Abstract
To address the growing demand for natural sources of drugs, in addition to chemical ones, the present study aimed to explore the phytochemical and biological activity of acetone stem bark extract of Albizia lebbeck. The phytoconstituents of the derivatized acetone stem bark extract were analyzed using Gas Chromatography–Mass Spectrometry (GC-MS), while the phenolic and flavonoid compounds were analyzed using High-Performance Liquid Chromatography (HPLC). Six bacterial strains (Serratia marcescens, Acinetobacter johnsonii, Agrobacterium tumefaciens, Bacillus subtilis, Erwinia carotovora, Escherichia coli) and three fungal strains (Rhizoctonia solani, Penicillium italicum, Fusarium oxysporum) were evaluated for antimicrobial activity. Furthermore, in vitro cytotoxicity was assessed against three cancer cell lines (PC-3, Caco-2, and MCF-7). Our findings indicated that the acetone extract of A. lebbeck stem bark was rich in fatty acids, with a predominance of oleic acid (19.2%). Additionally, eight phenolic acids, primarily cinnamic acid, and eight flavonoids, primarily chrysoeriol and hesperidin, were identified. It was found that the acetone extract of the A. lebbeck stem bark exhibited a high potential antibacterial effect against B. subtilis and S. marcescens and evident antifungal activity against F. oxysporum. Based on the calculated selectivity index, PC-3 cells were found to have the highest value (2.95), followed by Caco-2 cells (1.92) and MCF-7 cells (1.34). These results suggest the richness of A. lebbeck stem bark in phytochemicals with promising antimicrobial and cytotoxic properties.
1. Introduction
Albizia lebbeck (L.) Benth, commonly known as the lebbeck tree, belongs to Fabaceae and is one of several plant species with ornamental and potential medicinal values. It is native to India but has been introduced and cultivated in many tropical and subtropical regions throughout Asia, Africa, and the Middle East [1,2]. Traditional uses of bark include tanning fishing nets, treating boils, making soap, treating bronchitis, toothache, leprosy, asthma, skin diseases, erysipelas, allergies, infectious diarrhea, and anxiety [3,4]. Previous studies have dealt with the phytochemicals of seeds, including nutrients [5], glycosides [6], terpenoids, steroids, saponins [7], phenolics [8], and tannins [9]. Moreover, the flowers have an anti-pulmonary activity [10,11,12]. However, only a few investigations have studied the phytochemicals of stem bark and its biological activity, including antioxidant, antimicrobial, and antiproliferative activities [13,14,15,16,17].
The principal secondary metabolites of A. lebbeck include saponins, macrocyclic alkaloids, phenolic glycosides, and flavonoids [4]. Previous reports that studied active constituents of stem bark revealed the presence of fatty acids with the prevalence of oleic, palmitic, capric, lauric, myristic, stearic, and linoleic acids together with n-tritricontane and β- sitosterol [13,18]. Several authors have also detected phenolic compounds in stem bark extract. For example, Abd El-Ghany et al. [13] reported that e-vanillic acid was the principal compound in the ethyl acetate and butanol fractions, while syringic acid was in the total alcoholic extract. Abdul-Hafeez et al. [19] reported that A. lebbeck bark extract had the highest phenolic content (148.00 mg/g gallic acid equivalents) compared with other extracts. The flavonoids isolated from the bark include catechin [20], geraldone, luteolin, and isookanin [21]. Likewise, several triterpenoid saponin compounds have been isolated from stem bark [22,23]. The study conducted by Ibrahim et al. [24] examined nine tree species, including A. lebbeck, for their antimicrobial activity and antioxidant activity using DPPH radical scavenging to study their aqueous, ethanol, methanol, and acetone stem bark extracts in vitro, where acetone extracts had the lowest EC50.
The biological activity of various components identified or isolated from A. lebbeck has been reported in previous literature, although a few reports have dealt with extracts from stem bark with insufficient information about the active components responsible for their biological activity. Antiproliferative activity has been demonstrated on various cancer cell lines for the bark extract [13,14], triterpenoid saponins and steroidal derivatives through inducing apoptosis through the upregulation of caspase 8 cells [4,22,25,26], sterols and their glucosides [13,27,28], polyphenolic compounds and flavonoids [1,29,30], saponins [22,31] together with fatty acids and their esters [32,33,34,35,36,37].
The antimicrobial activity of A. lebbeck stem bark extract was evident against several Gram-positive and Gram-negative bacterial strains, such as Pseudomonas syringae, P. aeruginosa, Xanthomonas campestris, E. coli [13,14,15,16], Bacillus subtilis, Staphylococcus aureus, Vibrio mimicus, Salmonella typhi, and Shigella dysenteriae [16,17]. The bark extract has also shown antifungal activity against Penicillium chrysogenum and F. oxysporum [14], Aspergillus niger, and Candida albicans [13,17]. Unfortunately, the majority of these studies did not provide information about the bioactive components of the tested extracts and their potential antimicrobial properties. On the other hand, antimicrobial properties have been demonstrated for long-chain saturated fatty acids, which have been reported in A. lebbeck stem bark in some previous studies. Examples of these fatty acids include palmitic acid [36,38,39,40,41], methyl palmitate hexadecanoic acid methyl ester [42] and oleic acid [43].
Herein, the current work targets the characterization of stem bark extract of Albizia lebbeck using HPLC and GC-MS and the investigation of its antimicrobial activity against several bacterial and fungal strains and its antiproliferative activity against MCF-7, PC-3, Caco2, and normal Vero cell lines. The employed bacterial and fungal strains included a collection of the standard species most frequently studied, with varying susceptibility to different extracts in previous literature.
2. Materials and Methods
2.1. Chemicals and Reagents
Standard chemicals and GC-grade solvents were obtained from Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany. PDA (Potato dextrose agar) and NSA (nutrient-sucrose agar) media were purchased from HiMedia, India. In order to carry out the cytotoxicity assay, all chemicals were obtained from Bio Basic Canada Inc., including RPMI-1640 medium (Roswell Park Memorial Institute), MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), fetal bovine serum, DMSO (dimethyl sulfoxide), and PBS (phosphate-buffered saline).
2.2. Preparation of A. lebbeck Extract
The stem bark was collected from A. lebbeck trees growing near the campus of Assiut University, Egypt. Senior staff members (Prof. Dr. Gamal T. Mousa and Prof. Dr. Ismaiel El-Sallami) at Assiut University’s Department of Ornamental Plants and Landscape Gardening verified the plant samples’ authenticity. An acetone extract was prepared from the shade-dried ground bark samples. A mixture of 100 g of stem bark powder was macerated in 100 mL of acetone in water (80:20, respectively) with continued shaking for three days in the dark at room temperature of 23–25 °C, followed by filtering and collecting the macerate, then repeating the same procedure twice more. A mixture of the three mixed filtrates from each step was combined and concentrated at 50 °C using a rotary evaporator (Hidolph VV2000, Heidolph Instruments, Kehlheim, Germany), which was then freeze-dried for 48 h in a Telstar-LyoQuest plus-55 lyophilizer (TELSTAR, Terrassa, Spain). It was estimated that the dry extract yielded 4.78% of the stem bark powder, and the sample was stored at −20 °C until further analysis could be completed.
2.3. Extract Derivatization and Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
A sample of 10 mg of the powder extract was weighed into a vial containing H2SO4-methanol 2% (v/v) and heated at 80 °C with occasional shaking before adding 0.25 mL of neutralized aqueous solution (1 M NaOH). After neutralization, an aliquot of 1 mL of 0.8 M KCl was added and vigorously shaken. The top organic layer was then collected for GC-MS analysis. A trace GC-TSQ mass spectrometer (Triple Quadrupole GC-MS/MS System, Thermo Scientific, Austin, TX, USA) was employed with a TG-5MS capillary column (30 m × 0.25 mm × 0.25 µm film thickness). We set up the temperature program so that the oven temperature was 50 °C, then increased by 5 °C/min to 250 °C, and held at that temperature for 2 min. Finally, we increased the temperature to 300 °C by 30 °C/min and held it there for 2 min. We set the injector and MS transfer lines at 270 °C and 260 °C, respectively. As a carrier gas, helium was used at a 1 mL per minute flow rate. An autosampler (ASI300), with a solvent delay of 4 min, was used to inject the samples in GC split mode. The EI mass spectra were collected in full scan mode (m/z 50–650) at 200 °C ion source temperature. Then, using WILEY 09 and the National Institute of Standards and Technology (NIST) 14 databases, the mass spectra of the detected components were compared.
2.4. High-Performance Liquid Chromatography (HPLC) Analysis
In order to determine the extract level of phenols and flavonoids, dry extract (0.1 g) was dissolved in methanol (2 mL) to obtain a 50 mg/mL sample concentration. As part of the analysis, HPLC-(Agilent 1100) was used, which consisted of two LC pumps, a UV/Vis detector, and a C18 column (125 mm by 4.60 mm, 5 µm particle size). The phenolic acids were separated using a gradient mobile phase consisting of solvent A (methanol) and solvent B (acetic acid in water 1:25), where the detection wavelength was 250 nm. The flavonoids were separated with acetonitrile (A) and aqueous formic acid (B) using an isocratic elution program (70:30). The detection wavelength was 360 nm. A gradient program began with 100% solvent B, which was maintained for 3 min at this concentration. Following this, a 50% eluent of solvent A was added for 5 min. Next, the concentration of solvent A was increased to 80% for 2 min and then reduced to 50% for 5 min. Based on calibration curves for standard compounds, the concentrations of the detected compounds were calculated in the acetone extract (µg/mL), dry extract (µg/g) and dry stem bark (µg/g). All standards were purchased from Sigma-Aldrich (Chemie GmbH, Taufkirchen, Germany): catechol, syringic acid, cinnamic acid, caffeic acid, gallic acid, salicylic acid, ellagic acid, protocatechuic acid, naringin, rutin, quercetin, kaempferol, luteolin, hisperdin, catechin, and chrysoeriol.
2.5. Antibacterial Activity
Following Brulez and Zeller [44] and Ibrahim et al. [45], agar diffusion was used to test the antibacterial effect of A. lebbeck stem bark extract on six bacterial strains deposited at Assiut University Department of Plant Pathology (Agrobacterium tumefaciens 614, Serratia marcescens 2039, and Acinetobacter johnsonii 6005, Bacillus subtilis KNKI-2030, Erwinia carotovora MFB20, and Escherichia coli 1110) After spreading the tested bacterial suspensions on NSA medium and allowing them to dry, the extracts were applied in different concentrations in a 9 mm punch (15.75, 31.25, 62.5, 125, 250, 500, and 1000 µg/mL). The positive control was amoxicillin (62.5 µg/mL), and all treatments were repeated four times. Two days after incubation at 27 °C, the zone of inhibition was recorded for the control (A) and treatment (B). Using Equation (1), growth inhibition percentages were calculated, and the MIC was determined as the minimum extract concentration necessary to suppress bacterial growth.
2.6. Antifungal Activity
In accordance with the method previously reported by Abdel-Hafez et al. [46] and Ibrahim et al. [45], three fungus species obtained from the Department of Plant Pathology, Assiut University, Egypt (Rhizoctonia solani 301, Penicillium italicum 309, and Fusarium oxysporum 339) were tested in vitro for in vitro activity. The extract was prepared in PDA medium at various concentrations (15.6, 31.3, 62.5, 125, 250, 500, and 1000 µg/mL) and poured into Petri dishes along with hymexazol at 1000 µg/mL (positive control). Each plate was inoculated with a fungus plug of 2 mm, which was left to grow for 10 days at 28 °C. As soon as the control plates (A) were fully covered by the mycelia of the tested fungus, the diameter of the fungal colonies on the treated plates (B) was measured, and the percentage of growth inhibition was calculated using Equation (1).
2.7. Cytotoxicity Test In Vitro
2.7.1. Cell Cultures
Three human cancer cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). These included prostate (PC-3, ATCC CRL-1435), breast (MCF-7, ATCC HTB-22), colorectal adenocarcinoma (Caco-2, ATCC ATB-37), and normal Vero cells (ATCC CCL-81). The cells were grown in RPMI medium with 10% fetal bovine serum, 100 units/mL penicillin G, and 100 mg/mL streptomycin sulfate. Cells were incubated at 37 °C in a CO2 incubator before being harvested with trypsin 0.25% and EDTA-2Na 0.025 in PBS.
2.7.2. MTT (3-[4,5-Dimethylthiazol-2-yl]-2,5 Diphenyl Tetrazolium Bromide) Assay
Our previously published work [45,47] described the detailed method for studying the in vitro cytotoxicity of A. lebbeck stem bark extract. The harvested cells were inoculated at 100 µL/well (1 × 105 cells/mL) in 96-well plates and incubated for 24 h at 37 °C in a CO2 incubator. A fresh growth medium was used to wash the developed cell monolayer twice. In a maintenance medium (RPMI-1640 medium augmented with 2% fetal bovine serum), extract concentrations of 31.25, 62.5, 125, 250, 500, and 1000 µg/mL were prepared. Incubation of different extract concentrations for 48 h was followed by doxorubicin as the positive control. After removing the medium, 20 mL of MTT solution (5 mg/mL) was added to each well and incubated for 4 h in the dark. Once the MTT had been decanted, 200 µL of DMSO was added per well and incubated for 30 min. An ELISA reader was used to estimate the plated cells’ optical density at 560 nm wavelength, and Equation (2) was used to calculate the cytotoxicity percentage.
For each cell line, the IC50 of the extract and doxorubicin was estimated, and the selectivity index (SI) was calculated using Equation (3).
2.8. Statistical Analysis
For the comparison of the extract effects on bacterial and fungus strains, ANOVA was applied, and the LSD test at 0.05 was used to compare means. An unpaired t-test was used when comparing the response of cancer cell lines. Data on antimicrobial activity were presented as the mean ± SD derived from four replicates, and those of the in vitro antiproliferative assay were presented as the mean ± SD derived from three replicates. Analysis was performed using Statistix software (ver. 8.1, Analytical Software, Tallahassee, FL, USA).
3. Results
3.1. GC-MS Analysis
The acetone extract of A. lebbeck stem bark was subjected to a derivatization process and then analyzed using GC-MS. As a result, 31 compounds were identified, as listed in Table 1 and Figure 1, of which oleic acid was the most prevalent, corresponding to ca. 19.2% of the total peak area. In addition, the compounds represented in the high concentration included β -sitosterol (11.84%, phytosterols), nonadecanoic acid methyl ester (11.66%, fatty acid esters), γ-tocopherol (8.56%, tocopherol), palmitic acid (7.19%, saturated fatty acids), dodecanoic acid methyl ester (6.3%, fatty acid esters), and 2-hydroxy-3-[(9E)-9-octadecenoyloxy] propyl (9E)-9-octadecenoate (6.18%, 1,3-diacylglycerols). In addition, five components were detected at a medium concentration, including ethyl iso-allocholate (4.58%, steroid derivatives), 2,6-bis(3,4-methylenedioxyphenyl)-3,7-dioxabicyclo(3.3.0)octane (3.62%, lignans), 1,4-benzenediol, 2-(1,1-dimethylethyl)-5-(2-propenyl)- (3.33%, phenolic compounds), docosanoic acid methyl ester (2.37%, fatty acid esters) and 7,10-octadecadienoic acid, and methyl ester (2.31%, fatty acid esters). The rest of the components were represented in areas less than 2.0%, of which the most predominant were .psi.,.psi.-Carotene, 1,1′,2,2′-tetrahydro-1,1′-dimethoxy (carotenoids)-; 4-methoxy-hexacosanoic acid (methoxylated fatty acids); 9,12,15-octadecatrienoic acid, 2,3-bis[(trimethylsilyl)oxy]propyl ester, (z,z,z)- (fatty acid esters), and Isopropyl myristate (fatty acid esters).

Table 1.
A list of the components detected by GC-MS in the acetone stem bark extract of Albizia lebbeck.
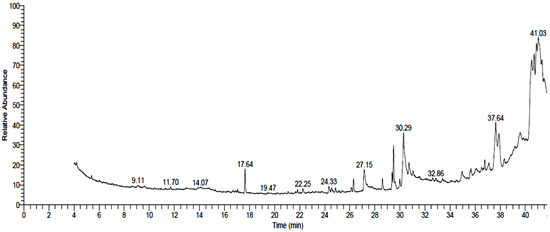
Figure 1.
GC–MS chromatogram of Albizia lebbeck stem bark extract.
3.2. HPLC Analysis
The results of HPLC quantitative analysis of phenolic and flavonoid components of A. lebbeck stem bark extract are presented in Table 2 and Table 3 and Figure 2. A total of eight phenolic compounds were detected and identified, with a total concentration of 1.53 mg/g dry extract equivalent to 73.1 μg/g dry stem bark. The most prevalent phenolic acid was cinnamic acid (28.3 μg/g stem bark). The other seven phenolic acids identified were in the following descending order: ellagic (14.3 μg/g), catechol (6.9 μg/g), salicylic (6.1 μg/g), caffeic (5.2 μg/g), syringic (5.1 μg/g), gallic (4.8 μg/g), and protocatechuic (2.3 μg/g).

Table 2.
Concentrations of phenolic components of Albizia lebbeck acetone stem bark extract screened by HPLC at a wavelength of 250 nm.

Table 3.
Concentrations of flavonoid components of Albizia lebbeck acetone stem bark extract by HPLC at a wavelength of 360 nm.
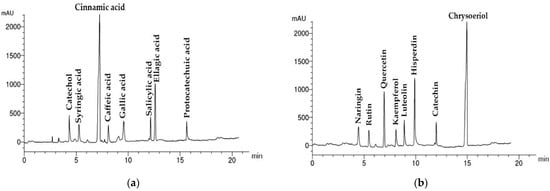
Figure 2.
HPLC chromatograms of Albizia lebbeck acetone stem bark extract: (a) phenolic components; (b) flavonoid components.
The flavonoids detected and identified were eight compounds listed in Table 3, with a total concentration of 1.61 mg/g dry extract equivalent to 77.1 μg/g dry stem bark. Chrysoeriol (23.0 μg/g stem bark), hesperidin (13.8 μg/g), and quercetin (10.9 μg/g) were the predominant flavonoids. The other flavonoids were represented in lower concentrations, which included naringin (6.8 μg/g), catechin (6.8 μg/g), rutin (5.9 μg/g), luteolin (5.9 μg/g), and kaempferol (4.0 μg/g).
3.3. Antibacterial Activity
Albizia lebbeck acetone stem bark extract was tested for its potential antibacterial properties against six bacterial strains. The results of the inhibition zone (mm) and the percentage of growth inhibition are illustrated in Figure 3. The extract showed a similar general trend against the six bacterial strains, where the percentage of growth inhibition exerted a concentration-dependent increase. However, the inhibitory effect of the extract was more pronounced against B. subtilis and S. marcescens, where the lowest extract concentration suppressing bacterial growth (MIC) was 62.5 µg/mL. However, a higher extract concentration was needed to suppress the growth of A. johnsonii and A. tumefaciens (125 µg/mL), E. carotovora (250 µg/mL), and E. coli (500 µg/mL). At its highest concentration (1000 µg/mL), the extract produced a maximum growth inhibition percentage of 238% compared with the positive control treatment, the amoxicillin antibiotic (483.3%). Meanwhile, E. coli was the least affected strain, showing growth inhibition only when subjected to the highest two concentrations (500 and 1000 µg/mL).
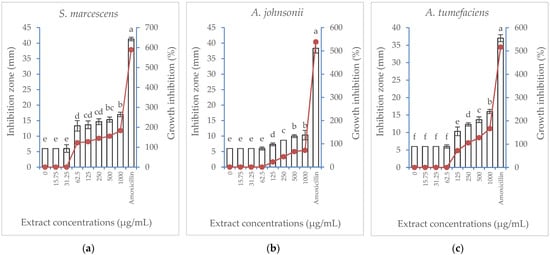
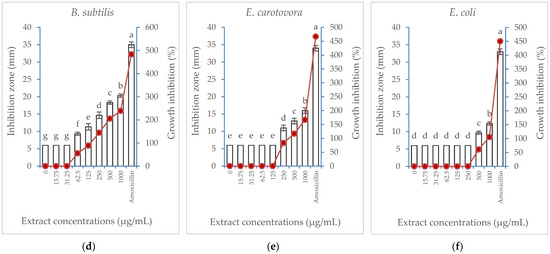
Figure 3.
Effect of Albizia lebbeck acetone stem bark extract at different concentrations compared with amoxicillin at 62.5 µg/mL on the inhibition zone (vertical bars on primary axis) and growth inhibition percentage (line on secondary axis) of six bacterial strains: (a) Serratia marcescens, (b) Acinetobacter johnsonii, (c) Agrobacterium tumefaciens, (d) Bacillus subtilis, (e) Erwinia carotovora, (f) Escherichia coli. The vertical bars indicate the SD values (n = 4). Different lowercase letters indicate significant differences between various treatments according to the LSD test at p = 0.05.
3.4. Antifungal Activity
Albizia lebbeck extract showed varying antifungal activity against three fungi species (R. solani, P. italicum, and F. oxysporum). Inhibition of mycelial growth was induced in the three fungi in a concentration-dependent manner (Figure 4). Fusarium oxysporum showed the highest growth inhibition, reaching the maximum value (42.95%) when the highest extract concentration (1000 µg/mL) was applied compared with 81.85% growth inhibition induced by the hymexazol treatment (the positive control). Rhizoctonia solani and P. italicum showed lower growth inhibition, with the latter being the most resistant.
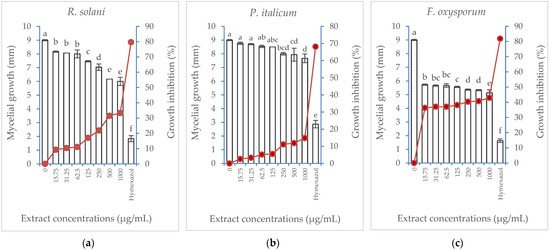
Figure 4.
Effect of Albizia lebbeck acetone stem bark extract at different concentrations compared with hymexazol at 1000 µg/mL on the mycelial growth (vertical bars on primary axis) and growth inhibition percentage (line on secondary axis) of three fungal species: (a) Rhizoctonia solani, (b) Penicillium italicum, (c) Fusarium oxysporum. The vertical bars indicate the SD values (n = 4). Different lowercase letters indicate significant differences between various treatments according to the LSD test at p = 0.05.
3.5. In Vitro Cytotoxic Activity
There were variable in vitro cytotoxic activities observed for A. lebbeck extract against the three cancer cell lines tested: the prostate (PC-3), breast (MCF-7), and colorectal adenocarcinoma (Caco-2), as compared with normal Vero cells (Figure 5). Cell viability was reduced in a concentration-dependent manner, resulting in varying values of IC50 for MCF-7 (203 µg/mL), PC-3 (92.29 µg/mL), and Caco-2 (142 µg/mL) compared with Vero cells (272.3). The calculated SI revealed the highest value for the PC-3 cell line (2.95), followed by Caco-2 (1.92) and MCF-7 (1.34).

Figure 5.
Cytotoxicity response of Albizia lebbeck acetone stem bark extract compared with doxorubicin on MCF-7, PC-3, Caco-2, and Vero cells: (a) cytotoxic effect of the extract, values are represented as mean (n = 3) ± SD, different lowercase letters with the same color indicate significant differences between various concentrations of the extract on the same cell line according to LSD test at p = 0.05; (b) IC50 (µg/mL) of the extract and doxorubicin; (c) selectivity index of the extract.
4. Discussion
Phytochemical screening using GC-MS and HPLC revealed the richness of A. lebbeck acetone stem bark extract with several bioactive compounds. GC-MS analysis of the derivatized extract revealed the prevalence of oleic acid, β -Sitosterol, nonadecanoic acid methyl ester, γ-tocopherol, palmitic acid, dodecanoic acid methyl ester, and 2-hydroxy-3-[(9E)-9-octadecenoyloxy] propyl (9E)-9-octadecenoate. The other components included ethyl iso-allocholate, 2,6-bis(3,4-methylenedioxyphenyl)-3,7-dioxabicyclo(3.3.0)octane, 1,4-benzenediol, 2-(1,1-dimethylethyl)-5-(2-propenyl)-, docosanoic acid methyl ester, 7,10-octadecadienoic acid, and methyl ester. In accordance with our findings, Abd El-Ghany et al. [13] reported the predominance of oleic (9-octadecanoic) and palmitic acids (hexadecanoic) together with capric (decanoic), lauric (dodecanoic), myristic (tetradecanoic), stearic (octadecanoic), linoleic (9,12-octadecadienoic), and β-sitosterol in total alcoholic extract of A. lebbeck stem bark. El-Hawary et al. [18] found that oleic and linoleic acids were the major fatty acids in the petroleum ether extract of A. lebbeck stem. In agreement with our results, they reported the predominance of β-sitosterol. However, they detected the incidence of n-tritricontane as a major component, which was not detected in our study. The variations in the reported results from the literature data could be explained based on the geographical and environmental conditions for individual plants from different origins, together with the differences in the extraction process.
Quantifying phenolic and flavonoid compounds revealed the presence of eight phenolic acids, principally cinnamic acid, and eight flavonoids, mainly chrysoeriol, hesperidin, and quercetin. All phenolic compounds identified in the current study have been previously reported in the stem bark extract of A. lebbeck by several authors, such as Abd El-Ghany et al. [13]. However, they identified e-vanillic acid as the principal compound in the ethyl acetate and butanol fractions, while syringic acid was present in the total alcoholic extract. In addition, the concentrations reported for phenolic acids were higher than those revealed in our study. The differences between solvents could justify these variations; acetone was used in the current study compared with methanol, ethyl acetate, and butanol.
Our findings indicated that the acetone extract of A. lebbeck stem bark was biologically active as an antibacterial agent against B. subtilis and S. marcescens and had moderate activity against A. johnsonii and A. tumefaciens, while E. carotovora and E. coli were the least affected. Antifungal activity was also evident against F. oxysporum, with inferior results against R. solani and P. italicum. These results are supported by several previous publications demonstrating the antimicrobial activity of stem bark extract from A. lebbeck. For example, a recent study by Arasu et al. [14] showed high antibacterial activity of the ethyl acetate, petroleum ether, and chloroform extracts of A. lebbeck bark against different bacterial strains, with Pseudomonas syringae being the most susceptible to the ethyl acetate and petroleum ether fractions and Xanthomonas campestris to the chloroform fraction. The extracts also showed strong antifungal activity against Penicillium chrysogenum (ethyl acetate extract) and F. oxysporum (chloroform extract). The authors claimed that the high content of total phenols and flavonoids was the reason behind the recorded antimicrobial activity. Abd El-Ghany et al. [13] reported moderate antibacterial activity of alcoholic and ethyl acetate extracts of A. lebbeck stem bark against several Gram-ve and Gram+ve bacterial strains; they attributed these results to the presence of sterols and sterol glucosides. Unlike our results, the extracts applied by the authors had better results against E. coli. They also showed antifungal activity against Aspragillus niger and Candida albicans. Ali et al. [17] found that the petroleum ether and ethyl acetate extracts A. lebbeck stem bark displayed moderate activity against Bacillus subtilis, Staphylococcus aureus, Vibrio mimicus, Salmonella typhi, Shigella dysenteriae, Candida arrizae, and Aspergillus niger, but the extract did not inhibit the growth of E. coli, which agrees with our findings. The authors ascribed the high activity of the petroleum ether extract to the presence of saponins. In accordance with Suruse et al. [15], the methanol extract of A. lebbeck stem bark displayed good antibacterial activity against Gram-positive and Gram-negative bacteria. They attributed this effect to the presence of bioactive phytochemicals in the extract, mainly oleic and palmitic acids, which accounted for 24.12 and 21.56% of the total content, respectively. A number of studies have shown that long-chain saturated fatty acids, including palmitic acid, possess antibacterial properties and can be used as antimicrobial food additives [36,39]. It was found that methyl palmitate hexadecenoic acid methyl ester exhibited the greatest antimicrobial activity against clinical pathogenic bacteria [42]. In their studies, Desbois and Smith [40] and Ngamakeue and Chitprasert [41] attributed their high antimicrobial activity to palmitic acid, which is an antimicrobial agent. Similar results were reported by Yff et al. [38], where palmitic acid was the most effective antibacterial agent against Gram-positive (B. subtilis and S. aureus) and Gram-negative bacteria (E. coli and Klebsiella pneumonia). In a similar manner, oleic acid derivatives have shown significant antimicrobial activity against a variety of microorganisms [43].
Furthermore, moderate in vitro cytotoxic activity was registered against the PC-3 cell line, as indicated by SI (2.95). Previous studies have examined the cytotoxic effects of A. lebbeck on a variety of cancer cell lines [48], with few reports on stem bark extracts. For example, Abd El-Ghany et al. [13] reported strong cytotoxic activity indicated by low LD50 for the methanolic extract of A. lebbeck stem bark against HePG-2 (5.2), HCT-116 (11.1), HEP-2 (11.7), HELA (44.0), and MCF-7 (48.9) cell lines. Additionally, Arasu et al. [14] detected a cytotoxic effect for ethyl acetate bark extract with LC50 of 69.5 μg/mL, which was accompanied by a high content of total phenols and flavonoids. Although more potent cytotoxic activity for A. lebbeck stem bark extract has been reported in these studies, no comparisons were made with normal cells. Therefore, it is impossible to calculate the selectivity index for a valid comparison. These recorded biological activities were accompanied by the richness of A. lebbeck extract in several bioactive compounds, as proved by the results of GC-MS and HPLC. These results could be attributed to the biological effects of sterols and their glucosides [13,28], polyphenolic compounds [1], and saponins [22,31]. In a number of previous studies, fatty acids and their esters have been shown to possess anticancer properties [32,33]. Hexadecanoic acid (palmitic acid) has been reported to exhibit anticancer activity in vitro by Bharath et al. [34]. An additional study found that palmitic acid isolated from Amphiroa zonata plants inhibited DNA topoisomerase without adversely affecting normal cells [35,36]. Similarly, Jiang et al. [37] found that oleic acid induced cell cycle arrest at G0/G1 in tongue squamous cell carcinoma cells. There has also been evidence that the sterols and steroidal derivatives present in the extract may act as anticancer agents [27]. As an example of bioactive steroidal derivatives, ethyl isoallocholate has shown anticancer activity against A549 lung cancer cells [25]. Additionally, flavone 4′-OH,5-OH,7-di-O-glucoside is an isoflavonoid that has antioxidant properties [29,30]. Another compound present in lower abundance in the A. lebbeck extract is 4-benzenediol, 2-(1,1-dimethy-lethyl)-5-(2-propenyl), a phenolic compound previously reported in GC-MS analysis of C. maritima ssp. aegyptiaca and has been claimed to have anticancer and apoptotic activity on various cancer cell lines [49].
5. Conclusions
The acetone extract of A. lebbeck stem bark was found to be rich in fatty acids, with oleic acid (19.2%) being the predominant fatty acid. Several phytochemicals were also detected, including phytosterols and tocopherols. Eight phenolic acids, primarily cinnamic acid, and eight flavonoids, mostly chrysoeriol, hesperidin, and quercetin, were quantified. It was found that A. lebbeck extract was biologically active as an antibacterial agent against B. subtilis and S. marcescens and had moderate activity against A. johnsonii and A. tumefaciens, while E. carotovora and E. coli were the least affected. Moreover, antifungal activity was observed against F. oxysporum, with less effectiveness against R. solani and P. italicum. According to the calculated selectivity index, the PC-3 cell line displayed the highest value (2.95), followed by Caco-2 (1.92) and MCF-7 (1.34). Based on these results, the acetone stem bark extract of A. lebbeck is a rich source of phytochemicals, indicating its potential cytotoxic, antibacterial, and antifungal activities. However, further research is needed to determine the biological activity of specific bioactive compounds identified in A. lebbeck extract.
Author Contributions
Conceptualization, O.H.M.I. and E.Y.A.-H.; data curation, O.H.M.I. and E.Y.A.-H.; investigation, O.H.M.I. and E.Y.A.-H.; methodology, O.H.M.I. and E.Y.A.-H.; project administration, O.H.M.I.; writing—original draft, O.H.M.I. and E.Y.A.-H.; writing—review and editing, O.H.M.I. and E.Y.A.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. (G: 73-155-1443).
Data Availability Statement
Not applicable.
Acknowledgments
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. (G: 73-155-1443). The authors, therefore, acknowledge with thanks DSR for their technical and financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mishra, S.S.; Gothecha, V.K.; Sharma, A. Albizia lebbeck: A short review. J. Herb. Med. Toxicol. 2010, 4, 9–15. [Google Scholar]
- Amog, P.U.; Manjuprasanna, V.N.; Yariswamy, M.; Nanjaraj Urs, A.N.; Joshi, V.; Suvilesh, K.N.; Nataraju, A.; Vishwanath, B.S.; Gowda, T.V. Albizia lebbeck seed methanolic extract as a complementary therapy to manage local toxicity of Echis carinatus venom in a murine model. Pharm. Biol. 2016, 54, 2568–2574. [Google Scholar] [CrossRef]
- Rashid, R.B.; Chowdhury, R.; Jabbar, A.; Hasan, C.M.; Rashid, M.A. Constituents of Albizzia lebbeck and antibacterial activity of an isolated flavone derivative. Saudi Pharm. J 2003, 11, 52–56. [Google Scholar]
- Noté, O.P.; Jihu, D.; Antheaume, C.; Zeniou, M.; Pegnyemb, D.E.; Guillaume, D.; Chneiwess, H.; Kilhoffer, M.C.; Lobstein, A. Triterpenoid saponins from Albizia lebbeck (L.) Benth and their inhibitory effect on the survival of high grade human brain tumor cells. Carbohydr. Res. 2015, 404, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, M.; Kundu, S.S.; Singh, S.; Misra, A.K. Cornell net carbohydrate and protein system for nutritional evluation of tree leaves, shrubs and grasses. Indian J Anim Sci. 2006, 76, 81–87. [Google Scholar]
- Varshney, I.P. Glycosides and carbohydrates from the members of the family Leguminosae. Univ. Indore Res. J. Sci. 1976, 4, 13–22. [Google Scholar]
- Pal, B.C.; Achari, B.; Yoshikawa, K.; Arihara, S. Saponins from Albizia lebbeck. Phytochemistry 1995, 38, 1287–1291. [Google Scholar] [CrossRef]
- Wati, M.; Khabiruddin, M. Comparision of antioxidants in phenol extract and methanol extract of Albizia lebbeck from two locations. Int. J. Pharm. Sci. Rev. Res. Int. 2017, 45, 78–82. [Google Scholar]
- Elshiekh, Y.H.; Alagbash, R.E.; Ali, R.A.; Saad, F.O.; Musharaf, M. Phytochemical constituents, antibacterial screening and antioxidant activity of Albizia lebbeck (L.) Benth (Seed). N. A. J. Adv. Res. Rev. 2020, 7, 035–040. [Google Scholar]
- Babu, N.P.; Pandikumar, P.; Ignacimuthu, S. Anti-inflammatory activity of Albizia lebbeck Benth., an ethnomedicinal plant, in acute and chronic animal models of inflammation. J. Ethnopharmacol. 2009, 125, 356–360. [Google Scholar] [CrossRef]
- Gul, F.; Shinwari, Z.K.; Afzal, I. Screening of indigenous knowledge of herbal remedies for skin diseases among local communities of north West Punjab, Pakistan. Pak. J. Bot. 2012, 44, 1609–1616. [Google Scholar]
- Farag, M.; El Gamal, A.; Kalil, A.; Al-Rehaily, A.; El Mirghany, O.; El Tahir, K. Evaluation of some biological activities of Albizia lebbeck flowers. Pharmacol. Pharm. 2013, 4, 473–477. [Google Scholar] [CrossRef]
- Abd El-Ghany, A.E.-S.; Dora, G.; Abdallah, R.H.; Hassan, W.; El-Salam, E.A. Phytochemical and biological study of Albizia lebbeck stem bark. J Chem Pharma Res 2015, 7, 29–43. [Google Scholar]
- Arasu, M.V.; Al-Dhabi, N.A.; Choi, K.C.; Vincent Bensy, A.D.; Rajaselvam, J. Bioactive potential of Albizia lebbeck extract against phytopathogens and protective properties on tomato plant against speck disease in greenhouse. Physiol. Mol. Plant Pathol. 2022, 117, 101750. [Google Scholar] [CrossRef]
- Suruse, P.B.; Bodele, S.B.; Duragkar, N.J.; Saundankar, Y.G. In-Vitro evaluation of antioxidant activity of Albizia Lebbeck bark. Int. J. Biol. Sci. Ayuveda Res. 2013, 1, 6–17. [Google Scholar]
- Abdul-Hafeez, E.Y.; Nguyen, T.N.; Karamova, N.S.; Ilinskaya, O.N. Antibacterial activity of certain medicinal plants on different bacterial strains associated with colorectral cancer. Int. J. Biosci. 2014, 5, 219–229. [Google Scholar]
- Ali, M.T.; Haque, S.T.; Kabir, M.L.; Rana, S.; Haque, M.E. A comparative study of in vitro antimicrobial, antioxidant and cytotoxic activity of Albizia lebbeck and Acacia nilotica stem bark. Bull. Fac. Pharm. Cairo Univ. 2018, 56, 34–38. [Google Scholar] [CrossRef]
- El-Hawary, S.; El-Fouly, K.; Sokkar, N.M.; Talaat, Z. A phytochemical profile of Albizia lebbeck (L.) Benth. cultivated in Egypt. Asian J. Biochem. 2011, 6, 122–141. [Google Scholar] [CrossRef]
- Abdul-Hafeez, E.Y.; Karamova, N.S.; Ilinskaya, O.N. Antioxidant activity and total phenolic compound content of certain medicinal plants. Int. J. Biosci. 2014, 5, 213–222. [Google Scholar]
- Desai, S.; Tatke, P.; Gabhe, S. Isolation of catechin from stem bark of Albizia lebbeck. Int. J. Res. Pharm. Biomed. Sci. 2014, 3, 31–35. [Google Scholar]
- Ahmed, D.; Kumar, V.; Sharma, M.; Verma, A. Target guided isolation, in-vitro antidiabetic, antioxidant activity and molecular docking studies of some flavonoids from Albizzia Lebbeck Benth. bark. BMC Complement Altern. Med. 2014, 14, 155. [Google Scholar] [CrossRef]
- Desai, T.H.; Joshi, S.V. Anticancer activity of saponin isolated from Albizia lebbeck using various in vitro models. J. Ethnopharmacol. 2019, 231, 494–502. [Google Scholar] [CrossRef]
- Viana, E.O.R.; Cruz, M.d.F.S.J.; da Silva, M.J.; Pereira, G.M.; da Silva, B.P.; Tinoco, L.W.; Parente, J.P. Structural characterization of a complex triterpenoid saponin from Albizia lebbeck and investigation of its permeability property and supramolecular interactions with membrane constituents. Carbohydr. Res. 2019, 471, 105–114. [Google Scholar] [CrossRef]
- Ibrahim, O.H.M.; Abdul-Hafeez, E.Y.; Mahmoud, A.F. Antimicrobial and Antioxidant Activities of Stem Bark Extracts of Different Ornamental Trees. Assiut J. Agric. Sci 2015, 46, 19–32. [Google Scholar]
- Thakur, R.S.; Ahirwar, B. A steroidal derivative from Trigonella foenum graecum L. that induces apoptosis in vitro and in vivo. J Food Drug Anal. 2019, 27, 231–239. [Google Scholar] [CrossRef]
- Noté, O.P.; Ngo Mbing, J.; Kilhoffer, M.-C.; Pegnyemb, D.E.; Lobstein, A. Lebbeckoside C, a new triterpenoid saponin from the stem barks of Albizia lebbeck inhibits the growth of human glioblastoma cells. Nat. Prod. Res. 2019, 33, 2292–2299. [Google Scholar] [CrossRef] [PubMed]
- Grattan, B.J. Plant Sterols as Anticancer Nutrients: Evidence for Their Role in Breast Cancer. Nutrients 2013, 5, 359–387. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kumar, P.; Jindal, A. Antibacterial potential of sterols of some medicinal plants. Int. J. Pharm. Pharm. Sci. 2012, 4, 159–162. [Google Scholar]
- Semwal, P.; Painuli, S.; Badoni, H.; Bacheti, R.K. Screening of phytoconstituents and antibacterial activity of leaves and bark of Quercus leucotrichophora A. Camus from Uttarakhand Himalaya. Clin. Phytoscience 2018, 4, 30. [Google Scholar] [CrossRef]
- Khan, N.; Ali, A.; Qadir, A.; Ali, A.; Warsi, M.H.; Tahir, A.; Ali, A. GC-MS analysis and antioxidant activity of Wrightia tinctoria R.Br. leaf extract. J. AOAC Int. 2021, 104, 1415–1419. [Google Scholar] [CrossRef]
- Jangwan, J.S.; Dobhal, M.; Kumar, N. New cytotoxic saponin of Albizzia lebbeck. Indian J. Chem. B 2010, 49, 123–126. [Google Scholar]
- Jóźwiak, M.; Filipowska, A.; Fiorino, F.; Struga, M. Anticancer activities of fatty acids and their heterocyclic derivatives. Eur. J. Pharm. 2020, 871, 172937. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, S.; Menzel, A. A subchronic 90-day oral rat toxicity study and in vitro genotoxicity studies with a conjugated linoleic acid product. Food Chem. Toxicol. 2003, 41, 1749–1760. [Google Scholar] [CrossRef]
- Bharath, B.; Perinbam, K.; Devanesan, S.; AlSalhi, M.S.; Saravanan, M. Evaluation of the anticancer potential of Hexadecanoic acid from brown algae Turbinaria ornata on HT–29 colon cancer cells. J. Mol. Struct. 2021, 1235, 130229. [Google Scholar] [CrossRef]
- Harada, H.; Yamashita, U.; Kurihara, H.; Fukushi, E.; Kawabata, J.; Kamei, Y. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res. 2002, 22, 2587–2590. [Google Scholar] [PubMed]
- Arora, S.; Kumar, G. Phytochemical screening of root, stem and leaves of Cenchrus biflorus Roxb. J. Pharm. Phytochem. 2018, 7, 1445–1450. [Google Scholar]
- Jiang, L.; Wang, W.; He, Q.; Wu, Y.; Lu, Z.; Sun, J.; Liu, Z.; Shao, Y.; Wang, A. Oleic acid induces apoptosis and autophagy in the treatment of tongue squamous cell carcinomas. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yff, B.T.S.; Lindsey, K.L.; Taylor, M.B.; Erasmus, D.G.; Jäger, A.K. The pharmacological screening of Pentanisia prunelloides and the isolation of the antibacterial compound palmitic acid. J. Ethnopharmacol. 2002, 79, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Yoo, J.-S.; Lee, T.-G.; Cho, H.-Y.; Kim, Y.-H.; Kim, W.-G. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005, 579, 5157–5162. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef]
- Ngamakeue, N.; Chitprasert, P. Encapsulation of Holy Basil Essential Oil in Gelatin: Effects of Palmitic Acid in Carboxymethyl Cellulose Emulsion Coating on Antioxidant and Antimicrobial Activities. Food Bioproc. Tech. 2016, 9, 1735–1745. [Google Scholar] [CrossRef]
- Shaaban, M.T.; Ghaly, M.F.; Fahmi, S.M. Antibacterial activities of hexadecanoic acid methyl ester and green-synthesized silver nanoparticles against multidrug-resistant bacteria. J. Basic Microbiol. 2021, 61, 557–568. [Google Scholar] [CrossRef]
- Novak, A.F.; Clark, G.C.; Dupuy, H.P. Antimicrobial activity of some ricinoleic acid oleic acid derivatives. JAOCS 1961, 38, 321–324. [Google Scholar] [CrossRef]
- Brulez, W.; Zeller, W. Seasonal changes of epiphytic Erwinia amylovora on ornamentals in relation to weather conditions and course of infections. Acta Hortic. 1981, 117, 37–43. [Google Scholar]
- Ibrahim, O.H.M.; Abo-Elyousr, K.A.M.; Asiry, K.A.; Alhakamy, N.A.; Mousa, M.A.A. Phytochemical characterization, antimicrobial activity and in vitro antiproliferative potential of Alchemilla vulgaris Auct root extract against prostate (PC-3), breast (MCF-7) and colorectal adenocarcinoma (Caco-2) cancer cell lines. Plants 2022, 11, 2140. [Google Scholar] [CrossRef]
- Abdel-Hafez, S.I.I.; Abo-Elyousr, K.A.M.; Abdel-Rahim, I.R. Effectiveness of plant extracts to control purple blotch and Stemphylium blight diseases of onion (Allium cepa L.) in Assiut, Egypt. Arch. Phytopathol. Plant Prot. 2014, 47, 377–387. [Google Scholar] [CrossRef]
- Ibrahim, O.H.M.; Mousa, M.A.A.; Asiry, K.A.; Alhakamy, N.A.; Abo-Elyousr, K.A.M. Azadirachta indica A. Juss fruit mesocarp epicarp extracts induce, antimicrobial antiproliferative effects against prostate, breast colorectal adenocarcinoma cancer cell lines through upregulation of proapoptotic genes. Plants 2022, 11, 1990. [Google Scholar] [CrossRef]
- Balkrishna, A.; Sakshi; Chauhan, M.; Dabas, A.; Arya, V. A Comprehensive Insight into the Phytochemical, Pharmacological Potential, and Traditional Medicinal Uses of Albizia lebbeck (L.) Benth. Evid. Based Complement. Altern. Med. 2022, 2022, 5359669. [Google Scholar] [CrossRef]
- Tawfik, M.M.; Galal, B.; Nafie, M.S.; El Bous, M.M.; El-Bana, M.I. Cytotoxic, apoptotic activities and chemical profiling of dimorphic forms of Egyptian halophyte Cakile maritima scop. J. Biomol. Struct. Dyn. 2023, 41, 147–160. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).