Rapid and Efficient In Vitro Propagation Protocol of Endangered Wild Prickly Pear Growing in Eastern Morocco
Abstract
1. Introduction
2. Materials and Methods
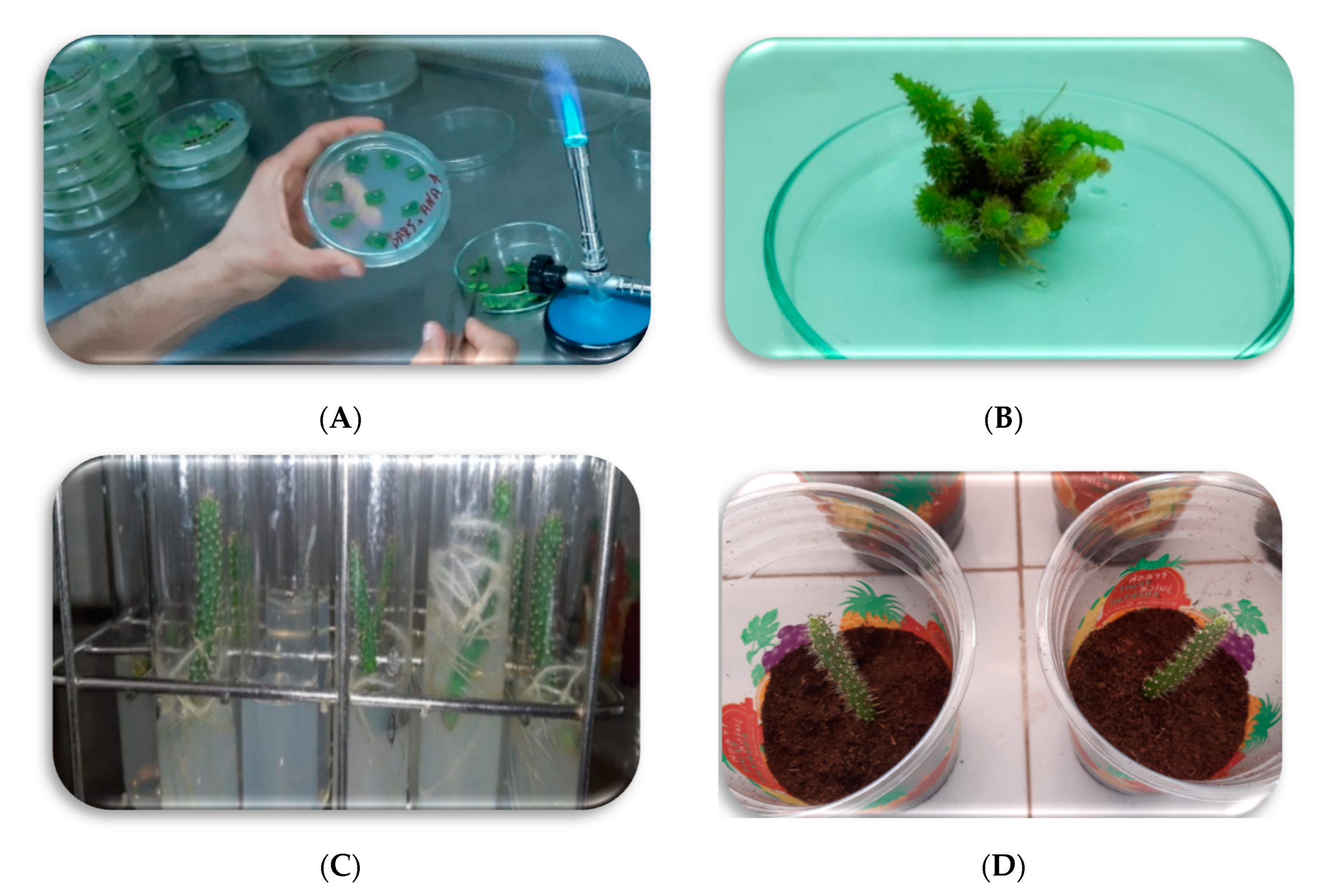
2.1. Explant Sampling and Disinfection
2.2. Initiation of Culture and Incubation Conditions
2.3. Proliferation Phase
2.4. Rooting of Proliferated Shoots
2.5. Acclimatization
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trivedi, S.; Raval, M.P. A critical review on the wonders of cactus. Rising-A J. Res. 2017, 1, 5–25. [Google Scholar]
- Pérez-Molphe-Balch, E.; Santos-Díaz, M.D.S.; Ramírez-Malagón, R.; Ochoa-Alejo, N. Tissue culture of ornamental cacti. Sci. Agric. 2015, 72, 540–561. [Google Scholar] [CrossRef]
- Radi, H.; Bouchiha, F.; El Maataoui, S.; Oubassou, E.-Z.; Rham, I.; Alfeddy, M.N.; Aissam, S.; Mazri, M.A. Morphological and physio-biochemical responses of cactus pear (Opuntia ficus indica (L.) Mill.) organogenic cultures to salt and drought stresses induced in vitro. Plant Cell Tissue Organ Cult. (PCTOC) 2023, 174, 1–14. [Google Scholar] [CrossRef]
- El Finti, A.; El Boullani, R.; El Ayadi, F.; Ait Aabd, N.; El Mousadik, A. Micropropagation in vitro of Opuntia ficus-indica in south of Morocco. Int. J. Chem. Biochem. Sci. 2012, 1, 6–10. [Google Scholar]
- Nefzaoui, A.; Salem, H.B. Opuntia: A Strategic Fodder and Efficient Tool to Combat Desertification in the WANA (West Asia/North Africa) Region; FAO Plant Production and Protection Paper (FAO): Rome, Italy, 2001. [Google Scholar]
- Sbaghi, M.; Bouharroud, R.; Boujghagh, M.; Bouhssini, M.E. Sources de résistance d’Opuntia spp. Contre la cochenille à carmin, Dactylopius opuntiae, au Maroc. EPPO Bull. 2019, 49, 585–592. [Google Scholar] [CrossRef]
- Inglese, P.; Mondragon, C.; Nefzaoui, A.; Saenz, C. Crop Ecology, Cultivation and Uses of Cactus Pear; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2017. [Google Scholar]
- Mazzeo, G.; Nucifora, S.; Russo, A.; Suma, P. Dactylopius opuntiae, a new prickly pear cactus pest in the Mediterranean: An overview. Entomol. Exp. et Appl. 2019, 167, 59–72. [Google Scholar] [CrossRef]
- Bouharroud, R.; Amarraque, A.; Qessaoui, R. First report of the Opuntia cochineal scale Dactylopius opuntiae (Hemiptera: Dactylopiidae) in Morocco. EPPO Bull. 2016, 46, 308–310. [Google Scholar] [CrossRef]
- El Aalaoui, M.; Sbaghi, M. Life history of Dactylopius opuntiae (Hemiptera: Dactylopiidae) on Moroccan resistant cactus germplasm. bioRxiv 2021, 89, 3–19. [Google Scholar]
- Stambouli-Essassi, S.; Harrabi, R.; Bouzid, S.; Harzallah-Skhiri, F. Evaluation of the Efficiency of Opuntia ficus-indica Cladode Cuttings for Vegetative Multiplication. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 521–527. [Google Scholar] [CrossRef]
- Stambouli-Essassi, S.; Zakraoui, M.; Bouzid, S.; Harzallah-Skhiri, F. Sexual Propagation of the Tunisian Spinescent Opuntia ficus-indica (L.) Mill., Morphogenetic Deployment and Polymorphism. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 50–58. [Google Scholar] [CrossRef]
- Zoghlami, N.; Bouamama, B.; Khammassi, M.; Ghorbel, A. Genetic stability of long-term micropropagated Opuntia ficus-indica (L.) Mill. plantlets as assessed by molecular tools: Perspectives for in vitro conservation. Ind. Crops Prod. 2012, 36, 59–64. [Google Scholar]
- McCown, B.; Sellmer, J. General media and vessels suitable for woody plant culture. In Cell and Tissue Culture in Forestry; Springer: Berlin/Heidelberg, Germany, 1987; pp. 4–16. [Google Scholar]
- Christianson, M.; Warnick, D. Temporal requirement for phytohormone balance in the control of organogenesis in vitro. Dev. Biol. 1985, 112, 494–497. [Google Scholar] [CrossRef]
- Gutiérrez-Quintana, L.; Zúñiga-Rizo, C.; Burgos, A.; Portillo, L. Micropropagation of prickly pear by axillary shoot proliferation. Bio-Protocol 2018, 8, e2923. [Google Scholar] [CrossRef]
- Shehu, U.I.; Sani, L.A.; Ibrahim, A.B. Auxin induced rooting of cactus pear (Opuntia ficus-indica L. Miller) cladodes for rapid on-farm propagation. Afr. J. Agric. Res. 2016, 11, 898–900. [Google Scholar]
- Lema-Rumińska, J.; Kulus, D. Micropropagation of cacti—A review. Haseltonia 2014, 2014, 46–63. [Google Scholar] [CrossRef]
- Bouzroud, S.; El Maaiden, E.; Sobeh, M.; Devkota, K.P.; Boukcim, H.; Kouisni, L.; El Kharrassi, Y. Micropropagation of Opuntia and Other Cacti Species Through Axillary Shoot Proliferation: A Comprehensive Review. Front. Plant Sci. 2022, 13, 926653. [Google Scholar] [CrossRef]
- Estrada-Luna, A.A.; Martínez-Hernández, J.D.J.; Torres-Torres, M.E.; Chablé-Moreno, F. In vitro micropropagation of the ornamental prickly pear cactus Opuntia lanigera Salm–Dyck and effects of sprayed GA3 after transplantation to ex vitro conditions. Sci. Hortic. 2008, 117, 378–385. [Google Scholar] [CrossRef]
- Giusti, P.; Vitti, D.; Fiocchetti, F.; Colla, G.; Saccardo, F.; Tucci, M. In vitro propagation of three endangered cactus species. Sci. Hortic. 2002, 95, 319–332. [Google Scholar] [CrossRef]
- El Finti, A.; El Boullani, R.; Naima Ai Msanda, F.; Serghini Ma El Mousadik, A. In vitro propagation of three Moroccan prickly pear cactus Opuntia and plant establishment in soil. Not. Sci. Biol. 2013, 5, 39–44. [Google Scholar] [CrossRef]
- Juárez, M.C.; Passera, C.B. In vitro propagation of Opuntia ellisiana Griff. and acclimatization to field conditions. Biocell 2002, 26, 319–324. [Google Scholar]
- Khalafalla, M.; Eltayeb, A. Micropropagation of cactus (Opuntia ficus-indica) as strategic tool to combat desertification in arid and semi arid regions. Int. J. Sustain. Crop Prod. 2007, 2, 1–8. [Google Scholar]
- Bouchiha, F.; Mazri, M.A. Micropropagation of cactus pear (Opuntia ficus indica) by organogenesis. Afr. Mediterr. Agric. J. Al Awamia 2022, 135, 1–15. [Google Scholar]
- Ghaffari, A.; Hasanloo, T.; Nekouei, M.K. Micropropagation of tuna (Opuntia ficus–indica) and effect of medium composition on proliferation and rooting. Int. J. Biosci. 2013, 3, 129–139. [Google Scholar]
- Angulo-Bejarano, P.I.; Paredes-López, O. Development of a regeneration protocol through indirect organogenesis in prickly pear cactus (Opuntia ficus-indica (L.) Mill). Sci. Hortic. 2011, 128, 283–288. [Google Scholar] [CrossRef]
- Garcıaa-Saucedo, P.A.; Valdez-Morales, M.; Elena Valverde, M.; Cruz-Hernandez, A.; Paredes-Lopez, O. Plant regeneration of three Opuntia genotypes used as human food. Plant Cell Tissue Organ Cult. 2005, 80, 215–219. [Google Scholar] [CrossRef]
- Costa, J.; Van de Pol, P. Own-Rooted Cuttings. In Encyclopedia of Rose Science; Elsevier Academic Press: Cambridge, MA, USA, 2003. [Google Scholar]
- Masekesa, T.; Gasura, E.; Ngadze, E.; Icishahayo, D.; Kujeke, G.T.; Chidzwondo, F.; Robertson, I. Efficacy of Zeatin, Kinetin and Thidiazuron in induction of adventitious root and shoot from petiole explants of sweetpotato cv. Brondal. South Afr. J. Bot. 2016, 104, 1–5. [Google Scholar] [CrossRef]
- Aseesh, P. Influence of kinetin on in vitro rooting and survival of banj oak (Quercus leucotrichophora L.). Afr. J. Biotechnol. 2012, 11, 12538–12545. [Google Scholar]
- Hesar, A.A.; Kaviani, B.; Tarang, A.; Zanjani, S.B. Effect of different concentrations of kinetin on regeneration of (‘Matthiola incana’). Plant Omics 2011, 4, 236–238. [Google Scholar]
- Read, P.; Preece, J. Cloning: Plants–micropropagation/tissue culture. Encyclopedia of Agriculture and Food Systems. Encycl. Agric. Food Syst. 2014, 2, 317–333. [Google Scholar]
- Leva, A. Innovative protocol for “ex vitro rooting” on olive micropropagation. Cent. Eur. J. Biol. 2011, 6, 352–358. [Google Scholar] [CrossRef]
- Clayton, P.W.; Hubstenberger, J.F.; Phillips, G.C.; Butler-Nance, S.A. Micropropagation of members of the Cactaceae subtribe Cactinae. J. Am. Soc. Hortic. Sci. 1990, 115, 337–343. [Google Scholar] [CrossRef]
- Beck, M.J.; Caponetti, J.D. The effects of kinetin and naphthaleneacetic acid on in vitro shoot multiplication and rooting in the fishtail fern. Am. J. Bot. 1983, 70, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pospíšilová, J.; Tichá, I.; Kadleček, P.; Haisel, D.; Plzáková, S. Acclimatization of micropropagated plants to ex vitro conditions. Biol. Plant. 1999, 42, 481–497. [Google Scholar] [CrossRef]

| Treatments (mg/L) | Shoot Number per Explant |
|---|---|
| Control (hormone free) | 0 a |
| BAP 1.5 | 2.83 b ± 1.22 |
| BAP 3 | 6.92 c ± 2.49 |
| BAP 5 | 19.42 d ± 2.52 |
| BAP 7.5 | 6.17 c ± 1.74 |
| BAP 5 + NAA 2 | 3.42 b ± 1.38 |
| Treatments (mg/L) | Root Number per Explant |
|---|---|
| Control | 1.58 a ± 0.62 |
| Kinetin 0.5 | 2.33 a ± 1.03 |
| Kinetin 1 | 5.33 b ± 1.56 |
| Kinetin 1.5 | 9.08 d ± 1.56 |
| Kinetin 2 | 8.75 d ± 1.68 |
| Kinetin 2.5 | 7.00 c ± 1.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marhri, A.; Tikent, A.; Garros, L.; Merah, O.; Elamrani, A.; Hano, C.; Abid, M.; Addi, M. Rapid and Efficient In Vitro Propagation Protocol of Endangered Wild Prickly Pear Growing in Eastern Morocco. Horticulturae 2023, 9, 491. https://doi.org/10.3390/horticulturae9040491
Marhri A, Tikent A, Garros L, Merah O, Elamrani A, Hano C, Abid M, Addi M. Rapid and Efficient In Vitro Propagation Protocol of Endangered Wild Prickly Pear Growing in Eastern Morocco. Horticulturae. 2023; 9(4):491. https://doi.org/10.3390/horticulturae9040491
Chicago/Turabian StyleMarhri, Ahmed, Aziz Tikent, Laurine Garros, Othmane Merah, Ahmed Elamrani, Christophe Hano, Malika Abid, and Mohamed Addi. 2023. "Rapid and Efficient In Vitro Propagation Protocol of Endangered Wild Prickly Pear Growing in Eastern Morocco" Horticulturae 9, no. 4: 491. https://doi.org/10.3390/horticulturae9040491
APA StyleMarhri, A., Tikent, A., Garros, L., Merah, O., Elamrani, A., Hano, C., Abid, M., & Addi, M. (2023). Rapid and Efficient In Vitro Propagation Protocol of Endangered Wild Prickly Pear Growing in Eastern Morocco. Horticulturae, 9(4), 491. https://doi.org/10.3390/horticulturae9040491











