Abstract
Pleurotus ostreatus is one of the most cultivated mushrooms worldwide. It is a lignocellulolytic fungus cultivated on different substrates, whose more common raw material is straw. The present study investigated the biological efficiency of Pleurotus ostreatus as affected by the different age of straw and the growing media preparation process in four production cycles. The content of organic carbon significantly decreased during the growing media preparation, while the content of total nitrogen, moisture, and ash, as well asthe pH value, showed an opposite trend. The first production cycle was characterized by the highest total and soluble sugar content. A dramatic reduction in soluble sugars was recorded at the end of the preparation of the growing media, regardless of the production cycle, while the total sugars were slightly reduced during the production cycle. The microbial population was significantly influenced by the growing media preparation, while only bacteria were slightly influenced by the straw’s age. In contrast, cellulase activity significantly increased in the old straw, while an opposite trend was observed for pectinase activity. The β-glucosidase activity was influenced only by the growing media preparation. As expected, cluster analysis showed that the microbial community changed in each phase of growing media preparation. Finally, the biological efficiency of Pleurotus ostreatus decreased from 26.28% to 15.49% with increasing age of the straw, which may presumably be ascribed to the higher content of sugars in fresh straw compared to the older ones. Therefore, fresh straw should be used to prepare the growing media of Pleurotus ostreatus in order to increase its biological efficiency.
Keywords:
wheat straw; mushroom; microbial community; soluble sugars; enzyme activity; PCR; cluster analysis 1. Introduction
Mushrooms belonging to the genus Pleurotus are the second most cultivated worldwide after Agaricus bisporus, due to their ability to adapt to a variety of substrates from different origins [1]. Furthermore, they require simpler substrates and are less sensitive to pathogens than other mushrooms [2]. Pleurotus ostreatus is considered a source of valuable proteins and can represent an important food in developing countries suffering protein shortages [3].
The application of best practices during the cultivation of mushrooms is essential for obtaining adequate quality and yield. Incorrect techniques can lead to negative consequences, such as: (i) diseases caused by molds that compete for the colonization of the substrate, inhibiting the growth of carpophores; (ii) bacterial infections, which cause a slowdown in development, a deformation of the carpophore, and a significant decrease in production; (iii) growth of animal parasites, which rapidly destroy the substrate by both trophic action and toxic action due to their byproducts; (iv) development of adverse conditions, which may affect the morphology of carpophores and productivity. As Pleurotus spp. mushrooms produce ligninolytic enzymes [4], they can be cultivated on many agricultural byproducts [5]. Although the main raw material used as the Pleurotus spp. growing substrate is wheat straw [1,6], several other low-value agricultural byproducts, including coffee husks, eucalyptus wood, corn cobs, banana stalks, rice straw, cotton waste, peanut shells, and spent mushroom substrate have been successfully tested to produce high-quality Pleurotus spp. [5,7,8,9,10]. However, the need to investigate the potentially toxic elements (heavy metals) present in these mushrooms has been raised recently to avoid any deleterious effect on human health [11].
Substrates cannot be inoculated directly with the fungi but require a previous pasteurization/sterilization. For example, Yang et al. [12] suggested high-pressure sterilization (121–123 °C for 2–3 h) or atmospheric pressure sterilization (95–100 °C for 8–12 h) as a pre-treatment for mushroom growing media, even if these treatments require a large quantity of energy. However, a more sustainable procedure to achieve pasteurization of substrates consists of their short-term prior composting (about 7–10 days), taking advantage of the natural thermophilic phase of this process [13,14]. A reduced composting time can limit the decomposition of organic matter, which thus remains available for microorganisms, improving the yield of mushrooms [12].
Furthermore, the agricultural practices followed during the wheat production cycle, the wheat variety, the method of harvesting, the moisture content, the storage conditions, and the age of the stored straw have also been shown to directly affect the quality of the wheat straw employed as substrate and, in turn, the production of the mushroom [15,16].
The aim of the present work was to monitor the biological efficiency (BE) of Pleurotus ostreatus as affected by two factors, i.e., the age of the straw substrate using gradually older straw and the procedure used in preparing the growing substrates during four production cycles.
2. Materials and Methods
2.1. Substrate Preparation and Experimental Design
The experiment was carried out at Fungo Puglia S.r.l., Rutigliano (Ba), Italy, a company that produces different types of edible mushrooms including Pleurotus ostreatus. Wheat straw (Triticum aestivum L.), stored in bales placed under appropriate canopies after wheat harvest (July 2019), was wetted, chopped into 1–6 cm pieces, and supplemented with a protein-rich soybean flour, which is a common practice in composting nitrogen-deficient biomasses aiming at the cultivation of mushrooms [17]. The mixture was kept in heaps (approximately 4 m by 2 m in cross-section) and subjected to a 5-day composting process, as suggested in [12] (Figure 1a). Then, the partially composted substrate was placed in tunnels and heated to 65 °C with steam for its complete pasteurization (Figure 1b). After cooling, the substrate was inoculated with Pleurotus ostreatus HK35 obtained from Sylvan Hungary Ltd. (Dunaharaszti, Hungary) (Figure 1c), pressed, divided into bales of about 20 kg each, and, finally, wrapped into plastic perforated foils (Figure 1d). Then, the bales were transported to the incubation rooms where temperature and humidity were maintained at the range 25 ± 1.0 °C and 80%, respectively, according to Melanouri et al. [1]. When the bales were completely colonized, they were transferred to another room and maintained at a temperature of about 17.5 ± 1.0 °C and 90% humidity, with cycles of 12 h of light, according to Economou et al. [8]. The substrate preparation steps are summarized in Table 1, whereas Table 2 shows the starting time of the four production cycles investigated. Samples of raw straw and growing media to be analyzed were collected randomly from the corresponding bales.

Figure 1.
Substrate preparation for mushroom growth: (a) short composting process; (b) steam pasteurization; (c) substrate inoculation; (d) bale preparation; (e) Pleurotus ostreatus fruitbody.

Table 1.
Sample preparation steps during substrate preparation.

Table 2.
Production cycles studied.
2.2. Chemical Analyses
Before analyses, samples were air-dried and ground to 0.5 mm. Moisture, expressed as percentage of the initial weight, was determined by drying samples at 105 °C overnight [18], whereas ashes were measured after their overnight incineration at 550 °C in a muffle. The pH was measured on sample/water extracts (1:20 w/v); the total nitrogen (TN) content was determined by the Kjeldahl method; and the organic carbon (OC) content was determined by the Springer–Klee method [19].
Total sugars were measured on samples subjected to total acid hydrolysis according to Englyst et al. [20] and Englyst and Cummings [21]. Briefly, each sample was incubated for 3 h at 35 °C in H2SO4 12 M (cellulose solubilization phase). The suspension was then diluted to a concentration of H2SO4 1 M and incubated for 4 h at 100 °C (acid hydrolysis). After centrifugation at 7000× g for 20 min, the supernatant was filtered through 0.20 µm filters and analyzed by ion exchange–high pressure liquid chromatography (IE-HPLC). The IE-HPLC apparatus was a Dionex DX 500 ion chromatograph equipped with a GP 40 pump, an ED 40 electrochemical detector used in amperometry integrated with the gold working electrode and the pH-Ag/AgCl reference electrode, a 25 µL fixed loop LC5 injector, a M10 Carbopack column, and an 18 mM NaOH mobile phase. The sugars were eluted at the flow rate of 1 mL min−1, using an aqueous solution of NaOH and CH3COONa as the mobile phase. The chromatographic run consisted of: (i) an initial phase of 10 min with 8.8 mM NaOH; (ii) the injection of the sample; (iii) an isocratic elution with 8.8 mM NaOH for 30 min; (iv) a first step of 10 min with 50 mM NaOH; (v) a second step of 10 min with 50 mM NaOH and 100 mM CH3COONa; and (vi) an isocratic elution with 8.8 mM NaOH for 30 min.
The determination of soluble sugars was performed by treating 0.2 g of 1-mm-size sample with 80% ethanol in a water bath for 7 min at 80 °C. Then, the samples were almost dried by using a rotavapor, filtered through Whatman 0.2 μm filters, and collected in a 10 mL flask before being analyzed with IE-HPLC as previously described.
2.3. Biochemical Analyses
2.3.1. Bacterial and Fungal Counts
Three replicates of 2 g subsamples (dry weight equivalent) of straw and growing substrates previously cut and homogenized in a Waring blender were suspended in 18 mL of deionized water prepared with a Mill-Q water purification system and 2 mL of 0.18% sodium pyrophosphate and sonicated for 2 min to disperse microbial cells. Nine mL of 1/4 strength Ringer solution (NaCl 2.25 g L−1, KCl 0.105 g L−1, CaCl2 0.045 g L−1, NaHCO3 0.05 g L−1 and citric acid 0.034 g L−1) were added to 1 mL of the liquid phase. Ten-fold serial dilutions of the supernatants were made in sterile 1/4 strength Ringer solution. For bacterial count, aliquots of the diluted supernatants were spread in triplicate on plates covered by a 1/10 strength TSA (Tryptic Soy Agar) medium augmented with 0.1 mg mL−1 cycloheximide. For fungal count, aliquots of the diluted supernatants were spread in triplicate on plates covered by a malt extract agar (MEA) medium containing 0.03 mg mL−1 streptomycin and 0.02 mg mL−1 tetracycline. The counts were performed after incubation at 28 °C for 72 h for total culturable bacteria (TCB) and 120 h for total culturable fungi (TCF) [22].
2.3.2. Enzymatic and Microbial Activities
The microbial activity was assessed by determining the enzymatic activity (β-glucosidase, cellulase, pectinase) by which complex substrates could be transformed in products easily usable for the growth of the cultivated fungus.
The total cellulase activity was measured by the Filter Paper assay. Briefly, an aliquot of 0.5 g of sample was augmented with 5 mL of 0.05 M citrate buffer at pH 4.8. After shaking at 4 °C for 24 h, the suspension was centrifuged for 5 min at 5000 rpm and for another 5 min at 11,000 rpm, and the supernatant enzymatic extract was recovered. Then, 500 µL of the extract were added to 500 µL of carboxy-methyl cellulose, and a blank was also prepared. After incubation at 50 °C for 24 h, the cellulase activity was determined by a Pharmacia Biotech Ultraspec 4000 spectrophotometer at a wavelength of 540 nm [23].
The β-glucosidase activity was determined by using ρ-nitrophenyl-β-glucoside essay [24]. Briefly, 1 mL of ρ-nitrophenyl-β-glucoside (5 mM) and 1.5 mL of 0.1 M acetate buffer, pH 4.8 at 50 °C, were thermostated for 5 min, to which 500 µL of enzyme solution were added. After 30 min and at the same temperature, the hydrolysis of the ρ-nitrophenyl-β-glucoside was blocked with 4 mL of 0.4 M glycine buffered at pH 10.8, allowing the development of the color of the ρ-nitrophenol, spectrophotometrically measurable at 400 nm.
The pectinase activity was measured according to Li et al. [25]. The enzyme and 0.05 M citrate buffer pH 4.8 was added to 50 µL of substrate (pectin or 1% polygalacturonic acid) with a final reaction volume of 1.5 mL. The samples, controls, and standards were incubated at 25 °C for 1 h, and after that, 3 mL of methyl-salicylic acid were added. The determination of the pectinase activity was done using a protocol similar to the β-glucosidase one.
2.3.3. DNA Extraction
The DNA extraction was performed by an indirect lysis method that involved the recovery of the microbial cells from the straw matrix thanks to a mechanical extractor (Fast Prep, BIO 101) and attached kit. This system operated at a speed of 5.5 m s−1 for 30 s, which warranted an efficient lysis of all microorganisms in the straw. The samples were then centrifuged to allow for the precipitation of proteins. Then, a matrix containing guanidine isothiocyanate was added to the supernatant to bind the DNA. Mini columns with filters allowed us to eliminate the contaminants and retained the DNA, which was recovered by eluting with 100 μL of water and stored at 4 °C.
2.3.4. PCR, Electrophoretic Profiles, and Cluster Analysis
The extracted DNA was amplified by a polymerization chain reaction using a Sprint thermocycler (Hyband) PCR at the following conditions: 10 min at 95 °C, 35 1-min cycles at 95 °C, 30 s at 45 °C, 2 min at 72 °C, and a final step of 5 min at 72 °C. The PCR products (3 µL) were separated by electrophoresis at 100 V for 60 min on a 1% (w/v) agarose gel and displayed with ethidium bromide (0.5 μg mL−1). The identification of bacteria was carried out upon partial sequencing of the 16S rDNA gene (16-23S region), using 968L-1401R as the primer. The identification of fungi was achieved upon partial sequencing of the 18S rDNA gene, using FR1-FF390 as the primer.
Amplified DNA fragments amounting to 0.5 Kb for bacteria and 0.4 Kb for fungi were analyzed by denaturing gradient gel electrophoresis (DGGE), using polyacrylamide 6% and urea-formamide 45–50% for bacteria, and polyacrylamide 7.5% and urea-formamide 45–50% for fungi. Briefly, about 30 µL of each amplified product were loaded on a parallel polyacrylamide gel, with an increasing gradient in the same direction as the electrophoretic migration. The electrophoretic run was performed for 16 h at constant temperature and voltage (60 °C and 100 V) in tris-glacial acetic acid-ethylenediaminetetraacetic acid (TEA) buffer. The electrophoretic patterns were visualized using SYBR Green I intercolorant that featured a high sensitivity for nucleic acids and produced a low fluorescent background. The gels were processed using the Bio-Rad Gel Doc 2000 system image acquirer and then analyzed by the Quantity One Software that allowed for the comparison and clusterization of the electrophoretic profiles using the UPGAMA method (unweighted pair group method using arithmetic averages).
2.4. Biological Efficiency/Mushroom Yields
Mushrooms were harvested when their cap surface was flat to slightly up rolled at the cap margins. The carpophores harvested from each bale at the end of each harvest period were weighed, and data were used to calculate the percentage of BE, as the percentage of the ratio of the fresh mushroom weight per bale and the dry substrate weight of bale, according to Zervakis et al. [26].
2.5. Statistical Analysis
All experimental data were tested against the normal distribution of variables and the homogeneity of variance using the RStudio software. The variables were then subjected to a suitable ANOVA and post-hoc test.
3. Results and Discussion
3.1. Chemical Analyses of Raw Straw and Growing Media
The chemical analysis results of the substrates of different cycles of production did not show any significant difference, whereas some significant differences were shown in the substrates after different steps of preparation (Table 3). As expected, the moisture content increased with increasing steps along the preparation of the growing substrates as a result of wetting bales, reaching values > 75% in the last two steps of the process. Such values are higher than the moisture content recommended at this composting phase, where humidity should be maintained in the range of 55–65% [27]. The pH value increased along the process from neutrality to medium alkalinity, possibly due to the proteolytic action of the microbial community that released ammoniacal N. Additionally, the ash content increased and, simultaneously, the OC content decreased along the substrate preparation due to the partial mineralization of growing media that occurred in the short-term composting phase. On the other hand, the low TN content of the raw straw (step A) increased by about 2% following the addition of the soy flour and remained almost constant until the end of the substrate preparation. Unfortunately, all substrates had a TN content well below 2%, a value considered optimal for fungal growth [28]. Accordingly, the C/N ratio decreased along the substrate preparation steps, but it was always higher than the values of 28–30 considered suitable for the growth of mushrooms [5,13,14]. Therefore, all production cycles shared some N deficiency, which affected mushroom growth and biological efficiency, since N is one of the main nutrients [28]. Nevertheless, even if the C/N ratio did not change with the age of the straw, the quality of carbon changed. In fact, fresh straw is richer in more easily degradable molecules containing C, such as cellulose and hemicellulose, than the older straw [29]. Therefore, similar C/N ratio values can derive from different carbon and nitrogen molecules, which can influence the biological efficiency of Pleurotus ostreatus differently.

Table 3.
Chemical analysis results of straw and substrates corresponding to different steps of preparation and different cycles of production. OC: organic carbon; TN: total nitrogen. Standard deviations are reported in parentheses. Different letters indicate significant differences among cycles (p < 0.05). n.s.: not significant.
Although moisture and C/N ratio are the main parameters for the growth of mushroom, other nutrients, such as phosphorus, magnesium, sulfur, calcium, iron, potassium, as well as vitamins, should be monitored and possibly added to the growing media to enhance the biological efficiency of Pleurotus ostreatus [5].
Table 4 reports the content of total and soluble sugars of raw straw and growing substrates recorded for all production cycles and within each production cycle. Among the total sugars, glucose and xylose were the two most represented sugars at the beginning of each cycle, while arabinose and galactose were in lower concentrations. With regards to the soluble sugars, which are promptly available to the fungus, sucrose and glucose were the most abundant sugars at the beginning of each cycle, followed by fructose, while arabinose, galactose, and xylose were present in very low concentrations. It is noteworthy that the first production cycle featured the highest total and soluble sugar content, possibly due to the initial fresh age of the straw, whereas the four production cycles occurred over several months with increasing age of the straw.

Table 4.
Total and soluble sugars at the end of different cycles of production and after different steps of preparation. ARA: Arabinose, GAL: Galactose, GLU: Glucose, XYL: Xylose, FRU: Fructose, SUC: Sucrose, TOT: Total. Standard deviations are reported in parentheses. Different letters indicate significant differences among cycles (p < 0.05). n.s.: not significant.
Within each production cycle, the concentration of total sugars decreased slightly from the first to the last step of the growing substrate preparation, apart from arabinose, but the content of the soluble sucrose, glucose, and fructose decreased dramatically (Table 4). Thus, along the preparation of the growing substrate, a portion of soluble sugars was consumed by the microbial community, and another portion was probably leached with wetting. Fewer available sugars mean fewer carbon sources for mushroom growth [30,31] and lower production of exoenzymes responsible for the degradation of recalcitrant lignin and polysaccharides to simple sugars [32]. Therefore, the BE of the various cycles ranged from 26.28% to 15.49%, with the lowest percentage recorded for the last cycle that used the oldest straw (Figure 2). These results were also confirmed by the high Pearson correlation coefficient between BE and soluble sugars (r = 0.910), while BE was less correlated with total sugars (r = 0.700).
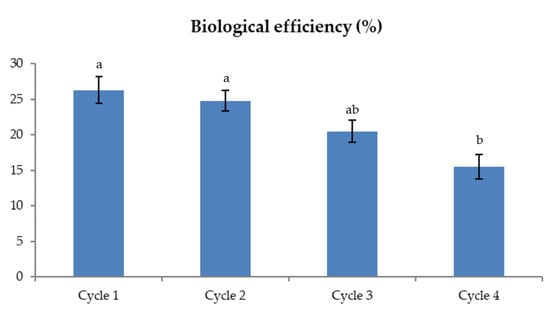
Figure 2.
Biological efficiency % of each production cycle. Different letters indicate significant differences among cycles (p < 0.05).
Previous studies reported that the BE varied according to the different raw materials used to prepare the substrate for the cultivation of mushrooms [17,33,34]. Fanadzo et al. [17] tested different growing media for the cultivation of Pleurotus ostreatus with different compositions, finding that BE ranged from 97% when using maize straw as a substrate without supplementation, to 15% when a supplement consisting of thatch grass and maize bran was provided. Frimpong–Manso et al. [35] evaluated the BE of Pleurotus ostreatus grown on composted sawdust with or without the supplementation of rice husk at different percentages and found that BE decreased from 75.2% to 40.7%, with the increasing percentage of rice husk.
3.2. Microbiological Analyses of Raw Straw and Growing Media
The TCB count showed a similar trend in the four cycles analyzed (Figure 3a). In particular, the TCB values in the initial substrate, i.e., raw straw, were low (about 107 CFU g−1 of dry straw), then successively increased by two orders of magnitude both in the wet straw samples after the addition of soy flour and in the substrates obtained at the end of 5 days of composting, and finally decreased again after pasteurization, reaching values similar to those of the starting substrates. These results were expected, as the addition of water and nitrogen in steps B and C better satisfied the requirements of the bacterial community in its growth and activity.
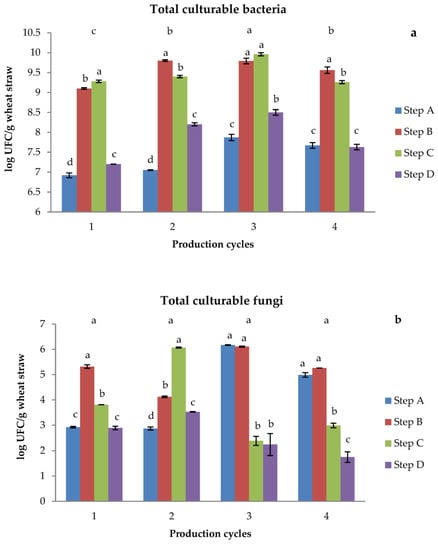
Figure 3.
Total culturable bacteria (a) and fungi (b) expressed as log UFC g−1 substrate. Different letters above production cycles indicate significant (p < 0.05) differences among them. Different letters above each bar indicate significant (p < 0.05) differences among steps of the same production cycle.
On the other hand, the values of TCF did not show a common trend in the four cycles (Figure 3b). In particular, the TCF trends shown by the first and second cycles were similar to those of TCB, while in the third and fourth cycles, the TCF values were very high for the initial two steps of substrate production and then decreased markedly in the last two steps.
In general, the content of TCF was two-thirds lower than that of TCB. Previous studies reported that bacteria dominate the initial stages of straw decomposition; as they prefer to decompose labile compounds such as carbohydrates [36,37], fungi dominate the last stages of the process as they are able to decompose more recalcitrant compounds [38,39]. In this study, the use of aged straw in cycles 3 and 4 showed a higher content of TCF at the beginning of substrate preparation (steps A and B) with respect to cycles 1 and 2, which started with fresh and less aged straw. As expected, in any cycle, the TCF content was reduced significantly at the end of the process due to pasteurization of the substrate.
The cluster analysis of the fingerprinting of bacteria resulted in a low similarity index, which ranged from 0.33 to 0.50 for the different steps of each cycle. A similar, even more marked, trend was observed for fungi, whose similarity indexes ranged from 0.16 to 0.22. Therefore, as expected, each phase was characterized by its own microbial community.
3.3. Enzymatic Analyses of Raw Straw and Growing Media
Cellulases are enzymes that catalyze the hydrolysis of cellulose to form cellobiose [40]. In general, the cellulase activity increased along the substrate preparation steps, reaching a significantly higher value at the end of pasteurization, when a larger release of such enzyme occurred (Figure 4a). Iqbal et al. [41] reported that cellulase activity was related to a complex relationship involving the inoculum size, the carbon source and cellulose quality, the pH value, temperature, aeration, and the growing time of microorganisms.
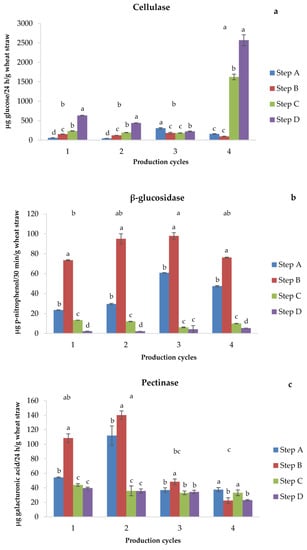
Figure 4.
Cellulase (a), β-glucosidase (b), and pectinase (c) activities. Different letters above production cycles indicate significant (p < 0.05) differences among them. Different letters above each bar indicate significant (p < 0.05) differences among steps of the same production cycle.
The β-glucosidase is an enzyme involved in the late steps of cellulose degradation by catalyzing the cleavage of cellobiose and the release of simple sugars easily available for microorganisms [42]. This enzyme is also active in breaking β-glycosidic bonds; thus, it can also act in the degradation of hemicelluloses and mucopolysaccharides. No significant difference in β-glucosidase activity was measured among the different cycles and among the same steps of the four cycles considered (Figure 4b). After the addition of soy flour and water (step B, Figure 4b), the β-glucosidase activity increased significantly in all cycles simultaneously with the increased microbial activity. The increased availability of nutrients generally promotes the growth of bacteria and fungi (Figure 3a,b), β-glucosidase activity (Figure 4b), and the production of enzymes with low substrate affinities [42].
Pectinases are enzymes able to degrade pectins, i.e., the main components of the middle lamellae of plant cells, to calcium and magnesium pectates [43], which allow microorganisms to extract nutrients from plant tissues. The pectinase activity was significantly higher in the first two cycles and, especially, in the first two steps of the latter (Figure 4c). Therefore, the activity of this enzyme would depend on the quality, i.e., age of the straw, which implies its greater activity in fresher straws that are richer in pectins that are, unlike cellulose, easily degradable polymers.
A positive correlation (r = 0.95; p = 0.045) was found between pectinase activity recorded in step D of substrate preparation and BE, which might be ascribed to the colonization phase associated with cell wall opening [44]. Apparently, this enzymatic phase anticipated the consequent decomposition of cellulose in cellobiose. On the other hand, no significant correlation was found between cellulase activity and BE, whereas a negative correlation occurred between β-glucosidase activity and BE (r = −0.97; p = 0.024) as both enzymes depend, in turn, on pectinase activity.
4. Conclusions
The results of this study suggest that the age of the straw and the process adopted to obtain the growing substrate for Pleurotus ostreatus affect the production of the mushroom. In particular, a reduction of sugars, especially the soluble ones, has been measured during the four substrate preparation cycles examined along the steps of each substrate preparation. However, the use of fresh straw (Cycle 1) resulted in the highest content of sugars. Furthermore, the two phases preceding the Pleurotus ostreatus inoculation drastically reduced the soluble sugar content, as these sugars were consumed by the microbial community and, possibly, also leached due to overwatering of the substrates during the brief initial composting process. Therefore, care should be taken in the storage of the fresh raw straw to reduce its degradation due to aging as much as possible or supplement the growth substrate with other sources of sugars as the straw ages. At the same time, particular attention should be devoted to controlling the C/N ratio and moisture content of the substrate, so that fungi can grow in the best possible conditions to enhance the biological efficiency and, in turn, the final yield of mushroom. In the present study, N deficiency was recorded in all growing media, as the addition of the N source did not reduce the C/N ratio to the values required by fungus. Therefore, the N deficiency should be balanced with organic N compounds such as urea, hydrolyzed proteins, amino acids, yeast extract, or ammonium or nitrate-based fertilizers.
Author Contributions
Conceptualization, G.B., A.T., F.D.M., C.C. and F.M.; methodology, G.B., A.T., F.D.M., C.C. and F.M.; software, A.T. and F.D.M.; validation, G.B. and C.C.; formal analysis, A.T., F.D.M. and F.M.; investigation, G.B., A.T., F.D.M. and C.C.; resources, G.B.; data curation, A.T. and F.D.M.; writing—original draft preparation, A.T. and F.D.M.; writing—review and editing, A.T., F.D.M., C.C. and G.B.; visualization, C.C. and G.B.; supervision, C.C. and G.B.; project administration, G.B.; funding acquisition, G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
Authors acknowledge Emeritus Nicola Senesi for his valuable contribution to the revision of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Melanouri, E.M.; Dedousi, M.; Diamantopoulou, P. Cultivating Pleurotus ostreatus and Pleurotus eryngii mushroom strains on agro-industrial residues in solid-state fermentation. Part II: Effect on productivity and quality of carposomes. Carbon Resour. Convers. 2022, 5, 52–60. [Google Scholar] [CrossRef]
- Muswati, C.; Simango, K.; Tapfumaneyi, L.; Mutetwa, M.; Ngezimana, W. The Effects of Different Substrate Combinations on Growth and Yield of Oyster Mushroom (Pleurotus ostreatus). Int. J. Agron. 2021, 2021, 9962285. [Google Scholar] [CrossRef]
- Abou Fayssal, S.; Alsanad, M.A.; Yordanova, M.H.; El Sebaaly, Z.; Najjar, R.; Sassine, Y.N. Effect of olive pruning residues on substrate temperature and production of oyster mushroom (Pleurotus ostreatus). Acta Hortic. 2021, 1327, 245–252. [Google Scholar] [CrossRef]
- Wang, D.; Sakoda, A.; Suziki, M. Biological efficiency and nutritional value of Pleurotus ostreatus cultivated on spent beer grain. Bioresour. Technol. 2001, 78, 293–300. [Google Scholar] [CrossRef]
- Bellettini, M.B.; Fiorda, F.A.; Maieves, H.A.; Teixeira, G.L.; Ávila, S.; Hornung, P.S.; Maccari Júnior, A.; Hoffmann Ribani, R. Factors affecting mushroom Pleurotus spp. Saudi J. Biol. Sci. 2019, 26, 633–646. [Google Scholar] [CrossRef]
- Yang, W.; Guo, F.; Wan, Z. Yield and size of oyster mushroom grown on rice/wheat straw basal substrate supplemented with cotton seed hull. Saudi J. Biol. Sci. 2013, 20, 333–338. [Google Scholar] [CrossRef]
- Getachew, A.; Negassa, A.K.; Aga, M.C. Production of Oyster Mushroom (Pleurotus ostreatus) on Substrate Composed from Wheat Straw, Waste Paper and Cotton Seed Waste. Int. J. Microbiol. Biotechnol. 2019, 4, 38–44. [Google Scholar] [CrossRef]
- Economou, C.N.; Diamantopoulou, P.A.; Philippoussis, A.N. Valorization of spent oyster mushroom substrate and laccase recovery through successive solid state cultivation of Pleurotus, Ganoderma, and Lentinula strains. Appl. Microbiol. Biotechnol. 2017, 101, 5213–5222. [Google Scholar] [CrossRef]
- Zied, D.C.; Prado, E.P.; Dias, E.S.; Pardo, J.E.; Pardo-Gimenez, A. Use of peanut waste for oyster mushroom substrate supplementation-oyster mushroom and peanut waste. Braz. J. Microbiol. 2019, 50, 1021–1029. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, V.; Eid, E.M.; AL-Huqail, A.A.; Adelodun, B.; Abou Fayssal, S.; Goala, M.; Arya, A.K.; Bachheti, A.; Andabaka, Ž.; et al. Spatial Assessment of Potentially Toxic Elements (PTE) Concentration in Agaricus bisporus Mushroom Collected from Local Vegetable Markets of Uttarakhand State, India. J. Fungi 2022, 8, 452. [Google Scholar] [CrossRef]
- Yang, Y.R.; Guo, Y.X.; Wang, Q.Y.; Hu, B.Y.; Tian, S.Y.; Yang, Q.Z.; Cheng, Z.A.; Chen, Q.J.; Zhang, G.Q. Impacts of composting duration on physicochemical properties and microbial communities during short-term composting for the substrate for oyster mushrooms. Sci. Total Environ. 2022, 847, 157673. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.X.; Chen, Q.J.; Qin, Y.; Yang, Y.R.; Yang, Q.Z.; Wang, Y.X.; Cheng, Z.A.; Cao, N.; Zhang, G.Q. Succession of the microbial communities and function prediction during short-term peach sawdust-based composting. Bioresour. Technol. 2021, 332, 125079. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Sun, B.; Zhang, J.; Zhang, Y.; Gu, L.; Bao, L.; Liu, S. Metagenomic analysis revealed the succession of microbiota and metabolic function in corncob composting for preparation of cultivation medium for pleurotus ostreatus. Bioresour. Technol. 2020, 306, 123156. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.K.; Kumar, U.; Mishra, V.K.; Chand, R.; Chatrath, R.; Naik, R.; Biradar, S.; Singh, R.P.; Budhlakoti, N.; Devulapalli, R.; et al. Variations in straw fodder quality and grain-Straw relationships in a mapping population of 287 diverse spring wheat lines. Field Crops Res. 2019, 243, 107627. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.G.; Mourtzinis, S.; Gaska, J.M.; Mueller, B.; Roth, A.; Smith, D.L.; Conley, S.P. Wheat grain and straw yield, grain quality, and disease benefits associated with increased management intensity. Agron. J. 2021, 113, 308–320. [Google Scholar] [CrossRef]
- Fanadzo, M.; Zireva, D.T.; Dube, E.; Mashingaidze, A.B. Evaluation of various substrates and supplements for biological efficiency of Pleurotus sajor-caju and Pleurotus ostreatus. Afr. J. Biotechnol. 2010, 9, 2756–2761. [Google Scholar] [CrossRef]
- Trinchera, L.; Leita, P.; Sequi, P. Metodi di Analisi per i Fertilizzanti; Ministero delle Politiche Agricole Alimentari e Forestali: Rome, Italy, 2006. [Google Scholar]
- Ciavatta, C.; Antisari, L.V.; Sequi, P. Determination of organic carbon in soils and fertilizers. Commun. Soil Sci. Plant Anal. 1989, 20, 759–773. [Google Scholar] [CrossRef]
- Englyst, H.; Cummings, J.H. Simplified method for the measurement of total non-starch polysaccharides by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst 1984, 109, 937–942. [Google Scholar] [CrossRef]
- Englyst, H.; Wiggins, H.S.; Cummings, J.H. Determination of the non-starch polysaccharides in plant foods by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst 1982, 107, 307–318. [Google Scholar] [CrossRef]
- De Mastro, F.; Traversa, A.; Brunetti, G.; Debiase, G.; Cocozza, C.; Nigro, F. Soil culturable microorganisms as affected by different soil managements in a two year wheat-faba bean rotation. Appl. Soil Ecol. 2020, 149, 103533. [Google Scholar] [CrossRef]
- Yu, X.; Liu, Y.; Cui, Y.; Cheng, Q.; Zhang, Z.; Lu, J.H.; Meng, Q.; Teng, L.; Ren, X. Measurement of filter paper activities of cellulase with microplate-based assay. Saudi J. Biol. Sci. 2016, 23, S93–S98. [Google Scholar] [CrossRef] [PubMed]
- Mangalassery, S.; Mooney, S.; Sparkes, D.; Fraser, W.; Sjögersten, S. Impacts of zero tillage on soil enzyme activities, microbial characteristics and organic matter functional chemistry in temperate soils. Eur. J. Soil Biol. 2015, 68, 9–17. [Google Scholar] [CrossRef]
- Li, Q.; Coffman, A.M.; Ju, L.-K. Development of reproducible assays for polygalacturonase and pectinase. Enzym. Microb. Technol. 2015, 72, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Zervakis, G.I.; Koutrotsios, G.; Katsaris, P. Composted versus raw olive mill waste as substrates for the production of medicinal mushrooms: An assessment of selected cultivation and quality parameters. BioMed. Res. Int. 2013, 2013, 546830. [Google Scholar] [CrossRef] [PubMed]
- Cocozza, C.; Parente, A.; Zaccone, C.; Mininni, C.; Santamaria, P.; Miano, T. Comparative management of offshore posidonia residues: Composting vs. energy recovery. Waste Manag. 2011, 31, 78–84. [Google Scholar] [CrossRef]
- Miles, P.G.; Chang, S.T. Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 95–101. [Google Scholar]
- Kim, M.; Kim, W.S.; Tripathi, B.M.; Adams, J. Distinct bacterial communities dominate tropical and temperate zone leaf litter. Microbial. Ecol. 2014, 67, 837–848. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Teixeira, J.A. Lignocellulose as Raw Material in Fermentation Processes. Curr. Res. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 2, 897–907. [Google Scholar]
- Ma, N.L.; Khoo, S.C.; Peng, W.; Ng, C.M.; Teh, C.H.; Park, Y.-K.; Lam, S.S. Green application and toxic risk of used diaper and food waste as growth substitute for sustainable cultivation of oyster mushroom (Pleurotus ostreatus). J. Clean. Prod. 2020, 268, 122272. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Wei, J.-K.; Wang, Q.-H.; Yang, R.; Gao, X.-J.; Sang, Y.-X.; Cai, P.-P.; Zhang, G.-Q.; Chen, Q.-J. Lignocellulose utilization and bacterial communities of millet straw based mushroom (Agaricus bisporus) production. Sci. Rep. 2019, 9, 1151. [Google Scholar] [CrossRef]
- Peksen, A.; Yakupoglu, G. Tea waste as a supplement for the cultivation of Ganoderma lucidum. World J. Microbiol. Biotechnol. 2009, 25, 611–618. [Google Scholar] [CrossRef]
- Liang, Z.; Wu, C.; Shieh, Z.; Cheng, S. Utilisation of grass plants for cultivation of Pleurotus citrinopeleatus. Int. Biodeterior. Biodegrad. 2009, 63, 509–514. [Google Scholar] [CrossRef]
- Frimpong–Manso, J.; Obodai, M.; Dzomeku, M.; Apertorgbor, M.M. Influence of rice husk on biological efficiency and nutrient content of Pleurotus ostreatus (Jacq. ex. Fr.) Kummer. Int. Food Res. J. 2011, 18, 249–254. [Google Scholar]
- Wang, X.; He, P.; Xu, X.; Qiu, S.; Zhao, S. Characteristics of rice straw decomposition and bacterial community succession for 2 consecutive years in a paddy field in southeastern China. Sci. Rep. 2022, 12, 20893. [Google Scholar] [CrossRef]
- Fan, F.L.; Yin, C.; Tang, Y.; Li, Z.; Song, A.; Wakelin, S.A.; Zou, J.; Liang, Y. Probing potential microbial coupling of carbon and nitrogen cycling during decomposition of maize residue by 13C-DNA-SIP. Soil Biol. Biochem. 2014, 70, 12–21. [Google Scholar] [CrossRef]
- Paterson, E.; Osler, G.; Dawson, L.A.; Gebbing, T.; Sim, A.; Ord, B. Labile and recalcitrant plant fractions are utilized by distinct microbial communities in soil: Independent of the presence of roots and mycorrhizal fungi. Soil Biol. Biochem. 2008, 40, 1103–1113. [Google Scholar] [CrossRef]
- Marschner, P.; Umar, S.; Baumann, K. The microbial community composition changes rapidly in the early stages of decomposition of wheat residue. Soil Biol. Biochem. 2011, 43, 445–451. [Google Scholar] [CrossRef]
- Makhatov, Z.; Alibayev, N.; Konarbayeva, Z.; Makhatov, B.; Makhatova, A.; Doltayeva, B.; Torlanova, B.; Arystanbaev, K.; Shagrayeva, B. Enzymatic depolymerization of wheat straw polysaccharides. In Proceedings of the IOP Conference Series: Earth and Environmental Science, 2nd International Conference on Energetics, Civil and Agricultural Engineering 2021 (ICECAE 2021), Tashkent, Uzbekistan, 14–16 October 2021; Volume 939, p. 012005. [Google Scholar] [CrossRef]
- Iqbal, H.M.N.; Ahmed, I.; Zia, M.A.; Irfan, M. Purification and characterization of the kinetic parameters of cellulose produced from wheat straw by Trichoderma viride under SSF and its detergent compatibility. Adv. Biosci. Biotechnol. 2011, 2, 149–156. [Google Scholar] [CrossRef]
- De Mastro, F.; Brunetti, G.; Traversa, A.; Blagodatskaya, E. Fertilization promotes microbial growth and minimum tillage increases nutrient-acquiring enzyme activities in a semiarid agro-ecosystem. Appl. Soil Ecol. 2022, 177, 104529. [Google Scholar] [CrossRef]
- Chiliveri, S.R.; Koti, S.; Linga, V.R. Retting and degumming of natural fibers by pectinolytic enzymes produced from Bacillus tequilensis SV11-UV37 using solid state fermentation. SpringerPlus 2016, 5, 559. [Google Scholar] [CrossRef]
- Ćilerdžić, J.; Galić, M.; Stajić, M. From pomiculture waste to biotechnological raw material: Efficient transformation using ligninosomes and cellulosomes from Pleurotus spp. Bioresour. Bioprocess. 2022, 9, 66. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).