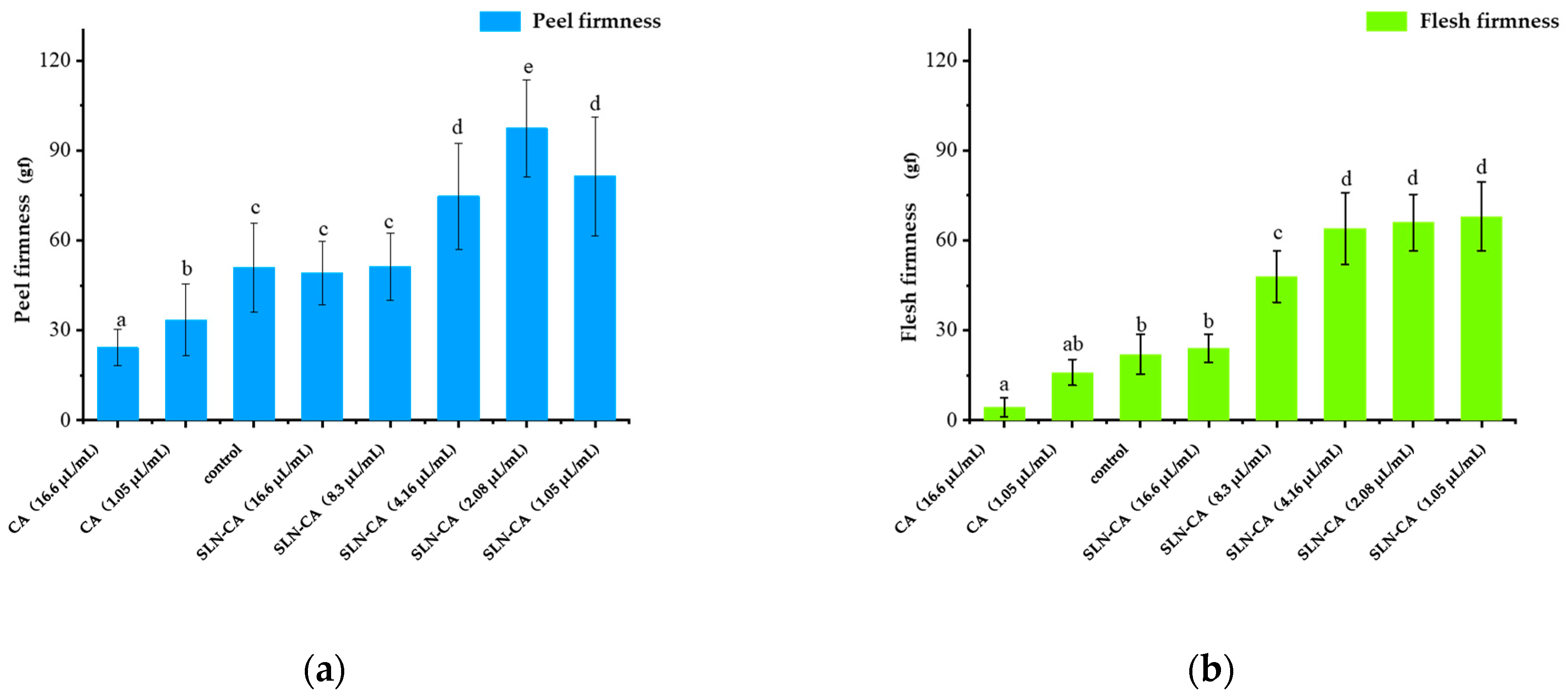
3.3. Firmness
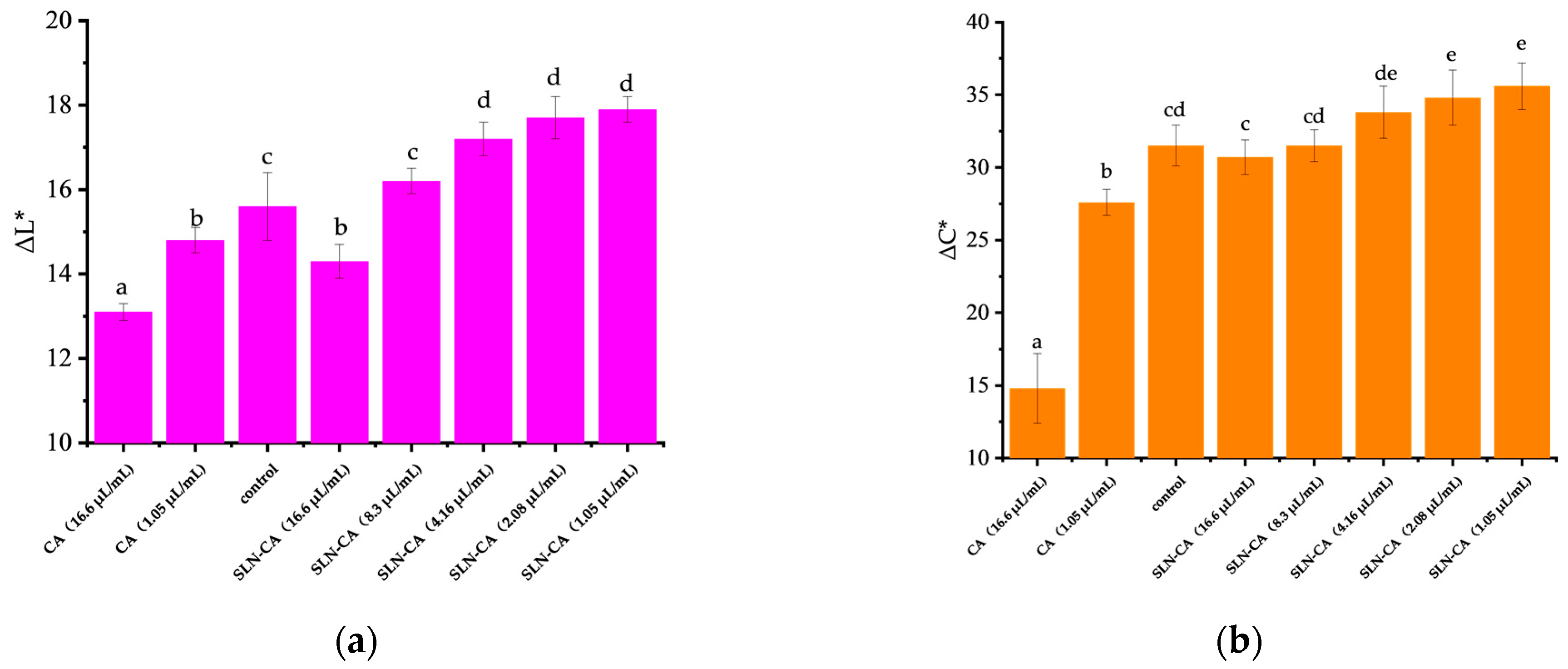
3.4. Colour Variation and Visual Appearance
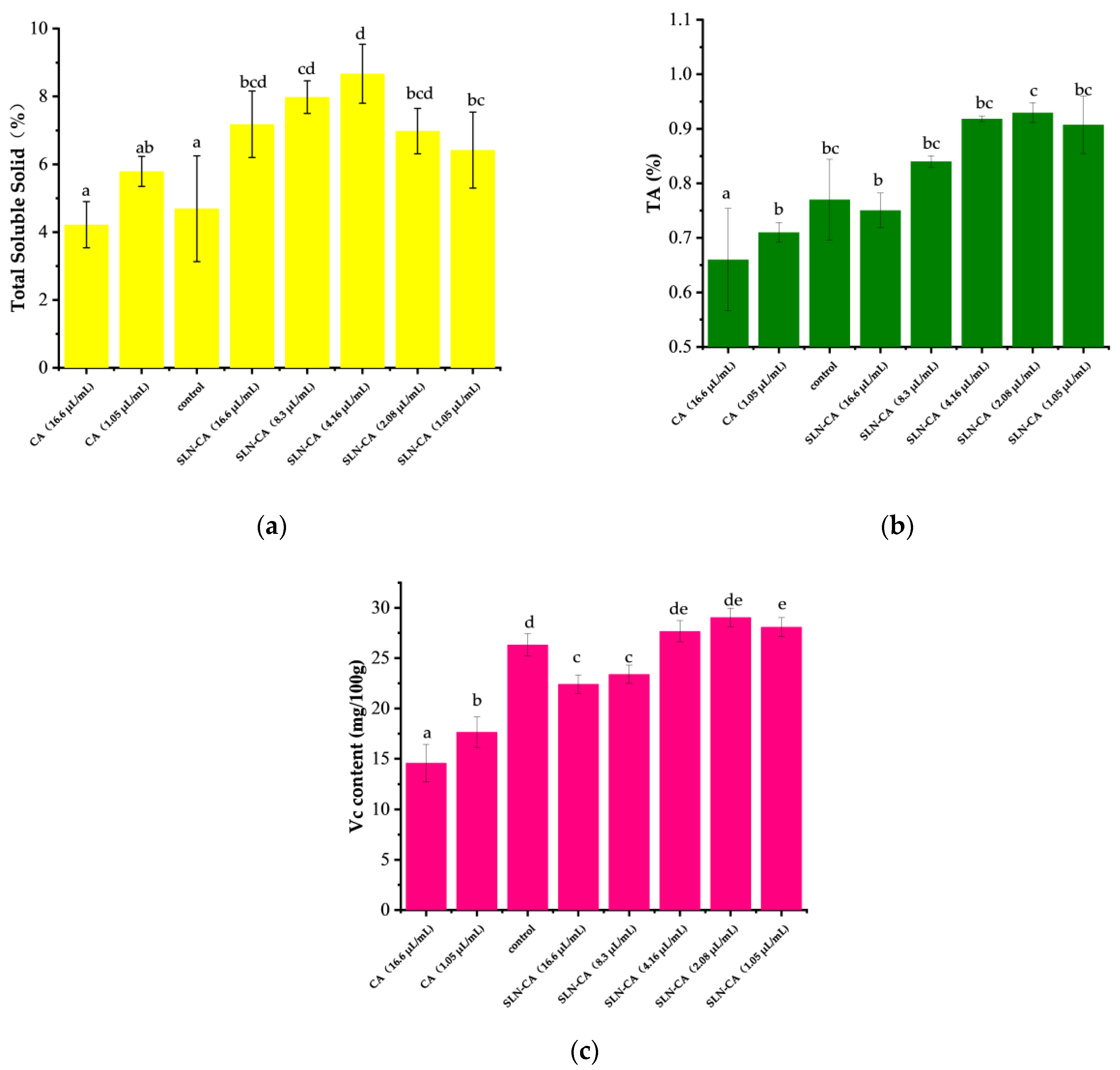
3.5. Vc, TSS, and TA
3.6. SOD, MDA, and CAT
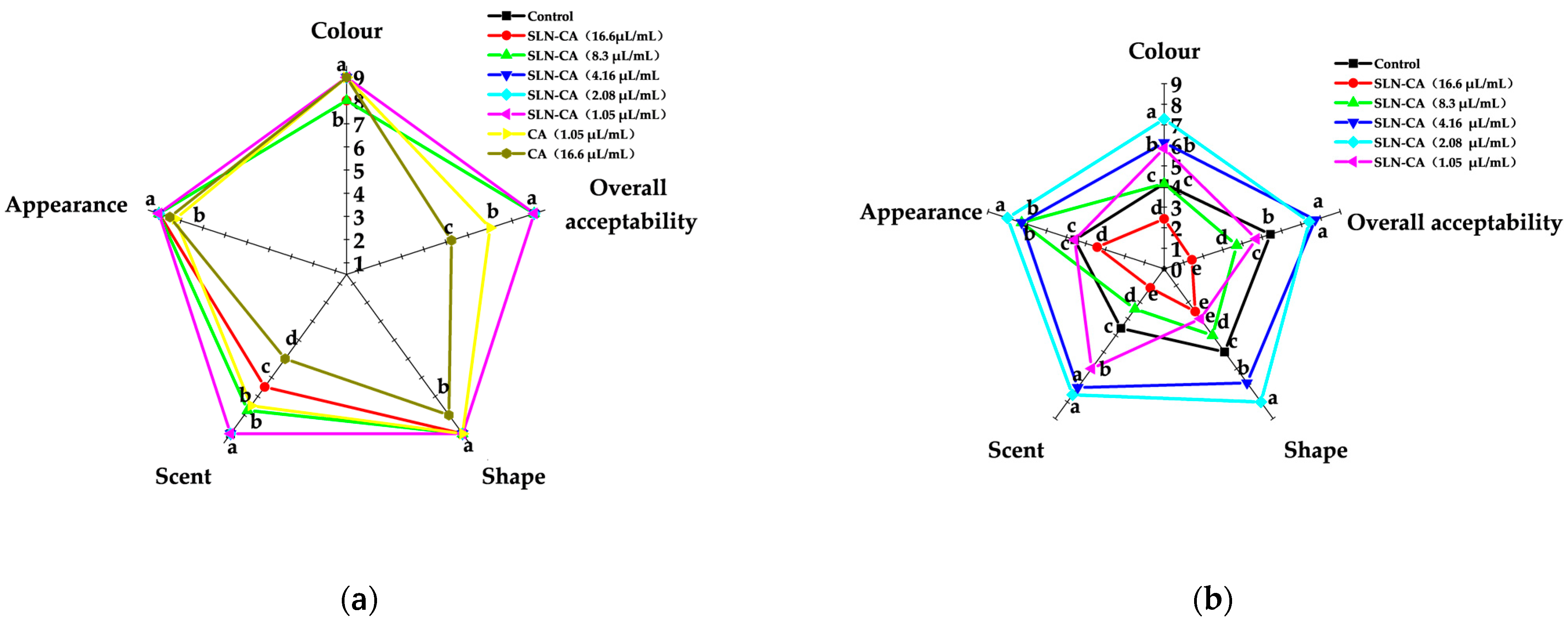
3.7. Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kallio, H.P.; Hakala, M.A.; Pelkkikangas, A.-M.; Lapveteläinen, A. Sugars and acids of strawberry varieties. Eur. Food Res. Technol. 2000, 212, 81–85. [Google Scholar] [CrossRef]
- Tulipani, S.; Marzban, G.; Herndl, A.; Laimer, M.; Mezzetti, B.; Battino, M.A. Influence of environmental and genetic factors on health-related compounds in strawberry. Food Chem. 2011, 124, 906–913. [Google Scholar] [CrossRef]
- Nunes, M.C.d.N.; Brecht, J.K.; Morais, A.M.M.B.; Sargent, S.A. Possible Influences of Water Loss and Polyphenol Oxidase Activity on Anthocyanin Content and Discoloration in Fresh Ripe Strawberry (cv. Oso Grande) During Storage at 1 °C. J. Food Sci. 2005, 70, 56. [Google Scholar] [CrossRef]
- Aday, M.S.; Buyukcan, M.B.; Caner, C. Maintaining the quality of strawberries by combined effect of aqueous chlorine dioxide with modified atmosphere packaging. J. Food Process. Preservat. 2013, 37, 568–581. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Temocico, G.; Fierascu, I.; Ortan, A.; Babeanu, N.E. Fragaria genus: Chemical composition and biological activities. Molecules 2020, 25, 498. [Google Scholar] [CrossRef]
- Jesmin, S.; Al-Jubayer, A.; Eusuf, S.b.; Kamal, A.H.M.A.; Islam, J.M.M.; Ferdoush, F.; Kabir, S.E.; Khan, M.A. Gamma Radiation Treated Chitosan Solution for Strawberry Preservation: Physico-Chemical Properties and Sensory Evaluation. Int. Lett. Nat. Sci. 2016, 60, 30–37. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, L.J.; Hu, Y.; Zhu, Z.; Zhuang, C.; Zhao, Y.; Zhong, Y. Electrostatic spraying of chitosan coating with different deacetylation degree for strawberry preservation. Int. J. Biol. Macromol. 2019, 139, 1232–1238. [Google Scholar] [CrossRef]
- Basu, A.; Nguyen, A.; Betts, N.M.; Lyons, T.J. Strawberry As a Functional Food: An Evidence-Based Review. Crit. Rev. Food Sci. Nutrit. 2014, 54, 790–806. [Google Scholar] [CrossRef]
- Nunes, M.C.d.N.; Morais, A.M.M.B.; Brecht, J.K.; Sargent, S.A.; Bartz, J.A. Prompt Cooling Reduces Incidence and Severity of Decay Caused by Botrytis cinerea and Rhizopus stolonifer in Strawberry. Hortechnology 2005, 15, 153–156. [Google Scholar] [CrossRef]
- Ceredi, G.; Antoniacci, L.; Montuschi, C.; De Paoli, E.; Mari, M.; Gengotti, S. Ten years of field trials on grey mold control on strawberries. Acta Hortic. 2009, 842, 327–330. [Google Scholar] [CrossRef]
- Duran, M.; Aday, M.S.; Zorba, N.N.D.; Temizkan, R.; Büyükcan, M.B.; Caner, C. Potential of antimicrobial active packaging ‘containing natamycin, nisin, pomegranate and grape seed extract in chitosan coating’ to extend shelf life of fresh strawberry. Food Bioprod. Process. 2016, 98, 354–363. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Jiyong, S.; Mahunu, G.K.; Zhai, X.; Mariod, A.A. Quality and postharvest-shelf life of cold-stored strawberry fruit as affected by gum arabic (Acacia senegal) edible coating. J. Food Biochem. 2018, 42, e12527. [Google Scholar] [CrossRef]
- Nunes, M.; Emond, J.; Brecht, J. Quality of strawberries as affected by temperature abuse during ground, in-flight and retail handling operations. In Proceedings of the International Conference on Quality in Chains; An Integrated View on Fruit and Vegetable Quality 604, Wageningen, The Netherlands, 6–9 July 2003; pp. 239–246. [Google Scholar]
- Doyle, A.A.; Stephens, J.C. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia 2019, 139, 104405. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Chemistry, Antimicrobial Mechanisms, and Antibiotic Activities of Cinnamaldehyde against Pathogenic Bacteria in Animal Feeds and Human Foods. J. Agric. Food Chem. 2017, 65, 10406–10423. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Shi, W.; Zheng, H.; Wang, L.; Li, L. Release of Cinnamaldehyde and Thymol from PLA/Tilapia Fish Gelatin-Sodium Alginate Bilayer Films to Liquid and Solid Food Simulants, and Japanese Sea Bass: A Comparative Study. Molecules 2021, 26, 7140. [Google Scholar] [CrossRef]
- Abdullah, S.Z.; Tong, W.Y.; Leong, C.R.; Ab Rashid, S.; Rozman, N.A.S.; Mohammad Hamid, N.H.; Karim, S.; Tumin, N.D.; Muda, S.A.; Yacob, L.S. Development of cinnamaldehyde loaded-alginate based film for food packaging. Asia Pacific J. Mol. Biol. Biotechnol. 2021, 10, 1150. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Chafer, M. Use of essential oils in bioactive edible coatings: A review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Xiong, S.; Wang, Y.-D.; Chen, Y.; Gao, M.; Zhao, Y.-X.; Wu, L. Effects of Drought Stress and Rehydration on Physiological and Biochemical Properties of Four Oak Species in China. Plants 2022, 11, 679. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, C.; Guan, X.; Lian, S.; Li, B.-h.; Wang, C.-x. Butylated Hydroxytoluene Induced Resistance Against Botryosphaeria dothidea in Apple Fruit. Front. Microbiol. 2021, 11, 599062. [Google Scholar] [CrossRef]
- Yue, M.; Jiang, L.; Zhang, N.; Zhang, L.; Liu, Y.; Wang, Y.; Li, M.; Lin, Y.; Zhang, Y.; Zhang, Y.; et al. Importance of FaWRKY71 in Strawberry (Fragaria × ananassa) Fruit Ripening. Int. J. Mol. Sci. 2022, 23, 12483. [Google Scholar] [CrossRef]
- Banerjee, A.; Qi, J.; Gogoi, R.; Wong, J.; Mitragotri, S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J. Control. Rel. Off. J. Controll. Rel. Soc. 2016, 238, 176–185. [Google Scholar] [CrossRef]
- Wang, C.; Lv, J.; Xue, C.-c.; Li, J.; Liu, Y.; Xu, D.; Jiang, Y.; Jiang, S.; Zhu, M.; Yang, Y.; et al. Novel role of COX6c in the regulation of oxidative phosphorylation and diseases. Cell Death Discov. 2022, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Ngwuluka, N.C.; Kotak, D.J.; Devarajan, P.V. Design and Characterization of Metformin-Loaded Solid Lipid Nanoparticles for Colon Cancer. AAPS PharmSciTech. 2017, 18, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in vitro release study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Brandl, M.; Bachmann, D.; Drechsler, M.; Bauer, K.H. Liposome Preparation by a New High Pressure Homogenizer Gaulin Micron Lab 40. Drug Dev. Ind. Pharm. 1990, 16, 2167–2191. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, D.; Sundaresan, S.; Iyadurai, A.; Subramanian, K.S.; Janavi, G.J.; Paliyath, G.; Subramanian, J. Hexanal Vapor Induced Resistance against Major Postharvest Pathogens of Banana (Musa acuminata L.). Plant Pathol. J. 2020, 36, 133–147. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, R.; Sun, X.; Yu, X.; Xiao, Y.; Wang, L.; Hu, W.; Zhong, T. The p-Anisaldehyde/β-cyclodextrin inclusion complexes as fumigation agent for control of postharvest decay and quality of strawberry. Food Control 2021, 130, 108346. [Google Scholar] [CrossRef]
- Mohammed, M.; Sallam, A.; Alqahtani, N.; Munir, M. The Combined Effects of Precision-Controlled Temperature and Relative Humidity on Artificial Ripening and Quality of Date Fruit. Foods 2021, 10, 2636. [Google Scholar] [CrossRef]
- Peryam, D.R.; Pilgrim, F.J. Hedonic scale method of measuring food preferences. Food Technol. 1957, 11, 9–14. [Google Scholar]
- Hu, W.; Feng, K.; Xiu, Z.; Jiang, A.; Lao, Y. Thyme oil alginate-based edible coatings inhibit growth of pathogenic microorganisms spoiling fresh-cut cantaloupe. Food Biosci. 2019, 32, 100467. [Google Scholar]
- Perdones, Á.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of chitosan–lemon essential oil coatings on storage-keeping quality of strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Hu, W.; Feng, K.; Xiu, Z.; Jiang, A.; Lao, Y. Efficacy of thyme oil-alginate-based coating in reducing foodborne pathogens on fresh-cut apples. Int. J. Food Sci. Technol. 2019, 54, 3128–3137. [Google Scholar]
- Dhital, R.; Mora, N.; Watson, D.G.; Kohli, P.; Choudhary, R. Efficacy of limonene nano coatings on post-harvest shelf life of strawberries. LWT 2018, 97, 124–134. [Google Scholar] [CrossRef]
- Asghari, M.; Hasanlooe, A.R. Methyl jasmonate effectively enhanced some defense enzymes activity and Total Antioxidant content in harvested “Sabrosa” strawberry fruit. Food Sci. Nutrit. 2016, 4, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Feliziani, E.; Romanazzi, G. Postharvest decay of strawberry fruit: Etiology, epidemiology, and disease management. J. Berry Res. 2016, 6, 47–63. [Google Scholar] [CrossRef]
- Moreira, M.R.; Cassani, L.; Martín-Belloso, O.; Soliva-Fortuny, R. Effects of polysaccharide-based edible coatings enriched with dietary fiber on quality attributes of fresh-cut apples. J. Food Sci. Technol. 2015, 52, 7795–7805. [Google Scholar] [CrossRef]
- Scollard, J.; Francis, G.A.; O’Beirne, D. Some conventional and latent anti-listerial effects of essential oils, herbs, carrot and cabbage in fresh-cut vegetable systems. Postharvest Biol. Technol. 2013, 77, 87–93. [Google Scholar] [CrossRef]
- Khalifa, I.; Barakat, H.; El-Mansy, H.A.; Soliman, S.A. Improving the shelf-life stability of apple and strawberry fruits applying chitosan-incorporated olive oil processing residues coating. Food Packag. Shelf Life 2016, 9, 10–19. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fotopoulos, V.; Nahar, K.; Fujita, M. Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Ji, H.; Kim, H.; Ryu, J.-H. Antimicrobial Activities of Gaseous Essential Oils against Xerophilic Mold (Penicillium corylophilum). In Proceedings of the IAFP 2018 Annual Meeting, Salt Lake City, UT, USA, 8–11 July 2018; pp. 49–54. [Google Scholar]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Martín-Belloso, O. Edible alginate-based coating as carrier of antimicrobials to improve shelf-life and safety of fresh-cut melon. Int. J. Food Microbiol. 2008, 121, 313–327. [Google Scholar] [CrossRef]

| Processing Means | Weight before (g) | Weight afterwards (g) | Weight Loss Rate (%) |
|---|---|---|---|
| CA (1.05 μL/mL) | 12.14 ± 2.05 a | D | D |
| CA (16.6 μL/mL) | 12.92 ± 1.83 a | D | D |
| SLN-CA (1.05 μL/mL) | 12.38 ± 2.23 a | 10.317 ± 2.2 b | 16.72 ± 1.33 a |
| SLN-CA (2.08 μL/mL) | 12.45 ± 2.19 a | 9.83 ± 2.14 b | 21.04 ± 2.22 b |
| SLN-CA (4.16 μL/mL) | 12.63 ± 2.62 a | 10.01 ± 2.49 b | 20.72 ± 4.95 b |
| SLN-CA (8.32 μL/mL) | 12.76 ± 1.95 a | 9.93 ± 1.88 b | 22.17 ± 3.36 b |
| SLN-CA (16.6 μL/mL) | 12.29 ± 1.18 a | 8.57 ± 1.12 a | 28.98 ± 3.11 c |
| Control | 12.18 ± 1.65 a | 9.51 ± 1.584 b | 21.98 ± 4.33 b |
| Processing Means | SOD (U/g) | CAT (U/g) | MDA (nmol/g) |
|---|---|---|---|
| CA (1.05 μL/mL) | 207.25 ± 1.62 a | 88.21 ± 7.62 bc | 577.75 ± 20.68 de |
| CA (16.6 μL/mL) | 197.86 ± 1.83 b | 64.98± 8.69 a | 598.63 ± 17.56 e |
| SLN-CA (1.05 μL/mL) | 214.77 ± 0.65 e | 92.91 ± 5.11 bc | 546.83 ± 11.43 c |
| SLN-CA (2.08 μL/mL) | 212.28 ± 1.08 cd | 98.6 ± 6.87 b | 463.07 ± 6.92 a |
| SLN-CA (4.16 μL/mL) | 213.02 ± 1.34 de | 94.6 ± 6.42 bc | 523.23 ± 6.51 b |
| SLN-CA (8.32 μL/mL) | 210.87 ± 0.86 c | 88.45 ± 3.08 bc | 565.93 ± 8.66 cd |
| SLN-CA (16.6 μL/mL) | 202.08 ± 1.06 b | 82.65 ± 7.16 b | 580.69 ± 8.93 de |
| Control | 210.42 ± 0.39 c | 94.56 ± 5.44 bc | 558.48 ± 7.65 cd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Chen, J.; Liu, Y.; Zheng, Q.; Tan, W.; Feng, X.; Feng, K.; Hu, W. Application of Cinnamaldehyde Solid Lipid Nanoparticles in Strawberry Preservation. Horticulturae 2023, 9, 607. https://doi.org/10.3390/horticulturae9050607
Li S, Chen J, Liu Y, Zheng Q, Tan W, Feng X, Feng K, Hu W. Application of Cinnamaldehyde Solid Lipid Nanoparticles in Strawberry Preservation. Horticulturae. 2023; 9(5):607. https://doi.org/10.3390/horticulturae9050607
Chicago/Turabian StyleLi, Shangjian, Jiajia Chen, Yuntong Liu, Qinhua Zheng, Weijian Tan, Xiaolin Feng, Kexin Feng, and Wenzhong Hu. 2023. "Application of Cinnamaldehyde Solid Lipid Nanoparticles in Strawberry Preservation" Horticulturae 9, no. 5: 607. https://doi.org/10.3390/horticulturae9050607
APA StyleLi, S., Chen, J., Liu, Y., Zheng, Q., Tan, W., Feng, X., Feng, K., & Hu, W. (2023). Application of Cinnamaldehyde Solid Lipid Nanoparticles in Strawberry Preservation. Horticulturae, 9(5), 607. https://doi.org/10.3390/horticulturae9050607





