Unraveling Arbuscular Mycorrhizal Fungi Interactions in the Exotic Plant Nicotiana glauca Graham for Enhanced Soil Fertility and Alleviation of Metal Pollution
Abstract
1. Introduction
2. Materials and Methods
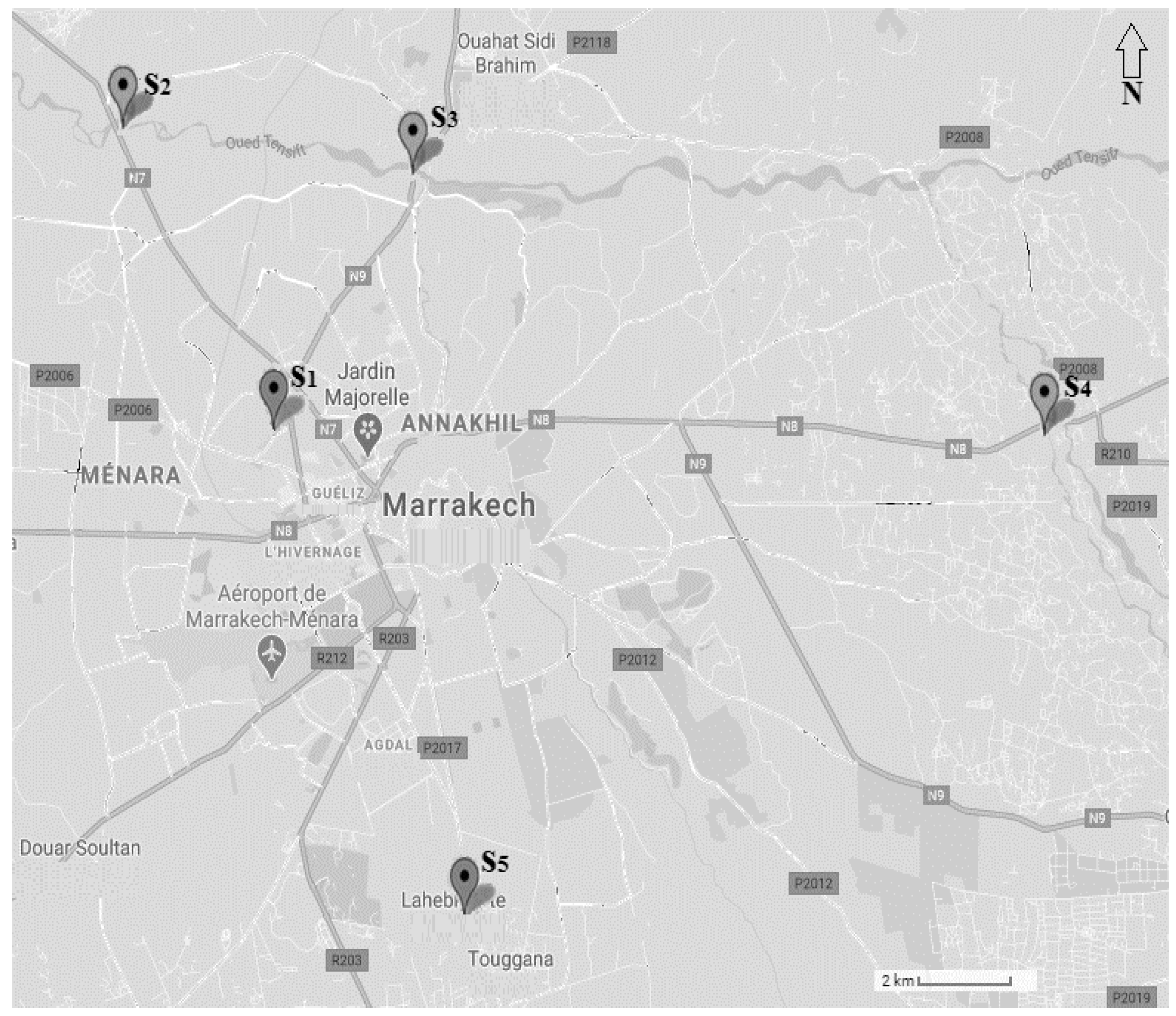
2.1. Sampling Sites
2.2. Soil Physicochemical Analysis
2.3. Estimated Seed Production of N. glauca
2.4. Soil Sampling and Enumeration of AMF Spores in Soils
2.5. Morphological Description of AMF Spores Associated with N. glauca
2.6. Determination of Mycorrhizal Traits in the Roots of N. glauca
2.7. Mycorrhizal Soil Infectivity in the Rhizhospheric Soil of N. glauca
2.8. Heavy Metal Analysis in N. glauca Rhizhospheric Soils
2.9. The Pollution Load Index
2.10. Statistical Analysis
3. Results
3.1. Physicochemical Characteristics of the Rhizospheric Soil of N. glauca and Bare Soil
3.2. Estimate of N. glauca Seeds Production
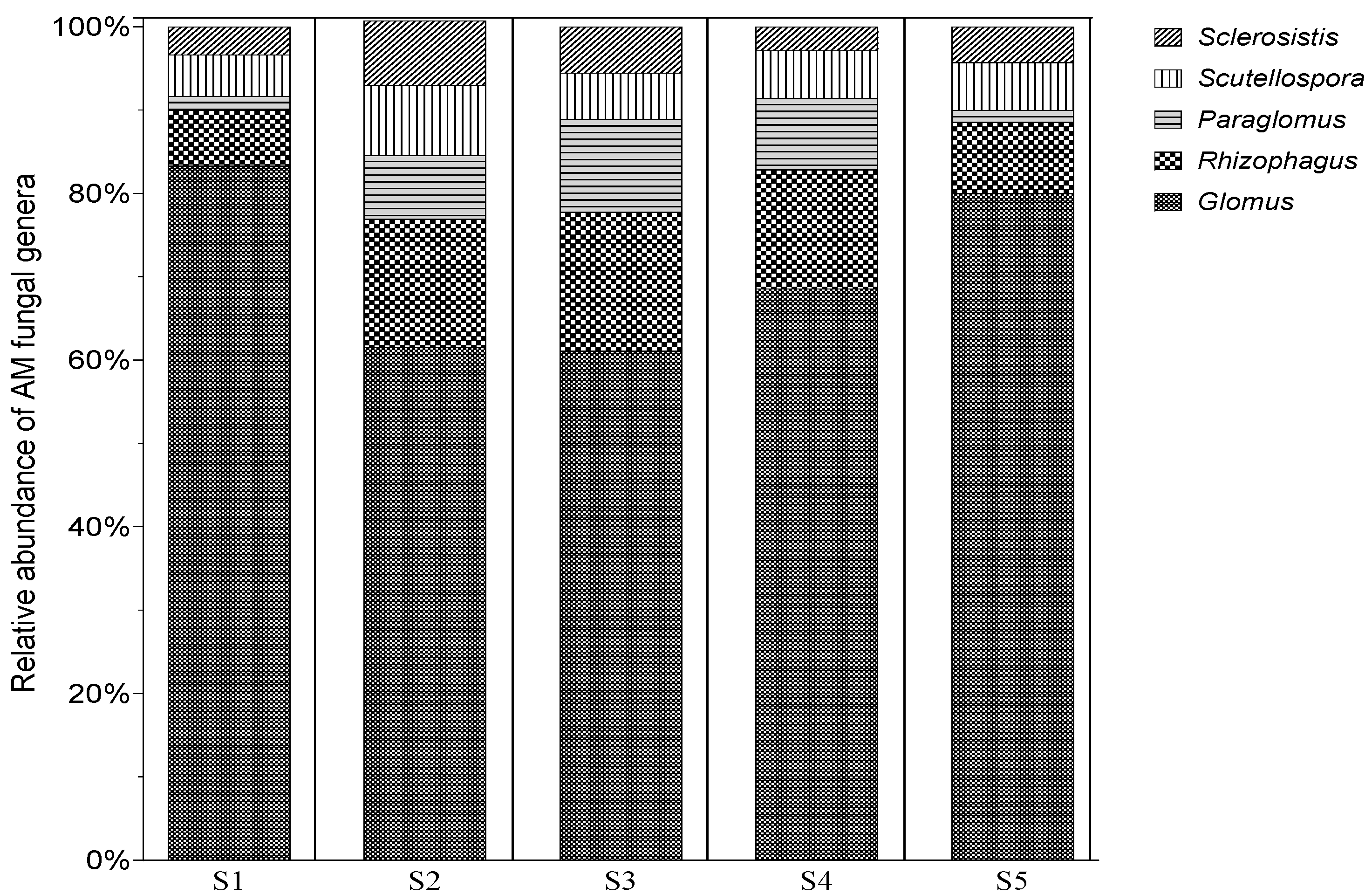
3.3. Counting the Spores of AMF Associated with N. glauca
3.4. Morphological Differentiaition of AMF Spores Morphotypes Associated with N. glauca
3.5. Determination of Mycorrhizal Traits in N. glauca Plant Roots
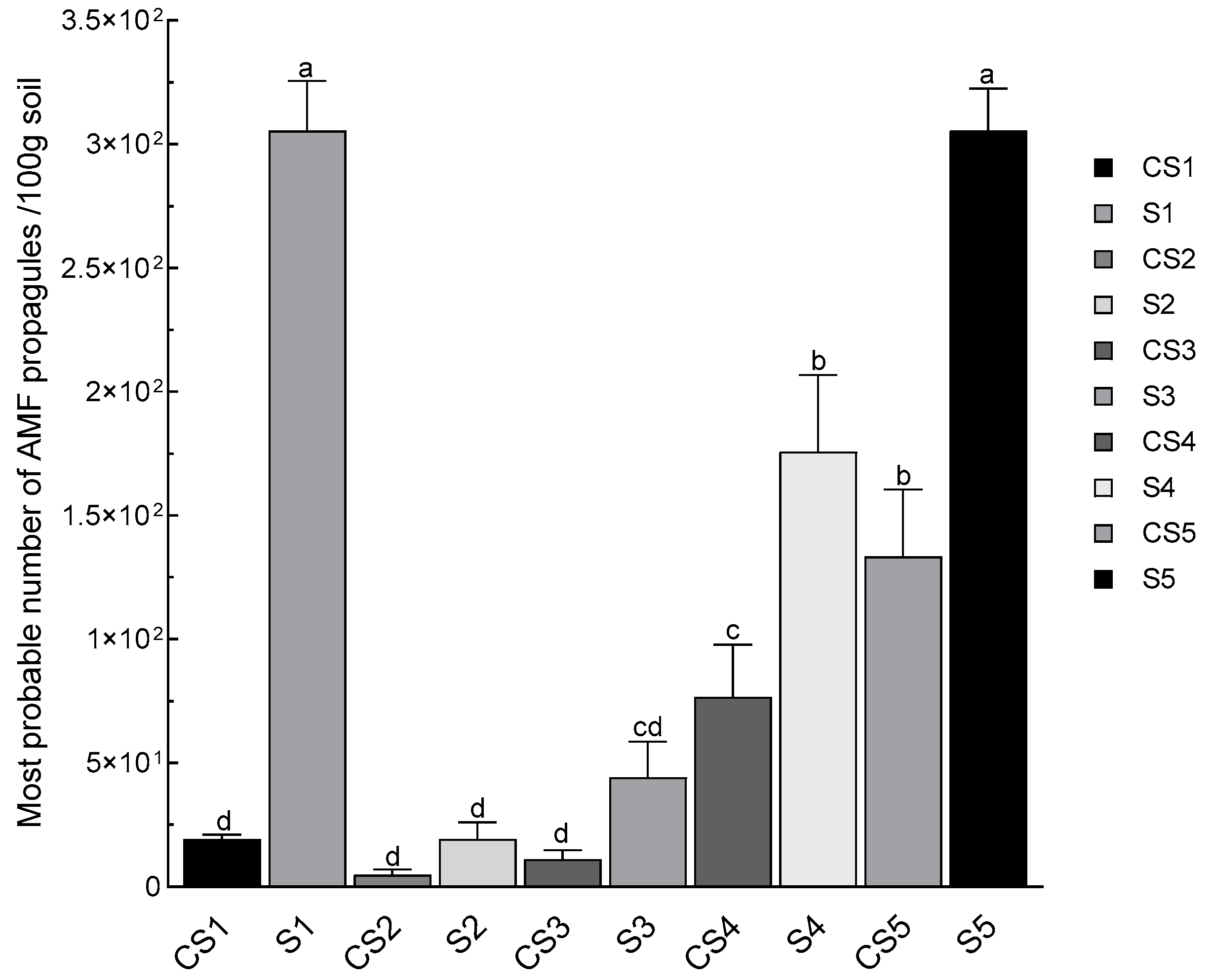
3.6. Mycorrhizal Potential in Rhizospheric Soils of N. glauca (MPN)
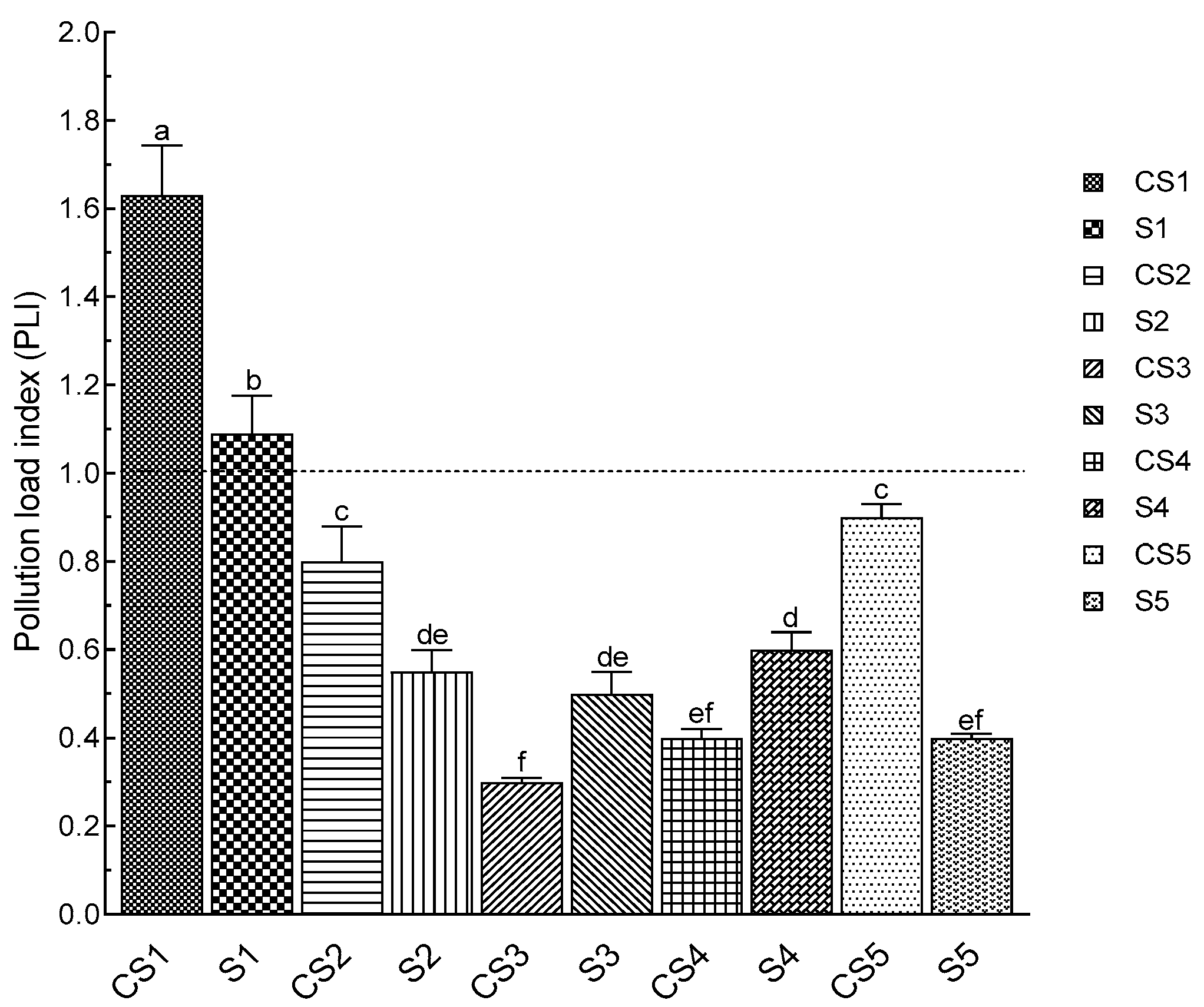
3.7. Heavy Metal Analysis and Pollution Load Index (PLI)
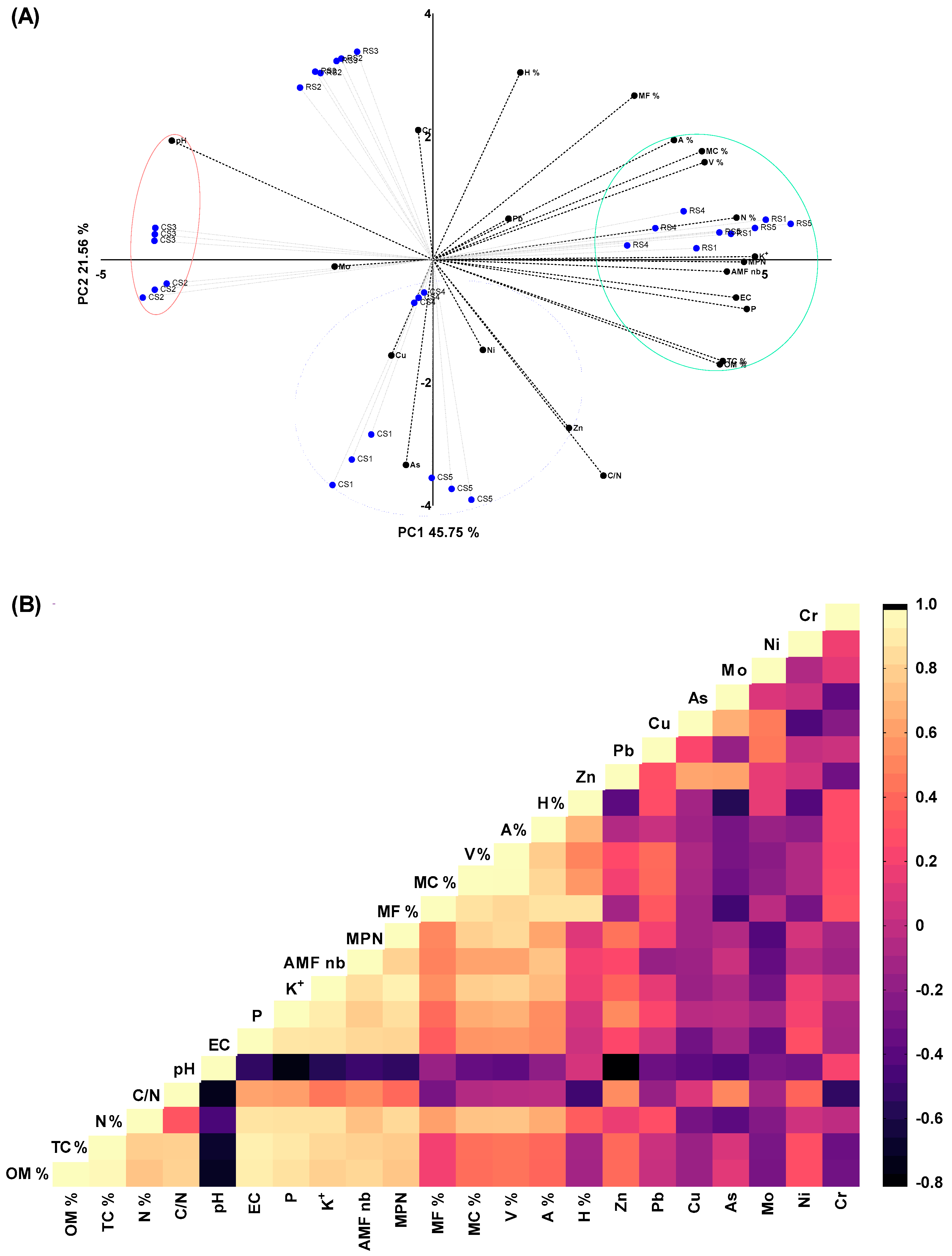
3.8. Inter-Dependencies between all Studied Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Wilgen, B.; Richardson, D.; Higgins, S.I. Integrated control of invasive alien plants in terrestrial ecosystems. L Use Water Resour. Res. 2001, 1, 1732-2016-140256. [Google Scholar]
- Usma, A.; Ahmad, M.; Zafar, M.; Sultana, S.; Ullah, F.; Saqib, S.; Ayaz, A.; Zaman, W. Palynological Study of Weed Flora from Potohar Plateau. Agronomy 2022, 12, 2500. [Google Scholar] [CrossRef]
- Cronk, Q.C.B.; Fuller, J.L. Plant Invaders: The Threat to Natural Ecosystems; Earthscan Publications Ltd. Routledge: London, UK, 2014; ISBN 1134203586. [Google Scholar] [CrossRef]
- Richardson, D.M.; Pyšek, P.; Rejmánek, M.; Barbour, M.G.; Panetta, F.D.; West, C.J. Naturalization and invasion of alien plants: Concepts and definitions. Divers. Distrib. 2000, 6, 93–107. [Google Scholar] [CrossRef]
- Ollerton, J.; Watts, S.; Connerty, S.; Lock, J.; Parker, L.; Wilson, I.; Schueller, S.; Nattero, J.; Cocucci, A.A.; Izhaki, I. Pollination ecology of the invasive tree tobacco Nicotiana glauca: Comparisons across native and non-native ranges. J. Pollinat. Ecol. 2012, 9, 85–95. [Google Scholar] [CrossRef]
- Jameson, R.; Jardine, W.; Rogers, H.D. The Edinburgh New Philosophical Journal: Exhibiting a View of the Progressive Discoveries and Improvements in the Sciences and the Arts; A. and C. Black: London, UK, 1841; Volume 30. [Google Scholar]
- Bogdanović, S.; Mitić, B.; Ruščić, M.; Dolina, K. Nicotiana glauca Graham (Solanaceae), a new invasive plant in Croatia. Acta Bot. Croat. 2006, 65, 203–209. [Google Scholar]
- Florentine, S.K.; Westbrooke, M.E.; Gosney, K.; Ambrose, G.; O’Keefe, M. The arid land invasive weed Nicotiana glauca R. Graham (Solanaceae): Population and soil seed bank dynamics, seed germination patterns and seedling response to flood and drought. J. Arid Environ. 2006, 66, 218–230. [Google Scholar] [CrossRef]
- Steenkamp, P.A.; Van Heerden, F.R.; Van Wyk, B.-E. Accidental fatal poisoning by Nicotiana glauca: Identification of anabasine by high performance liquid chromatography/photodiode array/mass spectrometry. Forensic Sci. Int. 2002, 127, 208–217. [Google Scholar] [CrossRef]
- Kunkel, G. Biogeography and Ecology in the Canary Islands; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 30, ISBN 940101566X. [Google Scholar]
- Quezel, P.; Santa, S. Nouvelle Flore de l’Algérie et des Régionsdésertiques et Méridionales, Tome II; Edition CNRS: Paris, France, 1963. [Google Scholar]
- Jahandiez, E.; Maire, R. Catalogue Des Plantes Du Maroc:(Spermatophytes et Ptéridophytes). Tome Premier. Ptéridophytes, Gymnospermes et Monocotylédones; Minerva: Alger, Algeria, 1931; 150p. [Google Scholar]
- Taleb, A.; Bouhache, M. Etat actuel de nosconnaissances sur les plantesenvahissantes au Maroc. Invasive Plants Mediterr. Type Reg. World 2006, 99–107. [Google Scholar]
- Schueller, S.K. Self-pollination in island and mainland populations of the introduced hummingbird-pollinated plant. Nicotiana glauca (Solanaceae). Am. J. Bot. 2004, 91, 672–681. [Google Scholar] [CrossRef]
- Furer, V.; Hersch, M.; Silvetzki, N.; Breuer, G.S.; Zevin, S.; Zevin, S. Nicotiana glauca (Tree Tobacco) Intoxication—Two Cases in One Family. J. Med. Toxicol. 2011, 7, 47–51. [Google Scholar] [CrossRef]
- Pickett, B.; Maltz, M.; Aronson, E. Impacts of invasive plants on soil fungi and implications for restoration. In Diversity and Ecology of Invasive Plants; IntechOpen: London, UK, 2019; pp. 1–18. [Google Scholar]
- Sally, E.; Smith, D.J.R. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2008; Volume 137, ISBN 9780123705266. [Google Scholar]
- Kapoor, R.; Sharma, D.; Bhatnagar, A.K. Arbuscular mycorrhizae in micropropagation systems and their potential applications. Sci. Hortic. 2008, 116, 227–239. [Google Scholar] [CrossRef]
- Zardak, S.G.; Dehnavi, M.M.; Salehi, A.; Gholamhoseini, M. Effects of using arbuscular mycorrhizal fungi to alleviate drought stress on the physiological traits and essential oil yield of fennel. Rhizosphere 2018, 6, 31–38. [Google Scholar] [CrossRef]
- El Kinany, S.; Achbani, E.; Faggroud, M.; Ouahmane, L.; El Hilali, R.; Haggoud, A.; Bouamri, R. Effect of organic fertilizer and commercial arbuscular mycorrhizal fungi on the growth of micropropagated date palm cv. Feggouss. J. Saudi. Soc. Agric. Sci. 2019, 18, 411–417. [Google Scholar] [CrossRef]
- Neuenkamp, L.; Moora, M.; Öpik, M.; Davison, J.; Gerz, M.; Männistö, M.; Jairus, T.; Vasar, M.; Zobel, M. The role of plant mycorrhizal type and status in modulating the relationship between plant and arbuscular mycorrhizal fungal communities. New Phytol. 2018, 220, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfeld, J.G. Ecosystem consequences of biological invasions. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 59–80. [Google Scholar] [CrossRef]
- Kulmatiski, A.; Beard, K.H. Long-term plant growth legacies overwhelm short-term plant growth effects on soil microbial community structure. Soil Biol. Biochem. 2011, 43, 823–830. [Google Scholar] [CrossRef]
- Cuddington, K. Legacy effects: The persistent impact of ecological interactions. Biol. Theory 2011, 6, 203–210. [Google Scholar] [CrossRef]
- Ater, M.; Ennabili, A. Flore (“Pteridophyta” et “Spermatophyta”) des zones humides du Maroc Méditérranéen: Inventaire et écologie. Acta Bot. Malacit. 1996, 21, 221–239. [Google Scholar]
- Bammi, J.; Mouhiddine, M.; Fassi, D.; Douira, A. Contribution à la connaissance de la végétation des Doukkala-Abda (Maroc Atlantique): Approcheéco-géomorphologique. J. Anim. Plant Sci. 2014, 20, 3202–3211. [Google Scholar]
- Fennane, M.; Tattou, M.I. Flore vasculaire du Maroc: Inventaire et chorologie. Trav. Inst. Sci. Rabat Sér. Botanique 2005, 1, 483. [Google Scholar]
- González, A.; Tezara, W.; Rengifo, E.; Herrera, A. Ecophysiological responses to drought and salinity in the cosmopolitan invader Nicotiana glauca. Brazilian J. Plant Physiol. 2012, 24, 213–222. [Google Scholar] [CrossRef]
- Curt, M.D.; Fernández, J. Production of Nicotiana glauca RC Graham aerial biomass in relation to irrigation regime. Biomass 1990, 23, 103–115. [Google Scholar] [CrossRef]
- Brandes, D. Nicotiana glauca als invasive Pflanze auf Fuerteventura. Adventivpflamen. Beiträgezu Biol. Vor. und Ausbreitungsdynamik von Archäophyten und NeophytenMitteleuropa. Tag. Des Braunschw. Kolloqu. Vom 2001, 3, 39–57. [Google Scholar]
- Kjeldahl, J. A New Method for the Determination of Nitrogen in Organic Matter. Z. Für Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Olsen, R.S.; Watanabe, F.S. A Method to Determine a Phosphorous Adsorption Maximum of Soils as Measured by Langmuir Isotherms. Soil Sci. Soc. Am. J. 1957, 21, 144–149. [Google Scholar] [CrossRef]
- Gaines, T.P.; Mitchell, G.A. Boron determination in plant tissue by the azomethine H. method. Commun. Soil Sci. Plant Anal. 1979, 10, 1099–1108. [Google Scholar] [CrossRef]
- Brundrett, M.; Bougher, N.; Dell, B.; Grove, T.; Malajczuk, N. Working with mycorrhizas in forestry and agriculture (ACIAR Monograph 32). Canberra Aust. Aust. Cent. Int. Agric. Res. 1996, 374. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158-IN18. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular—Arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Ouahmane, L.; Hafidi, M.; Plenchette, C.; Kisa, M.; Boumezzough, A.; Thioulouse, J.; Duponnois, R. Lavandula species as accompanying plants in Cupressus replanting strategies: Effect on plant growth, mycorrhizal soil infectivity and soil microbial catabolic diversity. Appl. Soil Ecol. 2006, 34, 190–199. [Google Scholar] [CrossRef]
- Porter, W.M. The ‘Most Probable Number’ method for enumerating infective propagules of vesicular arbuscular mycorrhizal fungi in soil. Soil Res. 1979, 17, 515–519. [Google Scholar] [CrossRef]
- Fisher, R.A.; Yates, F. Statistical Tables: For Biological, Agricultural and Medical Research; Oliver and Boyd: Edinburgh, UK, 1938. [Google Scholar]
- Cochran, W.G. Estimation of bacterial densities by means of the “most probable number”. Biometrics 1950, 6, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Bowen, H.J.M. Environmental Chemistry of the Elements; Academic Press: Cambridge, MA, USA, 1979; ISBN 0121204502. [Google Scholar]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresunters. 1980, 33, 566–575. [Google Scholar] [CrossRef]
- Youssef, S.; Lahcen, O.; Abdelaziz, A. Breaking seed dormancy in Cupressus atlantica Gaussen, an endemic and threatened coniferous tree in Morocco. J. For. Res. 2012, 23, 385–390. [Google Scholar] [CrossRef]
- Fabricante, J.R.; de Castro, R.A.; de Araujo, K.C.T.; de Siqueira-Filho, J.A. Ecological Attributes of Alien Nicotiana glauca Graham (Solanacea) And Assessment of The Ssusceptibility of The Species Ooccurring in Brazil. Cienc. Florest. 2015, 25, 959–967. [Google Scholar] [CrossRef]
- Henschel, J.R.; Parr, T. Population changes of alien invasive plants in the Lower Kuiseb River. Dinteria 2010, 31, 5–17. [Google Scholar]
- Florentine, S.K.; Weller, S.; Graz, P.F.; Westbrooke, M.; Florentine, A.; Javaid, M.; Fernando, N.; Chauhan, B.S.; Dowling, K. Influence of selected environmental factors on seed germination and seedling survival of the arid zone invasive species tobacco bush (Nicotiana glauca R. Graham). Rangel. J. 2016, 38, 417–425. [Google Scholar] [CrossRef]
- DiTomaso, J.M.; Kyser, G.B.; Oneto, S.R.; Wilson, R.G.; Orloff, S.B.; Anderson, L.W.; Wright, S.D.; Roncoroni, J.A.; Miller, T.L.; Prather, T.S. Weed control in natural areas in the western United States. Weed Res. Inf. Center Univ. Calif. 2013, 544. [Google Scholar]
- Nieto, A.C.; Pinós, M.C.C.; Cabot, P.; Pitet, M.; Estaún, V. Arbuscular mycorrhizal fungi associated with psammophilic vegetation in Mediterranean coastal sand dunes. Spanish J. Agric. Res. 2010, 8, 96–102. [Google Scholar]
- El Mrabet, S.; Ouahmane, L.; El Mousadik, A.; Msanda, F.; Abbas, Y. The Effectiveness of Arbuscular Mycorrhizal Inoculation and Bio-Compost Addition for Enhancing Reforestation with Argania spinosa in Morocco. Open J. For. 2014, 4, 14–23. [Google Scholar]
- Ouahmane, L.; Duponnois, R.; Hafidi, M.; Kisa, M.; Boumezouch, A.; Thioulouse, J.; Plenchette, C. Some Mediterranean plant species (Lavandula spp. and Thymus satureioides) act as potential ‘plant nurses’ for the early growth of Cupressus atlantica. Plant Ecol. 2006, 185, 123–134. [Google Scholar] [CrossRef]
- Elmostapha, O.; Hanane, D.; Rachid, B.; Lahcen, O. Application of Arbuscular Mycorrhizal Fungi Isolates from Semi-arid Mediterranean Ecosystems as Biofertilizers in Argan Tree Development. J. Soil Sci. Plant Nutr. 2021, 22, 944–955. [Google Scholar] [CrossRef]
- Azcón-Aguilar, C.; Palenzuela, J.; Roldán, A.; Bautista, S.; Vallejo, R.; Barea, J.M. Analysis of the mycorrhizal potential in the rhizosphere of representative plant species from desertification-threatened Mediterranean shrublands. Appl. Soil Ecol. 2003, 22, 29–37. [Google Scholar] [CrossRef]
- Lahcen, O.; Ibrahima, N.; Abdessadek, M.; Abderrahim, F.; Youssef, S.; Mohamed, A. Inoculation of Ceratonia siliqua L. with native arbuscular mycorrhizal fungi mixture improves seedling establishment under greenhouse conditions. Afr. J. Biotechnol. 2012, 11, 16422–16426. [Google Scholar]
- Diop, T.A.; Gueye, M.; Dreyfus, B.L.; Plenchette, C.; Strullu, D.G. Indigenous arbuscular mycorrhizal fungi associated with Acacia albida Del. in different areas of Senegal. Appl. Environ. Microbiol. 1994, 60, 3433–3436. [Google Scholar] [CrossRef]
- An, Z.-Q.; Hendrix, J.W.; Hershman, D.E.; Henson, G.T. Evaluation of the “most probable number”(MPN) and wet-sieving methods for determining soil-borne populations of endogonaceous mycorrhizal fungi. Mycologia 1990, 82, 576–581. [Google Scholar] [CrossRef]
- Bouazza, M.K.; Ighilharizn, Z.; de Lajudie, P.; Duponnois, R.; Bekki, A. Assessing th native arbuscular mycorrhizal symbioses to rehabilitate a degraded coastal sand dune in Algeria. Int. J. Agric. Crop. Sci. 2015, 8, 194–202. [Google Scholar]
- Maier, W.; Schmidt, J.; Nimtz, M.; Wray, V.; Strack, D. Secondary products in mycorrhizal roots of tobacco and tomato. Phytochemistry 2000, 54, 473–479. [Google Scholar] [CrossRef]
- Reynolds, H.L.; Packer, A.; Bever, J.D.; Clay, K. Grassroots ecology: Plant–microbe–soil interactions as drivers of plant community structure and dynamics. Ecology 2003, 84, 2281–2291. [Google Scholar] [CrossRef]
- Barazani, O.; Sathiyamoorthy, P.; Manandhar, U.; Vulkan, R.; Golan-Goldhirsh, A. Heavy metal accumulation by Nicotiana glauca Graham in a solid waste disposal site. Chemosphere 2004, 54, 867–872. [Google Scholar] [CrossRef]
- Shingu, Y.; Kudo, T.; Ohsato, S.; Kimura, M.; Ono, Y.; Yamaguchi, I.; Hamamoto, H. Characterization of genes encoding metal tolerance proteins isolated from Nicotiana glauca and Nicotiana tabacum. Biochem. Biophys. Res. Commun. 2005, 331, 675–680. [Google Scholar] [CrossRef]
- Gisbert, C.; Ros, R.; De Haro, A.; Walker, D.J.; Bernal, M.P.; Serrano, R.; Navarro-Aviñó, J. A plant genetically modified that accumulates Pb is especially promising for phytoremediation. Biochem. Biophys. Res. Commun. 2003, 303, 440–445. [Google Scholar] [CrossRef]


| Station | pH | EC µmol s−1 | Total Carbon (%) | Organic Matter (%) | N (%) | C/N | Olsen P (mg Kg−1) | K+ (mg Kg−1) |
|---|---|---|---|---|---|---|---|---|
| CS1 | 6.2 ± 0.08 e | 0.52 ± 0.02 c | 1.2 ± 0.13 b | 2.06 ± 0.05 c | 0.4 ± 0.04 cd | 3.0 ± 0.03 b | 62 ± 9.4 b | 100 ± 10 bc |
| S1 | 6.5 ± 0.13 de | 0.72 ± 0.04 a | 1.7 ± 0.1 a | 2.92 ± 0.09 ab | 0.7 ± 0.05 a | 2.43 ± 0.05 d | 86 ± 5.5 a | 210 ± 23 a |
| CS2 | 7.8 ± 0.2 a | 0.42 ± 0.01 d | 0.55 ± 0.01 c | 0.95 ± 0.05 e | 0.3 ± 0.02 d | 1.66 ± 0.08 e | 22 ± 3.7 d | 55 ± 3.0 d |
| S2 | 7.5 ± 0.14 a | 0.48 ± 0.03 cd | 0.7 ± 0.03 c | 1.20 ± 0.05 d | 0.5 ± 0.04 bc | 1.4 ± 0.06 f | 40 ± 4.2 c | 80 ± 3.8 bcd |
| CS3 | 7.8 ± 0.25 a | 0.42 ± 0.02 d | 0.5 ± 0.01 c | 0.86 ± 0.04 e | 0.3 ± 0.02 d | 1.67 ± 0.01 e | 22 ± 2.6 d | 60 ± 2.7 cd |
| S3 | 7.4 ± 0.12 ab | 0.48 ± 0.04 cd | 0.7 ± 0.04 c | 1.21 ± 0.04 d | 0.5 ± 0.04 bc | 1.4 ± 0.03 f | 40 ± 4.1 c | 85 ± 4.5 bcd |
| CS4 | 7.4 ± 0.14 ab | 0.7 ± 0.03 a | 1.6 ± 0.06 a | 2.75 ± 0.08 b | 0.6 ± 0.04 ab | 2.66 ± 0.03 c | 60 ± 2.2 b | 120 ± 13 b |
| S4 | 7 ± 0.7 bc | 0.74 ± 0.02 a | 1.8 ± 0.07 a | 3.1 ± 0.1 a | 0.6 ± 0.02 ab | 3.0 ± 0.04 b | 70 ± 5.7 b | 190 ± 15 a |
| CS5 | 6.8 ± 0.05 cd | 0.62 ± 0.01 b | 1.7 ± 0.14 a | 2.92 ± 0.07 ab | 0.5 ± 0.03 bc | 3.4 ± 0.07 a | 60 ± 3.9 b | 110 ± 8.5 b |
| S5 | 6.6 ± 0.13 cd | 0.74 ± 0.03 a | 1.8 ± 0.13 a | 3.12 ± 0.12 a | 0.7 ± 0.06 a | 2.57 ± 0.02 cd | 90 ± 7.1 a | 230 ± 29 a |
| Station | Mycorrhizal Frequency (%) | Mycorrhizal Colonization (%) | Vesicles (%) | Arbuscules (%) | Hyphae (%) |
|---|---|---|---|---|---|
| S1 | 100 ± 0.0 a | 62 ± 3.4 a | 60 ± 1.7 a | 10 ± 1.7 b | 30 ± 2.5 c |
| S2 | 100 ± 0.0 a | 25 ± 2.6 c | 20 ± 2.0 d | 10 ± 1.5 b | 70 ± 4.3 a |
| S3 | 100 ± 0.0 a | 25 ± 2.2 c | 20 ± 1.8 d | 10 ± 1.5 b | 70 ± 3.7 a |
| S4 | 100 ± 0.0 a | 38 ± 3.0 b | 30 ± 1.5 c | 20 ± 2.1 a | 50 ± 2.0 b |
| S5 | 100 ± 0.0 a | 50 ± 2.5 a | 50 ± 2.0 b | 20 ± 1.0 a | 30 ± 3.2 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dounas, H.; Bouskout, M.; Nafidi, H.-A.; Alsahli, A.A.; Bourhia, M.; Ouahmane, L. Unraveling Arbuscular Mycorrhizal Fungi Interactions in the Exotic Plant Nicotiana glauca Graham for Enhanced Soil Fertility and Alleviation of Metal Pollution. Horticulturae 2023, 9, 585. https://doi.org/10.3390/horticulturae9050585
Dounas H, Bouskout M, Nafidi H-A, Alsahli AA, Bourhia M, Ouahmane L. Unraveling Arbuscular Mycorrhizal Fungi Interactions in the Exotic Plant Nicotiana glauca Graham for Enhanced Soil Fertility and Alleviation of Metal Pollution. Horticulturae. 2023; 9(5):585. https://doi.org/10.3390/horticulturae9050585
Chicago/Turabian StyleDounas, Hanane, Mohammed Bouskout, Hiba-Allah Nafidi, Abdulaziz Abdullah Alsahli, Mohammed Bourhia, and Lahcen Ouahmane. 2023. "Unraveling Arbuscular Mycorrhizal Fungi Interactions in the Exotic Plant Nicotiana glauca Graham for Enhanced Soil Fertility and Alleviation of Metal Pollution" Horticulturae 9, no. 5: 585. https://doi.org/10.3390/horticulturae9050585
APA StyleDounas, H., Bouskout, M., Nafidi, H.-A., Alsahli, A. A., Bourhia, M., & Ouahmane, L. (2023). Unraveling Arbuscular Mycorrhizal Fungi Interactions in the Exotic Plant Nicotiana glauca Graham for Enhanced Soil Fertility and Alleviation of Metal Pollution. Horticulturae, 9(5), 585. https://doi.org/10.3390/horticulturae9050585







