Abstract
Peach (Prunus persica L.) is an economically important fruit crop worldwide due to its pleasant flavor. Volatile organic compounds (VOCs) are vital factors for assessing fruit quality. Here, we constructed the VOC profiles for the top eight popular commercial peach cultivars produced in Shanghai by combining gas chromatography-mass spectrometry (GC-MS), odor activity value and gas chromatograph-ion mobility spectrometry (GC-IMS). Seventy VOCs were detected using GC-MS, of which twenty-three were commonly found in eight peach cultivars, including hexanal, nonanal, benzaldehyde, 2-hexenal, butyl acetate, hexyl acetate, (Z)-3-hexen-1-yl acetate, linalool, β-myrcene, D-limonene, 1-hexanol, 3-hexenol, 2-hexenol, 2-ethyl-1-hexanol, γ-octalactone, δ-decalactone, γ-hexalactone, γ-decalactone, γ-dodecalactone, β-ionone, 2-octanone, 2-ethyl furan and 2,4-ditert-butyl phenol. A total of 17 VOCs were screened on the basis of OAV ≥ 1 and the top 5 of this contribution were γ-decalactone, β-ionone, hexanal, 2-hexenal and linalool. Lactones had the highest OAV in HJML and terpenoids had the highest OAV in JC. JXIU had the lowest OAV of lactones and terpenoids. Based on the range of their OAV values, the flavor evaluation standard of Shanghai high-quality peach cultivars can be established, which is also a reference for breeding excellent offspring. Twenty-six VOCs were detected using GC-IMS, and the largest proportion were aldehydes. Principal component analysis (PCA) showed that Hikawa Hakuho (HH) and Jinchun (JC) were distant from the other samples, indicating that their volatiles were more distinct. These results provide a foundation for improving our understanding of aroma compositions in these high-quality peach cultivars, which might also provide a reference for future design breeding to improve fruit flavor.
1. Introduction
The peach (Prunus persica (L.) Batsch) has become an important economic crop due to its soft and juicy flesh, attractive aroma and enriched nutrition, and it has been grown extensively at home and abroad [1]. As the originating country of the peach, China holds a large number of wild relatives and landraces [2]. ‘Chinese Cling’, the most influential founder in peach-breeding history, has derived many excellent cultivars such as ‘Elberta’, ‘J.H. Hale’, ‘Okubo’ and ‘Hakuho’ [3]. The elite commercial peach cultivars planted in Shanghai, such as ‘Hujingmilu’ (HJML), ‘Xinfengmilu’ (XFML) and ‘Jinxiu’ (JX), are also excellent offspring of ‘Chinese Cling’. These cultivars are popular in Shanghai for their high quality and were cultivated in 3100 hectares in 2021, accounting for approximately 82% of the total peach-cultivated area in Shanghai (data source: Shanghai Agricultural Technology Extension Service Center).
Aroma is an essential factor to determine consumers’ preference, which has received great attention from geneticists and breeders in recent years [4,5]. The aroma profiles in fruit result from the interactions of many volatiles. Currently, more than 100 volatiles have been identified in peaches, mainly including aldehydes, alcohols, esters, lactones, terpenes and ketones [6,7]. However, the compositions and content of volatiles are influenced by genetic backgrounds, fruit maturity and cultivation conditions. For instance, aldehydes and alcohols are the major contributors of VOCs in immature fruits and their contents decrease gradually during maturation, while the contents of lactones increase with peach fruit maturation [8]. Studies on several varieties have shown many differences in the composition and content of volatiles. Wang et al. have reported that the Chinese wild peach ‘Wutao’ has the highest content of total volatiles, while ‘Ruipan 14’ and ‘Babygold 7’ show high contents of lactones, and cultivars of American and European origin mainly contain high levels of linalool [7]. Several studies have also shown that cultivation conditions can also influence the composition and content of volatiles, such as fertilization and bagging [9,10,11]. Although more than 100 VOCs can be detected in peaches, only around 25 of them significantly contribute to flavor quality, and these are recognized as characteristic compounds [12]. The odor activity value (OAV) is commonly used to evaluate the contribution of a compound to the overall aroma, and it represents the ratio of the actual concentration in the sample to the threshold value in water, and was first proposed by Rothe [13]. Compounds with OAV ≥ 1 are not only considered major contributors to flavor, but also good indicators for the breeding of good-quality peaches.
Gas chromatography-mass spectrometry (GC-MS) is a commonly used technique that takes advantage of the high separation ability of GC and the superiority of mass spectrometry in the identification of substances, which allows it to be applied with high sensitivity and precision in the qualitative and quantitative analysis of VOCs in samples [14]. However, due to the complexity of most sample matrices, this method requires tedious and time-consuming sample pretreatment before analysis while working under vacuum conditions, making it unsuitable for rapid characterization studies of VOCs in complex samples [15,16]. Ion mobility spectrometry (IMS) is an atmospheric analytical chemistry method for the detection and identification of different types of substances based on the mobility of gas-phase ions in a weak electric field [15]. GC-IMS integrates the high separation ability of GC with the high sensitivity and fast response of IMS. Compared to GC-MS, it has the advantages of having a lower detection limit, no pre-treatment, convenience and can be operated at atmospheric pressure, which can maximize the authenticity of flavor in samples and reflect the original flavor information [16,17]. The method of combining various techniques to study VOCs in foods has become a hot topic, which can establish a more comprehensive and scientific aroma fingerprint. As far as we know, comparison analysis of volatiles in different commercial peach cultivars based on GC-MS and GC-IMS is rarely reported.
In the present study, the volatiles of eight commercial peach cultivars from Shanghai were analyzed by GC-MS, OAV and GC-IMS to comprehensively investigate their volatile compounds and aroma profiles. The results could improve our understanding of aroma compositions in these high-quality peach cultivars, which might also provide a reference for future design breeding to improve fruit flavor.
2. Materials and Methods
2.1. Plant Materials
Eight elite peach cultivars derived from ‘Chinese Cling’ were selected in this study, and all of them had a melting texture and displayed strong aromas. The characteristic information of each cultivar is listed in Figure 1 and Table 1. All trees were planted in the Peach Germplasm Repository of the Shanghai Academy of Agricultural Sciences, China (30°89′ N, 121°39′ E), and the orchard management procedures such as irrigation, pruning, disease control and fertilization were the same for all cultivars. Thirty fruits of each cultivar were picked at the commercial harvest stage in the summer of 2022 according to grounded skin color, fruit firmness and recorded maturity time. The fruits were picked from three trees and immediately transported to the laboratory. Fifteen fruits of each cultivar with uniform size were selected in each biological replicate. The mesocarp was cut into pieces, frozen immediately in liquid nitrogen, grounded into powder and stored at −80 °C for use.
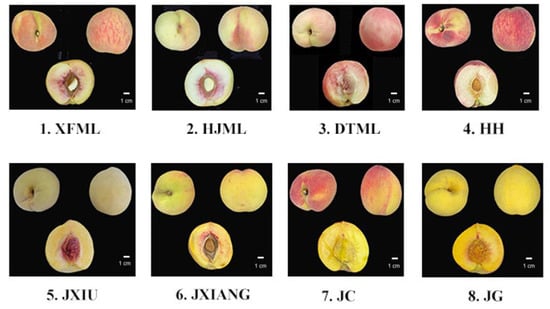
Figure 1.
Representative images of eight peach cultivars used in this study. The codes under each image are the abbreviation names for each cultivar.

Table 1.
The characteristic information of peach materials.
2.2. Chemicals
All qualification and quantitative standards were purchased from Sigma Aldrich (Shanghai, China), and their purity was above 95%.
2.3. GC-MS Analysis of Volatile Compounds
The method of sample pretreatment referred to a previous study [4], with the difference that each headspace vial in this study contained three grams of sample. A fiber (65 µm, PDMS/DVB, Supelco, Bellefonte, PA, USA) was inserted into the headspace of a vial to extract the volatile compounds at 40 °C for 30 min. At the end of extraction, the fiber was desorbed into the injection port of the GC for 5 min. The time of solvent delay was 3 min. The samples were analyzed using the Agilent gas chromatography mass spectrometry (GC-MS) instrument (7890–5975) with a DB-WAX (60 m × 250 μm × 0.25 μm, Agilent 122–7062) under splitless injection mode. Helium (99.999%) was used as a carrier gas at a constant flow (1 mL/min).
The GC oven temperature was initially held at 40 °C for 2 min, increased to 100 °C at 3 °C/min and then increased to 230 °C at 5 °C/min and held for 5 min. The mass detector was used in an electron impact mode at 70 eV, an ion source temperature of 230 °C and a mass spectrometry scan range of m/z 30–350. According to the NIST 08 (National Institute of Standards and Technology) mass spectrometry database, the volatile compound was identified by matching degree, retention time, retention index (RI) and standard chemicals. Compounds with matching degrees greater than 80% were selected as effective aroma components. In order to quantify the components, semi-quantification was performed using an internal standard method to calculate the relative concentration of volatile compounds in the samples. Measurements were repeated three times for each sample.
2.4. GC-IMS Analysis of Volatile Compounds
The VOCs of different peach cultivars were detected using HS-GC-IMS (FlavorSpec ®, G.A.S, Dortmund, Germany). Two grams of grounded sample from each cultivar were transferred into 20 mL headspace bottles. The headspace injection condition was set at the following parameters: incubation time was 15 min, incubation temperature was 40 °C, injection needle temperature was 85 °C and injection volume was 500 μL. The GC conditions were as follows: the chromatographic column was MXT-5 (15 m × 0.53 mm, 1 μm, Agilent Technology, Palo Alto, CA, USA)). Nitrogen of 99.99% purity was used as a carrier gas at a programmed flow as follows: 2 mL/min for 2 min, 10 mL/min for 8 min and 100 mL/min for 10 min. The IMS conditions were as follows: the drift tube temperature was 45 °C, and the drift gas velocity was 150 mL/min. All tests were repeated three times. Volatile compounds were preliminarily identified by comparing the RI and ion drift times (the time in milliseconds it takes for an ion to reach the collector through the drift tube) of standards in the GC-IMS library and the NIST database.
2.5. Identification of Characteristic Aroma Compounds in Samples by the Odor Activity Value Method
The OAVs of compounds were calculated using the following formula: OAV = Ci/OTi, where Ci indicates the concentration of a compound and OTi indicates the odor threshold. The odor thresholds of all compounds were obtained from the published literature [18]. For a compound with multiple thresholds, the standard we chose was its threshold in water and the most recent data.
2.6. Statistical Analysis
The experimental data derived from GC-MS were analyzed using Origin 2021 software (Microcal Software, Inc., Northampton, MA, USA). Analysis of variance (ANOVA) and Duncan’s multiple range test (p < 0.05) were used to analyze the significant differences among samples. Cluster analysis was performed using TB tools software (versions v1.1043). For the data obtained using GC-IMS, four software programs from the instrument were used for analyzing: (1) VOCal was used for viewing analytical spectra and qualitative and quantitative data; the NIST database and IMS database built into the application software can be used for qualitative analysis of compounds. (2) The Reporter plug-in for directly comparing the spectral differences between samples. (3) The Gallery Plot plug-in was used for fingerprint profile comparison. (4) The Dynamic PCA plug-in was used for dynamic principal component analysis.
3. Results and Discussion
3.1. Volatile Profile of Different Peach Cultivars Using GC-MS
3.1.1. The Construction of VOCs Profiling for Eight Peach Cultivars
By searching the NIST database and the chemical standard, a total of seventy VOCs were tentatively identified and quantified for eight peach cultivars using GC-MS. They were categorized into ten groups, including nine aldehydes, ten esters, eleven terpenoids, nine alcohols, six lactones, five ketones, five alkanes, eleven aromatic hydrocarbons, two furans and two other compounds (Figure 2, Table 2). Twenty-three VOCs were commonly identified in all cultivars, including hexanal, nonanal, benzaldehyde, 2-hexenal, butyl acetate, hexyl acetate, linalool, β-myrcene, D-limonene, 1-hexanol, 3-hexenol, 2-hexenol, 2-ethyl-1-hexanol, γ-octalactone, δ-decalactone, γ-hexalactone, γ-decalactone, γ-dodecalactone, β-ionone, 2-octanone, 2-ethyl furan and 2,4-ditert-butyl phenol. More than 40 VOCs were identified in HJML (44), DTML (42), HH (42) and JC (43). Fewer than 35 volatile compounds were identified in XFML (35), JXIU (29), JXIANG (35) and JG (32). Seventeen unique compounds were identified only in one cultivar. Differences were observed in the total content of volatile compounds among the eight peach cultivars (Table 2 and Figure 2).

Figure 2.
The total contents of each category of VOCs in eight peach cultivars. 3.1.2 Cluster Analysis of VOCs of eight peach cultivars.

Table 2.
The absolute and relative contents of VOCs in eight peach cultivars tested using GC-MS.
Aldehydes were regarded as one of the most significant contributors to the peaches’ green and grassy flavor, and have been reported to exist widely in immature plums, apples and pears [19,20]. The aldehydes detected in this experiment include hexanal, nonanal, benzaldehyde, 2-hexenal, 3-hexenal, (E)-2-nonenal, (E)-2-hexenal, (E)-2-heptenal and heptanal (Table 1). Among these, hexanal, nonanal, 2-hexenal and benzaldehyde were identified as major components and were detected in all the samples, which is consistent with previous studies [20,21]. Notably, the highest content of 2-hexenal among all samples was 5132.24 μg/kg in HH, while the lowest was 589.96 μg/kg in HJML; therefore, this compound may be the most important factor responsible for the differences in aldehydes among the samples. It is also clear from Figure 2 that the total content of aldehydes is highest in HH and lowest in HJML.
Alcohols are a group of compounds other than aldehydes that give peach fruit a green aroma. In this study, nine alcohols were found, namely 2-ethyl-1-hexanol, 3-methyl-3-nonanol, carbitol, 3-hexenol, hexanol, 2-hexenol, 3-methyl-3-buten-1-ol, α-terpineol and geraniol. Among them, 3-hexenol, hexanol, 2-hexenol and 2-ethyl-1-hexanol were detected in all samples at high levels (Table 2). JC had the highest level of alcohols and JXIANG had the lowest (Figure 2).
Esters are the main flavor components of most ripe fruits and provide the fruity notes, and particularly (Z)-3-hexen-1-yl acetate and hexyl acetate are considered to contribute significantly to the aroma characteristics of peaches [6,22,23]. A total of ten esters were detected in the eight peach cultivars. Among these, butyl acetate, (Z)-3-hexen-1-yl acetate and hexyl acetate was detected in all samples and the levels were significantly different between samples. (E)-2-hexen-1-yl acetate was also detected at high levels in most cultivars. The remaining unmentioned esters were present at less than 10 μg/kg in each sample, so these components were not the focus of this study.
A total of 11 terpenoids were identified, of which linalool, β-myrcene and D-limonene were detected in all peach cultivars with significant differences. Horvat et al. [24] identified linalool as one of the key aroma compounds of peach fruit that increased significantly with fruit ripening and has floral aroma properties [25,26]. The order of linalool content in all samples was as follows: JC > HH > JXIANG > HJML > XFML> DTML > JG > JXIU. Surprisingly, the linalool content in JC was more than twice that of HH. Previous studies have shown that nectarines contain higher levels of linalool than peaches [7]. Although the material in this study did not include nectarines, JC was derived from peach Jinxiu and nectarine Huyou 018, which may be the reason for its highest linalool content among the eight cultivars. β-myrcene is reported as a precursor of linalool and is one of the common terpenoids in peaches and nectarines [27,28]. Here, the content of β-myrcene ranked consistently with linalool in the eight cultivars.
More than 10 lactones have been found in peaches [6,27,29]. Here, we identified six lactones: γ-hexalactone, γ-decalactone, γ-octalactone, γ-dodecalactone, γ-heptalactone and δ-decalactone. Consistent with previous studies [7], γ-decalactone, δ-decalactone and γ-hexalactone were the leading lactones with the highest content in these elite cultivars, with the difference that δ-decalactone was the most abundant in the present study. As reported, mid- and later-ripening cultivars have higher lactone content than early-ripening cultivars [30]. However, in this study, some early- and mid-maturing cultivars such as HJML and JC had the highest total lactone content, while the late-maturing cultivar JXIU had the lowest content (Figure 2), which might be related to its genetic background and cultivation conditions.
Five ketones were detected, and β-ionone and 2-octanone were detected in all samples. The content of total ketone was highest in ‘XFML’ and lowest in ‘JX’ (Figure 2). Interestingly, dihydro-β-ionone was detected in all white flesh peaches, but not in all yellow flesh peaches; this result is similar to that of several researchers [31]. This is due to the fact that C-13-norisoprenoids such as dihydro-β-ionone in peach fruit are mainly synthesized via the isoprenoid pathway, and carotenoids are precursors for their synthesis. Carotenoids in white-flesh peaches are cleaved to disubstituted carotenoids via the action of carotenoid cleavage dioxygenase (CCD4), which in turn forms the volatile compounds such as dihydro-β-ionone, while the CCD4 enzyme gene in yellow-flesh peaches is mutated and cannot degrade carotenoids, so less dihydro-β-ionone is synthesized than in white-fleshed peaches [32,33].
To further understand the differences of volatiles in different peach cultivars, a cluster analysis heat map was constructed (Figure 3). The vertical axis of the figure represents different volatiles and the horizontal axis represents different peach cultivars. The red-to-blue and large-to-small circles indicate the abundance of volatiles from highest to lowest. According to the content of each of the VOCs, cluster analysis clustered JXIU, DTML, JG and XFML into one group. This indicates that the composition and content of their volatiles are similar. Horizontally, the numerous volatiles were roughly divided into three clusters. Cluster 1 covers most of the aldehydes and alcohols. In general, this cluster of compounds is relatively higher in XFML and JG. The substances in cluster 2 mainly include esters and lactones, and the HH and HJML shows a high abundance in this cluster. The most abundant compounds in cluster 3 are terpenoids and aromatic hydrocarbons, and JC shows the highest abundance in this cluster. Cluster analysis plots visually show the abundance of various substances in the sample.

Figure 3.
A clustered heatmap of VOCs among eight peach cultivars.
3.1.2. Analysis of Odor Activity Values of VOCs in Eight Peach Cultivars
OAV is the main method of aroma characterization and it is also an indicator of whether human can perceive VOCs in samples by olfactory. Here, by calculating the OAV for each compound, we grasped the compounds that contribute to the peaches. The sensory thresholds of each compound and their OAV in the samples are listed in Table 3, and considering that OAV ≥ 1 is required to have a contribution to flavor, we only analyzed this part of the data.

Table 3.
Comparison of odor activity values of different VOCs.
By statistical analysis, only 17 volatiles with OAV ≥ 1 were retained, including 4 aldehydes, 3 esters, 3 terpenoids, 1 ketones, 2 alcohols and 4 lactones. The importance of these compounds was determined by ranking them according to the minimum OAV in each of the eight cultivars: γ-decalactone > β-ionone > hexanal > 2-hexenal > linalool > nonanal > 3-hexenol > δ-decalactone > D-limonene > β-myrcene > 1-hexanol > γ-octalactone > γ-dodecalactone > (Z)-3-hexen-1-yl acetate > butyl acetate > isopentyl acetate > octanal. Although γ-decalactone was not the most abundant in quantity, it showed the highest OAV due to the low threshold value. As the main contributor of peach-like aroma, it is also an essential factor that distinguishes peaches from other fruits [34,35]. The OAV of γ-decalactone was highest in HJML. β-ionone and linalool are the two most dominant floral compounds in peach fruits with extremely low-threshold values. They can exhibit rose and violet flavor despite their low content. In our result, both the amount and OAV of linalool were high in the eight peaches. The OAV ranged from 17.09 to 3730.59, and the highest value was present in JC. Due to the 0.007 μg/kg threshold value, the range of OAV of β-ionone was from 61.43 to 290, and the highest value exhibited in HH. Regarding the grass/green-notes aldehydes and C6 alcohols, hexanal and 3-hexenol were the most active flavor compounds in most investigated cultivars. Their OAV ranged from 24.73 to 226.8 and 7.58 to 63.53. The highest value of these two compounds was observed in JC and JXIU, respectively.
3.2. Volatile Profile of Different Peach Cultivars Using GC-IMS
3.2.1. Differential Analysis of the Topographic Plots of VOCs in Eight Cultivars Using GC-IMS
The VOC profiles of eight peaches were also constructed using GC-IMS. The 3D topographic plot of the peach volatiles showed that the composition and signal intensities of VOCs varied considerably between different peach cultivars (Figure 4a). To observe the data more conveniently and intuitively, we normalized the ion migration time and the reaction ion peak, and obtained a two-dimensional top-view plot of the ion migration spectrum (Figure 4b). The red line at the horizontal coordinate 1.0 in the graph is the RIP peak. Each point to the right of the RIP peak represents a volatile and the color of the point represents the concentration of the compound, with white to red indicating low-to-high concentrations [36]. Most of the signal is concentrated between drift times of 1.0–1.9 ms and retention times of 100–700 s. It is clear from the graph that the signal intensity of VOCs appearing between retention times of 500–700 s varies considerably between samples. To compare the differences more obviously between samples, a difference comparison model can be used, where the spectrum of JC is selected as a reference and the spectra of the other samples are deducted from the reference. If the VOCs of both are the same, the background after deduction is white, while red means that the concentration of the substance is higher than the reference, and blue means that the concentration of the substance is lower than the reference [37]. JXIU, HJML and XFML showed significantly higher concentrations of VOCs in the retention time range of 500–600 s than the other samples. It is clearly seen that VOCs in the retention time range of 350–450 s and ion drift times of 1.6 ms are specific in the HH. The substances in the green-dotted box are significantly more concentrated in the HJML than in the other samples. The location and quantities of VOC peaks in the samples are approximately the same in the spectra, but there are differences in peak strength, indicating that the content of VOCs is determined by the peach cultivars.

Figure 4.
VOCs of eight peach cultivars determined by GC-IMS. (a) The 3D topographic plot of the peach VOCs. (b) The 2D topographic plots. (c) The difference comparison topographic plots.
3.2.2. The Volatile Fingerprints of Eight Cultivars Using GC-IMS
The results of the qualitative analysis of the VOCs of the sample using GC-IMS are shown in Table 4. According to the NIST gas phase retention index database and the IMS migration time database built into the flavor analyzer (FlavourSpec®) software, 53 signal peaks were detected and 26 typical volatiles were identified, including 11 aldehydes, 5 esters, 4 alcohols, 2 ketones, 2 terpenes and 2 furans. There are 12 substances that were not identified. The most abundant substances detected in peach fruit using GC-IMS in this study were aldehydes, while other researchers [31,38] have detected esters, which are closely related to factors such as peach ripeness and variety selection. The compounds of 2-hexenal, 1-hexanal, acetic acid ethyl ester, benzaldehyde, 1-hexanol, methyl acetate, 2-butanone, pentanal, 2-methylbutanal, 3-methyl butanal, (Z)-3-hexenyl acetate, 2-methylpropyl acetate and (E)-2-pentenal exhibited more than two peaks. This is due to the fact that during ionic drift, when the concentration of a substance increases, two or more molecules will share a proton or electron, forming a dimer or monomers [14,39]. The 53 signals obtained were used to draw fingerprint profiles based on peak intensities to compare the differences in volatiles between samples, which can give clearer results (Figure 5). Each row of the figure indicates the signal peaks of all volatiles in the same sample, and each column indicates the signal intensity of the same compounds in different samples. The areas where the signal is more pronounced are boxed out for easier analysis.

Table 4.
Identification of VOCs in eight peach cultivars using GC-IMS.
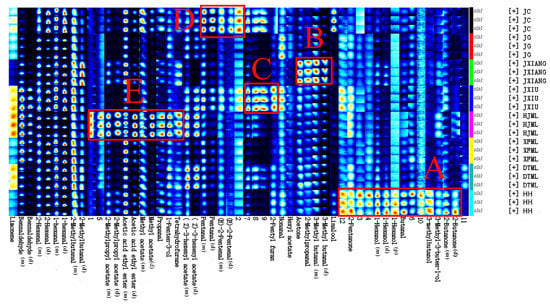
Figure 5.
The VOCs fingerprints of different peach cultivars using GC-IMS. Box (A) 14 compounds highly accumulated in cv. ‘HH’; Box (B) four compounds highly accumulated in ‘JXIANG’; Box (C) four compounds highly accumulated in ‘JXIIU’; Box (D) five compounds highly accumulated in cv. ‘JC’; Box (E) eleven compounds highly accumulated in ‘HJML’.
As shown in box A, some alcohols, ketones and unknown compounds were the most abundant in the HH, and these were the main reasons for the differences in the overall fingerprint profile of the HH compared to the other samples. Aldehydes, namely (E)-2-pentenal, 3-methyl butanal, 2-methylpropanal and pentanal, dominated in JC and JXIANG (box B and D), which differed from the GC-MS, where aldehydes were mainly dominated by 2-hexenal and hexanal. Among the overall peak signals of JXIU, box C had the strongest signals of substances that were not characterized; it is therefore necessary to characterize these unknown compounds in future research. Box E demonstrates that HJML had high concentrations of methyl acetate, acetic acid ethyl ester, 2-methylpropyl acetate, and the flavor of these compounds is discriminatingly fruity. None of these were found in GC-MS. As we can see, 12 unknown compounds have strong peak signals in the fingerprints, indicating that these compounds were present at high levels in the samples, and characterizing these substances is essential for the establishment of a complete GC-IMS-based peach fingerprint.
Combining Table 2 and Table 4, the types of VOCs detected by the two techniques differed significantly, with GC-MS detecting more than GC-IMS. Lactones, typical VOCs in peaches, were not found in GC-IMS due to their high molecular weight, which makes them difficult to be detected. Although both techniques are used in conjunction with GC, their focus is dissimilar. GC-MS works under a vacuum and at a high temperature and focuses on the accuracy of qualitative and quantitative information, while GC-IMS performs detection at atmospheric pressure and normal temperature and focuses more on reflecting the authenticity of the sample flavor [40,41].
3.2.3. Similarity Analysis of Samples between Different Peach Cultivars Based on PCA
PCA was performed on the volatile compound content (signal peak volume) of different cultivars of peaches using the Dynamic PCA plug-in, and the data were visualized to obtain a spatial–dimensional plot containing three principal components, where the X, Y and Z axes indicate the first, second and third principal components, respectively (Figure 6). The cumulative contribution rate of PC1, PC2 and PC3 was 65.7%. The results show that the eight peach cultivars were clearly distinguished based on the identified VOCs using GC-IMS. JC and JXIU are located on the right side of the PC1-axis (Figure 6a), indicating that the content of characteristic aroma compounds in these two cultivars were more similar, which might be caused by the fact that JC was an F1 offspring of JXIU. DTML, XFML, JG and JXIANG are close together and can be grouped together, while HH and HJML are located at the ends of the PC3 and PC2 axes, which means that they are relatively different from the other samples based on the VOC performance (Figure 6a). The difference between samples in a group is small, while the differences between groups are more obvious. Overall, the aroma profiles of JC, HJML and HH are more specific compared to the other samples. This result is highly consistent with that of the cluster analysis in Figure 3. The PCA loading plot showed that (E)-2-pentenal, 2-hexenal and pentanal highly contributed to the JC’s aroma, and 1-hexanol, 3-methylbutanol highly contributed to the HH aroma. These results are consistent with the GC-IMS fingerprints. These findings suggest that the aroma profiles of these eight popular peaches are quite different for selecting strong/specific aroma cultivars.

Figure 6.
Principal component analysis (PCA) score plot (a) and PCA loading plot of different peach cultivars (b).
4. Conclusions
The aroma profiles of eight popular peach cultivars produced in Shanghai were comprehensively analyzed using GC–MS, OAV determination and GCIMS. Seventy VOCs were detected using GC-MS, including nine aldehydes, ten esters, eleven terpenoids, nine alcohols, six lactones, five ketones, five alkanes, eleven aromatic hydrocarbons, two furans and two other compounds, of which twenty-three VOCs were commonly identified in eight peach cultivars. The most VOCs were detected in HJML and the least in JG. Cluster analysis classified XFML, JG, DTML and JXIU into one cluster, which means that their aroma profiles are more similar.
In addition, based on an OAV ≥ 1, a total of 17 key aroma compounds were screened, and their contributions were ranked as follows: γ- decalactone> β- ionone > hexanal > 2-hexenal > linalool > nonanal > 3-hexenol > δ-decalactone > D-limonene > β-myrcene > 1-hexanol > γ-octalactone > γ-dodecalactone > (Z)-3-hexen-1-yl acetate > butyl acetate > isopentyl acetate > octanal. The OAV of VOCs differed greatly among the cultivars. Lactones had the highest OAV in HJML, which indicates that HJML is the cultivar that contains the most fruity notes. Terpenoids had the highest OAV in JC, meaning that JC had the strongest floral notes. It is obvious that JXIU was the cultivar with the lightest aroma.
Furthermore, a total of twenty-six VOCs were detected using GC-IMS, among which aldehydes accounted for the largest proportion. GC-IMS has the advantage of data visualization, which can visualize the differences of volatiles among samples. It can be directly observed in the fingerprints that aldehydes have the strongest signals in samples. The PCA results showed that XFML, JG, DTML and JXIU were close to each other, whereas HJML, HH and JC were distant from other samples, which is similar to the results of the cluster analysis. The differences in focus and working conditions between GC-MS and GC-IMS led to significant differences in the composition of the VOCs detected by them. The combination of GC-MS and GC-IMS can capture relatively complete flavor information of samples, which is a hot research topic in the field of flavor at present and will be for a long time in the future.
Author Contributions
Conceptualization, X.L., X.Y. and Z.Y; methodology, X.W. and X.L.; software, X.W.; validation, X.L.; formal analysis, X.W. and X.L.; investigation, X.L.; resources, M.S., M.Z., J.D., H.Z. and X.Z.; data curation, X.W. and Y.H.; writing—original draft preparation, X.W. and X.L.; writing—review and editing, X.L., Z.Y. and X.Y.; visualization, X.W.; supervision, Z.Y. and X.Y.; project administration, X.L. and Z.Y.; funding acquisition, X.L. and Z.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funds from the National Key Research and Development Program of China (2019YFD1000801), the Shanghai Science and Technology committee Rising-Star Program (19QB1404600), Hu Nong Ke Chuang Zi-2021-1-1, the Shanghai Municipal Science and Technology Commission 2019 Science and Technology innovation Action Plan Agricultural Project (19391903400), the Outstanding Team Program of Shanghai Academy of Agricultural Science (Grant No. ZY2022–004), the Pangao Plan of Shanghai Academy of Agricultural Science, the Qiankehe Basics [2019]1408 and the Qiankehe Platform Talent [2018]5781.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, X.; Meng, X.; Jia, H.; Yu, M.; Ma, R.; Wang, L.; Cao, K.; Shen, Z.; Niu, L.; Tian, J.; et al. Peach genetic resources: Diversity, population structure and linkage disequilibrium. BMC Genet. 2013, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L. Genetic resources, breeding programs in China, and gene mining of peach: A review. Hortic. Plant J. 2020, 6, 205–215. [Google Scholar] [CrossRef]
- Aranzana, M.; Abbassi, E.; Howad, W.; Arús, P. Genetic Variation, population structure and linkage disequilibrium in peach commercial varieties. BMC Genet. 2010, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, J.; Zhang, L.; Yu, Y.; Ye, Z.; Wang, X.; Zhou, J.; Chai, M.; Zhang, H.; Arús, P.; et al. Identification of volatile and softening-related genes using digital gene expression profiles in melting peach. Tree Genet. Genomes. 2015, 11, 71. [Google Scholar] [CrossRef]
- Zhang, X.; Su, M.; Du, J.; Zhou, H.; Li, X.; Zhang, M.; Hu, Y.; Ye, Z. Analysis of the free amino acid content and profile of 129 peach (Prunus Persica (L.) Batsch) germplasms using LC-MS/MS without derivatization. J. Food Compos. Anal. 2022, 114, 104811. [Google Scholar] [CrossRef]
- Aubert, C.; Milhet, C. Distribution of the volatile compounds in the different parts of a white-fleshed peach (Prunus Persica L. Batsch). Food Chem. 2007, 102, 375–384. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Li, S.; Yang, L.; Wang, Y.; Zhao, J.; Jiang, Q. Volatile characteristics of 50 peaches and nectarines evaluated by HP–SPME with GC–MS. Food Chem. 2009, 116, 356–364. [Google Scholar] [CrossRef]
- Zhang, B.; Shen, J.; Wei, W.; Xi, W.; Xu, C.; Ferguson, I.; Chen, K. Expression of genes associated with aroma formation derived from the fatty acid pathway during peach fruit ripening. J. Agric. Food Chem. 2010, 58, 6157–6165. [Google Scholar] [CrossRef]
- Jia, H.; Araki, A.; Okamoto, G. Influence of fruit bagging on aroma volatiles and skin coloration of ‘Hakuho’ peach (Prunus Persica Batsch). Postharvest Biol. Technol. 2005, 35, 61–68. [Google Scholar] [CrossRef]
- Toselli, M.; Baldi, E.; Marangoni, B.; Noferini, M.; Fiori, G.; Bregoli, A.; Ndagijimana, M. Effects of mineral and organic fertilization and ripening stage on the emission of volatile organic compounds and antioxidant activity of “Stark redgold” nectarine. Acta Hortic. 2010, 868, 381–388. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Liu, C.; Xu, M.; Li, S.; Yang, L.; Wang, Y. Effects of bagging on volatiles and polyphenols in “Wanmi” peaches during endocarp hardening and final fruit rapid growth stages. J. Food Sci. 2010, 75, S455–S460. [Google Scholar] [CrossRef]
- Grosch, W. Evaluation of the key odorants of foods by dilution experiments, aroma models and omission. Chem. Senses. 2001, 26, 533–545. [Google Scholar] [CrossRef]
- Rothe, M.; Thomas, B. Aromastoffe des Brotes. Eur. Food Res. Technol. 1963, 119, 302–310. [Google Scholar] [CrossRef]
- Ruiz, M.; Rodríguez, M.; Flores, G.; Blanch, G. New method based on solid phase microextraction and multidimensional gas chromatography-mass spectrometry to determine pesticides in strawberry jam. LWT 2019, 99, 283–290. [Google Scholar] [CrossRef]
- Cavanna, D.; Zanardi, S.; Dall, C.; Suman, M. Ion mobility spectrometry coupled to gas chromatography: A rapid tool to assess eggs freshness. Food Chem. 2019, 271, 691–696. [Google Scholar] [CrossRef]
- Qi, H.; Ding, S.; Pan, Z.; Li, X.; Fu, F. Characteristic volatile fingerprints and odor activity values in different citrus-tea by HS-GC-IMS and HS-SPME-GC-MS. Molecules 2020, 25, 6027. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography–ion mobility spectrometry (GC–IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Van Gemert, L.J. Odour Thresholds-Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter & Partners: Utrecht, The Netherlands, 2003. [Google Scholar]
- Hadi, M.; Zhang, F.; Wu, F.; Zhou, C.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef]
- Mihaylova, D.; Popova, A.; Vrancheva, R.; Dincheva, I. HS-SPME-GC–MS volatile profile characterization of peach (Prunus Persica L. Batsch) Varieties grown in the eastern Balkan Peninsula. Plants 2022, 11, 166. [Google Scholar] [CrossRef]
- Xi, W.; Zheng, Q.; Lu, J.; Quan, J. Comparative Analysis of three types of peaches: Identification of the key individual characteristic flavor compounds by integrating consumers’ acceptability with flavor quality. Hortic. Plant J. 2017, 3, 1–12. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, Z. Characterization of the key aroma compounds in peach by gas chromatography–olfactometry, quantitative measurements and sensory analysis. Eur. Food Res. Technol. 2019, 245, 129–141. [Google Scholar] [CrossRef]
- Tan, F.; Wang, P.; Zhan, P.; Tian, H. Characterization of key aroma compounds in flat peach juice based on gas chromatography-mass spectrometry-olfactometry (GC-MS-O), odor activity value (OAV), aroma recombination, and omission experiments. Food Chem. 2022, 366, 130604. [Google Scholar] [CrossRef] [PubMed]
- Horvat, R.; Chapman, G.; Robertson, J.; Meredith, F.; Scorza, R.; Callahan, A.; Morgens, P. Comparison of the volatile compounds from several commercial peach cultivars. J. Agric. Food Chem. 1990, 38, 234–237. [Google Scholar] [CrossRef]
- Robertson, J.; Meredith, F.; Horvat, R.; Senter, S. Effect of cold storage and maturity on the physical and chemical characteristics and volatile constituents of peaches (Cv. Cresthaven). J. Agric. Food Chem. 1990, 38, 620–624. [Google Scholar] [CrossRef]
- Chapman, G.; Horvat, R.; Forbus, W. Physical and chemical changes during the maturation of peaches (Cv. Majestic). J. Agric. Food Chem. 1991, 39, 867–870. [Google Scholar] [CrossRef]
- Eduardo, I.; Chietera, G.; Bassi, D.; Rossini, L.; Vecchietti, A. Identification of key odor volatile compounds in the essential oil of nine peach accessions. J. Sci. Food Agric. 2010, 90, 1146–1154. [Google Scholar] [CrossRef]
- Brodkorb, D.; Gottschall, M.; Marmulla, R.; Lüddeke, F.; Harder, J. Linalool dehydratase-isomerase, a bifunctional enzyme in the anaerobic degradation of monoterpenes. J. Biol. Chem. 2010, 285, 30436–30442. [Google Scholar] [CrossRef]
- Engel, K.; Flath, R.; Buttery, R.; Mon, T.; Ramming, D.; Teranishi, R. Investigation of volatile constituents in nectarines. 1. analytical and sensory characterization of aroma components in some nectarine cultivars. J. Agric. Food Chem. 1988, 36, 549–553. [Google Scholar] [CrossRef]
- Mohammed, J.; Belisle, C.; Wang, S.; Itle, R.; Adhikari, K.; Chavez, D. Volatile Profile Characterization of Commercial Peach (Prunus Persica) Cultivars Grown in Georgia, USA. Horticulturae 2021, 7, 516. [Google Scholar] [CrossRef]
- Sun, P.; Xu, B.; Wang, Y.; Lin, X.; Chen, C.; Zhu, J.; Jia, H.; Wang, X.; Shen, J.; Feng, T. Characterization of volatile constituents and odorous compounds in peach (Prunus Persica L.) fruits of different varieties by gas chromatography–ion mobility spectrometry, gas chromatography–mass spectrometry, and relative odor activity value. Front. Nutr. 2022, 9, 965796. [Google Scholar] [CrossRef]
- Brandi, F.; Bar, E.; Mourgues, F.; Horváth, G.; Turcsi, E.; Giuliano, G.; Liverani, A.; Tartarini, S.; Lewinsohn, E.; Rosati, C. Study of “Redhaven” peach and its white-fleshed mutant suggests a key role of CCD4 carotenoid dioxygenase in carotenoid and norisoprenoid volatile metabolism. BMC Plant Biol. 2011, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Adami, M.; Defranceschi, P.; Brandi, F.; Liverani, A.; Giovannini, D.; Rosati, C.; Dondini, L.; Tartarini, S. Identifying a carotenoid cleavage dioxygenase (ccd4) gene controlling yellow/white fruit flesh color of peach. Plant Mol. Biol. Report. 2013, 31, 1166–1175. [Google Scholar] [CrossRef]
- Spencer, M.; Pangborn, R.; Jennings, W. Gas chromatographic and sensory analysis of volatiles from cling peaches. J. Agric. Food Chem. 1978, 26, 725–732. [Google Scholar] [CrossRef]
- Aubert, C.; Günata, Z.; Ambid, C.; Baumes, R. Changes in physicochemical characteristics and volatile constituents of yellow- and white-fleshed nectarines during maturation and artificial ripening. J. Agric. Food Chem. 2003, 51, 3083–3091. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, R.; Zhang, H.; Wang, S.; Chen, D.; Lin, S. Development of a flavor fingerprint by HS-GC–IMS with PCA for volatile compounds of Tricholoma Matsutake Singer. Food Chem. 2019, 290, 32–39. [Google Scholar] [CrossRef]
- Feng, T.; Sun, J.; Song, S.; Wang, H.; Yao, L.; Sun, M.; Wang, K.; Chen, D. Geographical differentiation of Molixiang table grapes grown in China based on volatile compounds analysis by HS-GC-IMS coupled with PCA and sensory evaluation of the grapes. Food Chem. X 2022, 15, 100423. [Google Scholar] [CrossRef]
- Leng, P.; Hu, H.; Cui, A.; Tang, H.; Liu, Y. HS-GC-IMS with PCA to analyze volatile flavor compounds of honey peach packaged with different preservation methods during storage. LWT 2021, 149, 111963. [Google Scholar] [CrossRef]
- Rodríguez, R.; Vyhmeister, E.; Meisen, S.; Rosales, A.; Kuklya, A.; Telgheder, U. Identification of terpenes and essential oils by means of static headspace gas chromatography-ion mobility spectrometry. Anal. Bioanal. Chem. 2017, 409, 6595–6603. [Google Scholar] [CrossRef]
- Louw, S. Recent trends in the chromatographic analysis of volatile flavor and fragrance compounds: Annual review 2020. Anal. Sci. Adv. 2021, 2, 157–170. [Google Scholar] [CrossRef]
- Li, S.; Yang, H.; Tian, H.; Zou, J.; Li, J. Correlation analysis of the age of brandy and volatiles in brandy by gas chromatography-mass spectrometry and gas chromatography-ion mobility spectrometry. Microchem. J. 2020, 157, 104948. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).