Thidiazuron Promoted Microspore Embryogenesis and Plant Regeneration in Curly Kale (Brassica oleracea L. convar. acephala var. sabellica)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Isolation of Microspores
2.3. Effect of Genotypes on the Embryogenesis
2.4. TDZ Treatments
2.5. Embryo Germination and Plantlet Regeneration
2.6. Effect of NAA on Root Regeneration
2.7. Ploidy Identification of Regenerated Plants
2.8. Horticultural Characteristics of the DH Lines
2.9. Data Collection and Statistical Analysis
3. Results
3.1. Donor Genotype Is the Primary Intrinsic Factor for the Microspore Embryogenesis
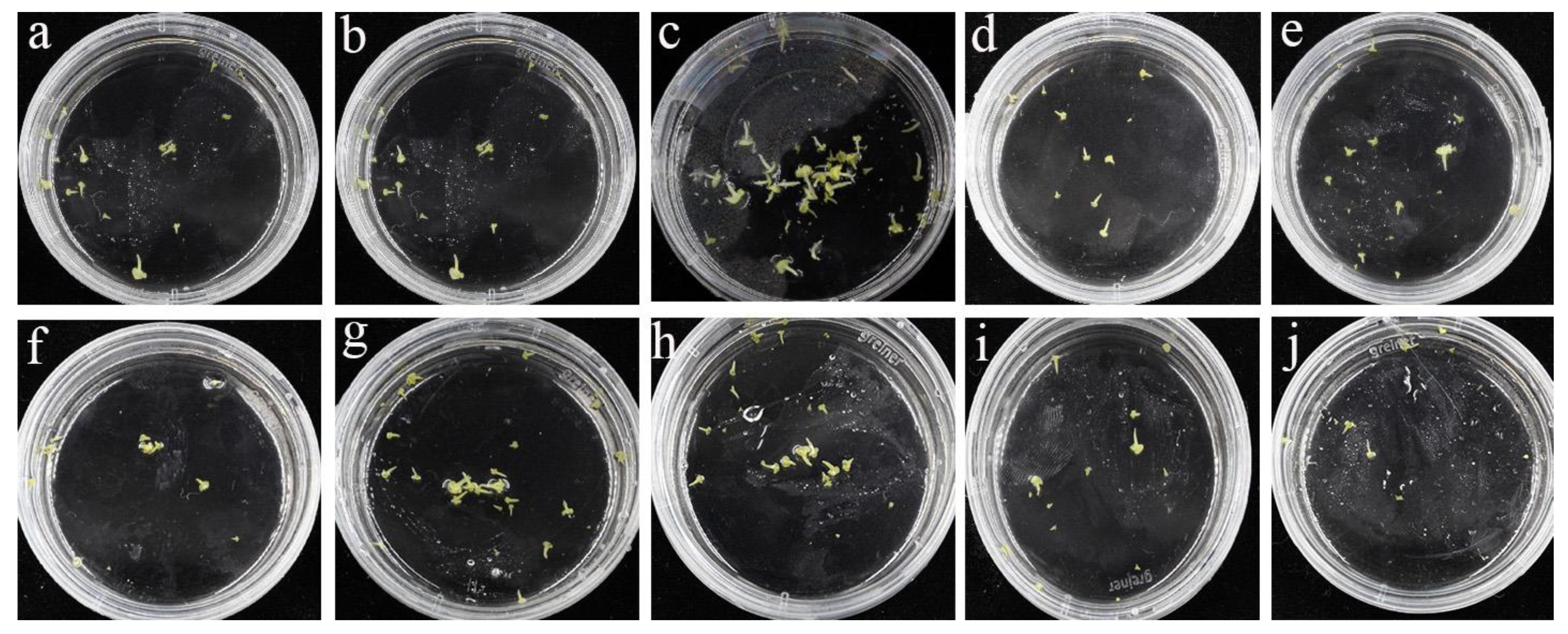
3.2. Effects of TDZ on Microspore-Derived Embryogenesis
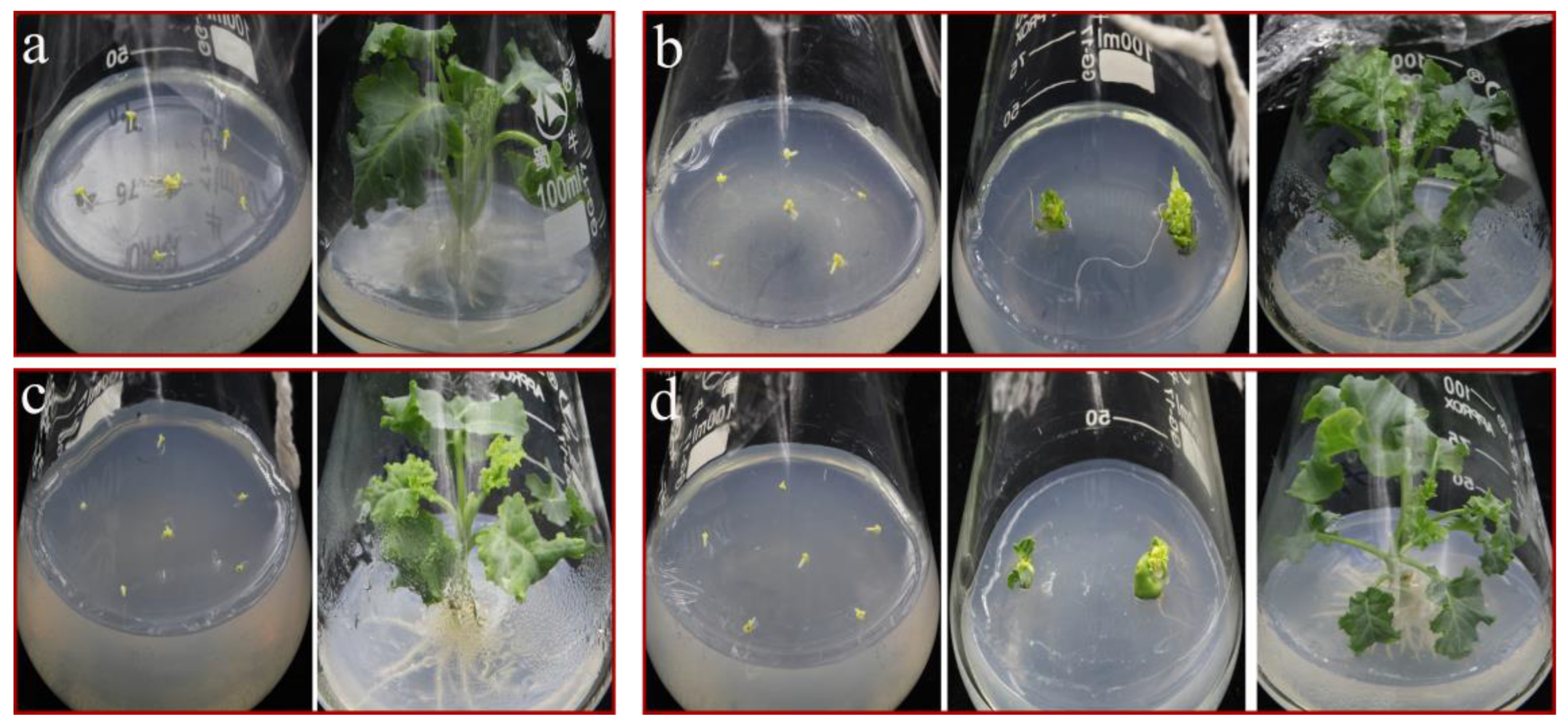
3.3. An Optimal Effect on Plantlet Regeneration was Achieved with 0.2 mg/L TDZ
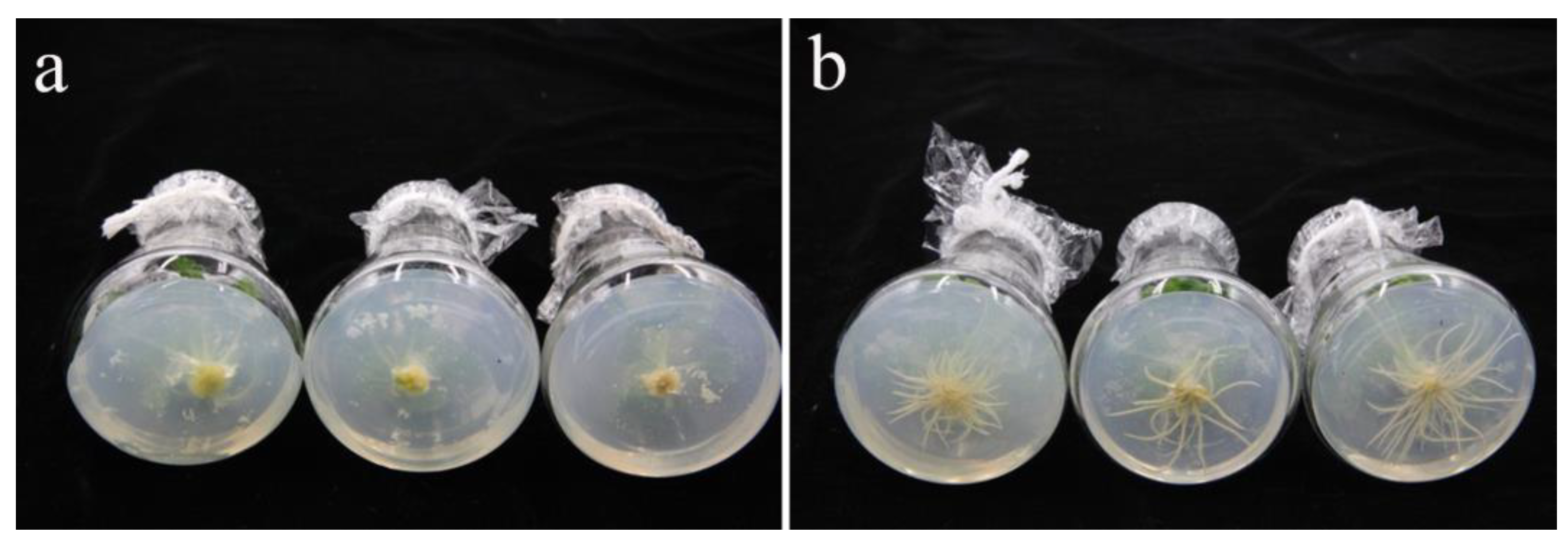
3.4. Half Strength MS Medium Is Beneficial to the Rooting of ‘Starbor F2’ Regenerated Seedlings
3.5. The Tested Genotypes Exhibited Similar Chromosome Doubling Efficiencies
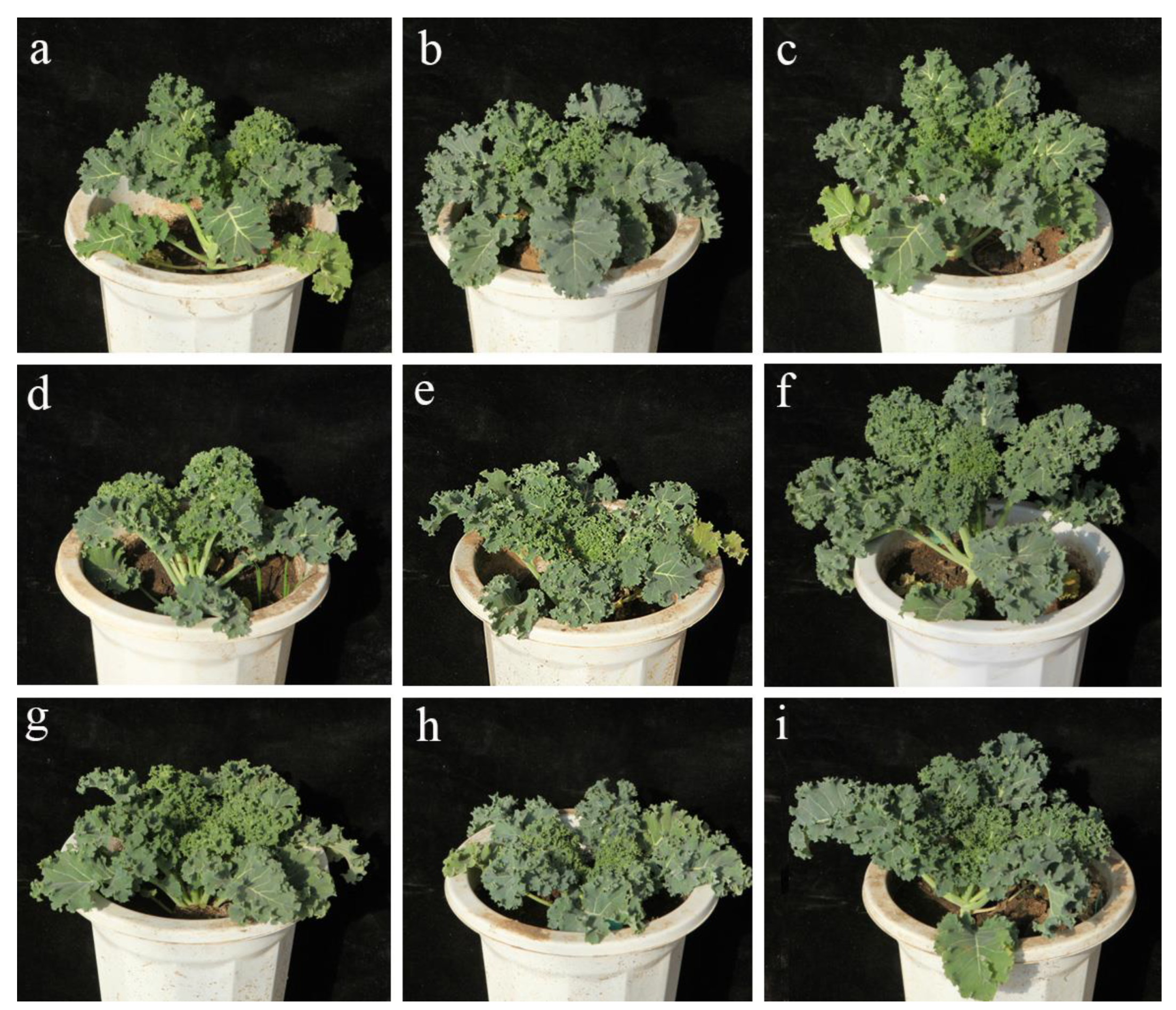
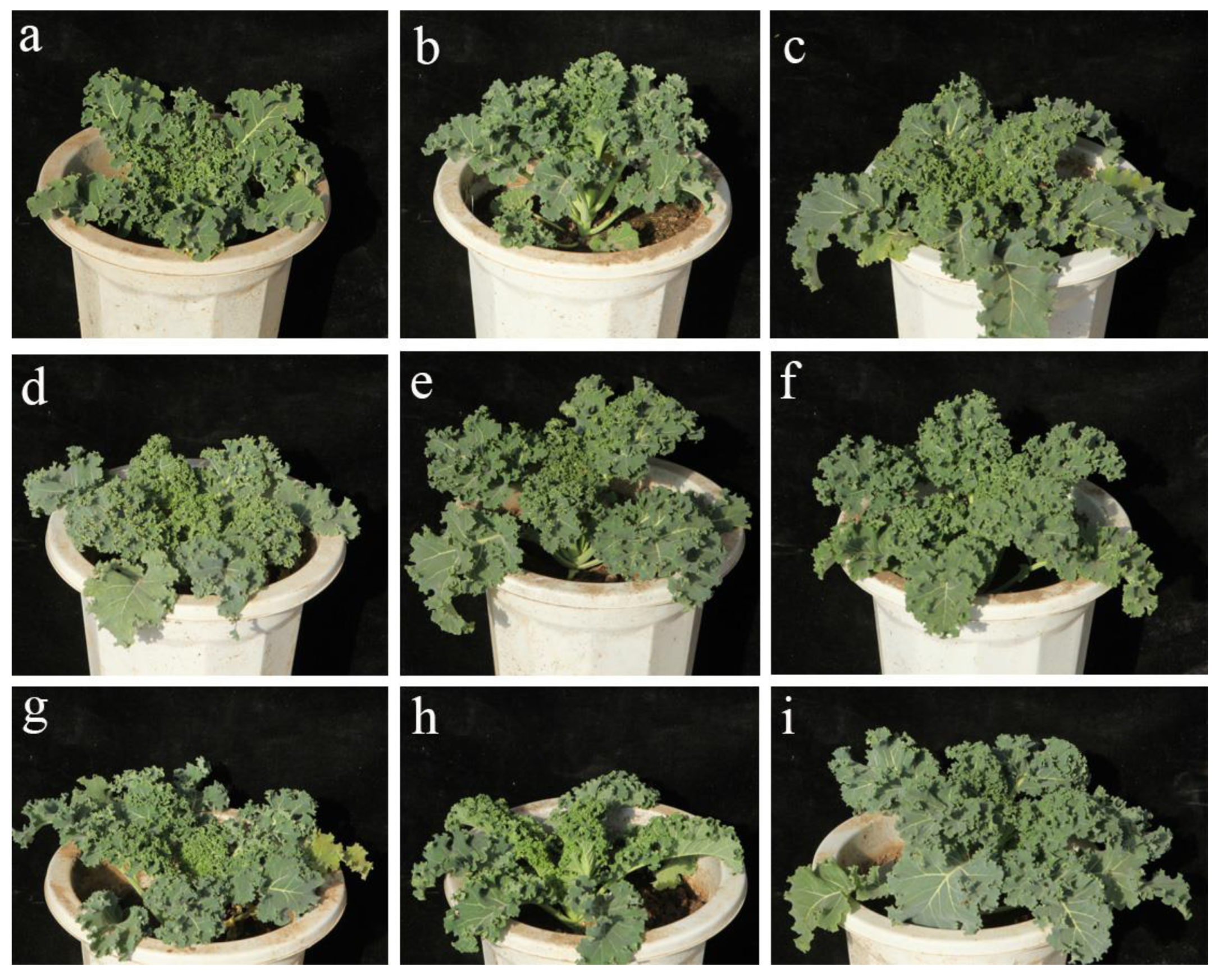
3.6. Horticultural Characteristics of the DH Lines
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Olsen, H.; Grimmer, S.; Aaby, K.; Saha, S.; Borge, G.I. Antiproliferative effects of fresh and thermal processed green and red cultivars of curly kale (Brassica oleracea L. convar. acephala var. sabellica). J. Agric. Food Chem. 2012, 60, 7375–7383. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Urlić, B.; Salopek-Sondi, B. Kale (Brassica oleracea var. acephala) as a superfood: Review of the scientific evidence behind the statement. Crit. Rev. Food Sci. Nutr. 2018, 59, 2411–2422. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.M.; Yu-Chun, C.; Kang-Mo, K. Glucosinolates, Carotenoids, and Vitamins E and K Variation from Selected Kale and Collard Cultivars. J. Food Qual. 2017, 2017, 5123572 . [Google Scholar]
- Korus, A. Amino Acid Retention and Protein Quality in Dried Kale (Brassica oleracea L. var. acephala). J. Food Process. Pres. 2014, 38, 1–8. [Google Scholar] [CrossRef]
- Sikora, E.; Bodziarczyk, I. Composition and antioxidant activity of kale (Brassica oleracea L. var. acephala) raw and cooked. Acta Sci. Pol. Technol. Aliment. 2012, 11, 239–248. [Google Scholar]
- Russell, L.B. Brassicas: Cooking the World`s Healthiest Vegetables: Kale, Cauliflower, Broccoli, Brussels Sprouts and More; Ten Speed Press: Berkeley, CA, USA, 2014. [Google Scholar]
- Ari, E.; Bedir, H.; Mutlu, N. Enhancement of embryogenesis in freshly isolated microspore cultures of ornamental kale through direct cold shock treatment. Sci. Hortic. 2021, 280, 1–5. [Google Scholar] [CrossRef]
- Olsen, H.; Aaby, K.; Borge, G.I. Characterization, quantification, and yearly variation of the naturally occurring polyphenols in a common red variety of curly kale (Brassica oleracea L. convar. acephala var. sabellica cv. ‘Redbor’). J. Agric. Food Chem. 2010, 58, 11346–11354. [Google Scholar] [CrossRef]
- Li, H.; Devaux, P. Isolated microspore culture overperforms anther culture for green plant regeneration in barley (Hordeum vulgare L.). Acta Physiol. Plant 2005, 27, 611–619. [Google Scholar] [CrossRef]
- Lichter, R. Induction of haploid plants from isolated pollen of Brassica napus . Z. Pfanzenphysiol. 1982, 105, 427–434. [Google Scholar] [CrossRef]
- Yuan, S.X.; Su, Y.B.; Liu, Y.M.; Li, Z.S.; Fang, Z.Y.; Yang, L.M.; Zhuang, M.; Zhang, Y.Y.; Lv, H.H.; Sun, P.T. Chromosome Doubling of Microspore-Derived Plants from Cabbage (Brassica oleracea var. capitata L.) and Broccoli (Brassica oleracea var. italica L.). Front. Plant. Sci. 2015, 6, 1118. [Google Scholar] [CrossRef]
- Bhatia, R.; Dey, S.S.; Sood, S.; Sharma, K.; Parkash, C.; Kumar, R. Effificient microspore embryogenesis in cauliflflower (Brassica oleracea var. Botrytis L.) for development of plants with different ploidy level and their use in breeding programme. Sci. Hortic. 2017, 216, 83–92. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, Y.; Feng, H. Effects of brassinolide on microspore embryogenesis and plantlet regeneration in pakchoi (Brassica rapa var. multiceps). Sci. Hortic. 2019, 252, 354–362. [Google Scholar] [CrossRef]
- Zhang, N.; Yin, J.M.; Lin, N.; Tang, Z.L.; Li, J.N. Free Microspore Culture and Plantlet Regeneration of Brassica alboglabra with Yellow Flowers. Guizhou Agric. Sci. 2009, 37, 43–47. [Google Scholar]
- Niu, L.J.; Shi, F.Y.; Feng, H.; Zhang, Y. Efficient doubled haploid production in microspore culture of Zengcheng flowering Chinese cabbage (Brassica campestris L. ssp. chinensis [L.] Makino var. utilis Tsen et Lee). Sci. Hortic. 2019, 245, 57–64. [Google Scholar] [CrossRef]
- Zhang, W.; Fu, Q.; Dai, X.G.; Bao, M.Z. The culture of isolated microspores of ornamental kale (Brassica oleracea var. acephala) and the importance of genotype to embryo regeneration. Sci. Hortic. 2008, 117, 69–72. [Google Scholar] [CrossRef]
- Chen, W.S.; Zhang, Y.; Huang, S.N.; Ren, J.; Feng, H. l-Ascorbic acid sodium salt promotes microspore embryogenesis and chromosome doubling by colchicine in ornamental kale (Brassica oleracea var. acephala). Plant. Cell Tiss. Organ. Cult. 2022, 149, 753–765. [Google Scholar] [CrossRef]
- Dai, X.G.; Shi, X.P.; Bao, M.Z. Improvement of isolated microspore culture of ornamental kale (Brassica oleracea var. acephala): Effects of sucrose concentration, medium replacement, and cold pre-treatment. J. Hortic. Sci. Biotech. 2009, 84, 519–525. [Google Scholar] [CrossRef]
- Liu, C.H.; Song, G.X.; Fang, B.; Liu, Z.Y.; Zou, J.Q.; Dong, S.Y.; Du, S.; Ren, J.; Feng, H. Suberoylanilide hydroxamic acid induced microspore embryogenesis and promoted plantlet regeneration in ornamental kale (Brassica oleracea var. acephala). Protoplasma 2022, 260, 117–129. [Google Scholar] [CrossRef]
- Gao, Y.; Jia, J.X.; Cong, J.L.; Ma, Y.Y.; Feng, H.; Zhang, Y. Non-ionic surfactants improved microspore embryogenesis and plant regeneration of recalcitrant purple flowering stalk (Brassica campestris ssp. chinensis var. purpurea Bailey). In Vitro Cell Dev. Biol. Plant 2020, 56, 207–214. [Google Scholar] [CrossRef]
- Popova, T.; Grozeva, S.; Todorova, V.; Stankova, G.; Anachkov, N.; Rodeva, V. Effects of low temperature, genotype and culture media on in vitro androgenic answer of pepper (Capsicum annuum L.). Acta Physiol. Plant 2016, 38, 273. [Google Scholar] [CrossRef]
- Takahashi, Y.; Yokoi, S.; Takahata, Y. Effects of genotypes and culture conditions on microspore embryogenesis and plant regeneration in several subspecies of Brassica rapa L. Plant Biotechnol. Rep. 2012, 6, 297–304. [Google Scholar] [CrossRef]
- Yang, S.; Liu, X.L.; Fu, Y.; Zhang, X.; Li, Y.F.; Liu, Z.Y.; Feng, H. The effect of culture shaking on microspore embryogenesis and embryonic development in Pakchoi (Brassica rapa L. ssp. chinensis). Sci. Hortic. 2013, 152, 70–73. [Google Scholar] [CrossRef]
- Bhatia, R.; Dey, S.S.; Parkash, C.; Sharma, K.; Sood, S.; Kumar, R. Modification of important factors for efficient microspore embryogenesis and doubled haploid production in field grown white cabbage (Brassica oleracea var. capitata L.) genotypes in India. Sci. Hortic. 2018, 233, 178–187. [Google Scholar] [CrossRef]
- Cao, M.Q.; Charlot, F.; Dore, C. Embryogenesis and plant regeneration of sauerkraut cabbage (Brassica oleracea L. ssp. capitata) via in vitro isolated microspore culture. Comptes Rendus Lacadémie Sci. 1990, 310, 203–209. [Google Scholar]
- Kuginuki, Y.; Nakamura, K.; Hida, K.I.; Yosikawa, H. Varietal Differences in Embryogenic and Regenerative Ability in Microspore Culture of Chinese Cabbage (Brassica rapa L. ssp. pekinensis). Breed. Sci. 1997, 47, 341–346. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, W.F.; Li, J.; Qi, X.H.; Feng, H.; Zhang, Y. Effects of genotype and sodium p-nitrophenolate on microspore embryogenesis and plant regeneration in broccoli (Brassica oleracea L. var. italica). Sci. Hortic. 2022, 293, 110711. [Google Scholar] [CrossRef]
- Esteves, P.; Belzile, F. Improving the efficiency of isolated microspore culture in six-row spring barley: I-optimization of key physical factors. Plant Cell Rep. 2014, 33, 993–1001. [Google Scholar] [CrossRef]
- Khan, S.; Naz, S.; Ali, K.; Zaidi, S. Direct organogenesis of Kalanchoe tomentosa (Crassulaceae) from shoot-tips. Pak. J. Bot. 2006, 38, 977–981. [Google Scholar]
- Varga, A.; Thoma, L.H.; Bruinsma, J. Effects of auxins and cytokinins on epigenetic instability of callus propagated Kalanchoe blossfeldiana Poelln. Plant Cell Tiss. Organ. Cult. 1988, 15, 223–231. [Google Scholar] [CrossRef]
- Ahmadi, B.; Shariatpanahi, M.E.; Teixeira da Silva, J.A. Efficient induction of microspore embryogenesis using abscisic acid, jasmonic acid and salicylic acid in Brassica napus L. Plant Cell Tiss. Organ. Cult. 2014, 116, 343–351. [Google Scholar] [CrossRef]
- Gu, H.H.; Sheng, X.G.; Zhao, Z.Q.; Yu, H.F.; Wang, J.S. Initiation and development of microspore embryogenesis and plant regeneration of Brassica nigra . In Vitro Cell Dev. Biol. Plant 2014, 50, 534–540. [Google Scholar] [CrossRef]
- Cappelletti, R.; Sabbadini, S.; Mezzetti, B. The use of TDZ for the efficient in vitro regeneration and organogenesis of strawberry and blueberry cultivars. Sci. Hortic. 2016, 207, 117–124. [Google Scholar] [CrossRef]
- Srivastava, S.; Krishna, R.; Sinha, R.P.; Singh, M. TDZ-induced plant regeneration in Brassica oleracea L. var. botrytis: Effect of antioxidative enzyme activity and genetic stability in regenerated plantlets. In Vitro Cell Dev. Biol. Plant 2017, 53, 598–605. [Google Scholar] [CrossRef]
- Taha, R.A.; Allam, M.A.; Hassan, S.A.M.; Bakr, B.M.M.; Hassan, M.M. Thidiazuron-induced direct organogenesis from immature inflorescence of three date palm cultivars. J. Genet. Eng. Biotechnol. 2021, 19, 14. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, N.V.; Rivas, E.B.; Cardoso, J.C. Somatic embryogenesis from flower tepals of Hippeastrum aiming regeneration of virus-free plants. Plant Sci. 2022, 317, 111191. [Google Scholar] [CrossRef]
- Jia, J.X.; Zhang, Y.; Cui, L.W.; Feng, H. Effect of thidiazuron on microspore embryogenesis and plantlet regeneration in Chinese flowering cabbage (Brassica rapa. var. parachinenis). Plant Breed. 2019, 138, 916–924. [Google Scholar] [CrossRef]
- Xiong, Y.P.; Chen, S.Y.; Wu, T.; Wu, K.L.; Li, Y.; Zhang, X.H.; da Silva, J.A.T.; Zeng, S.J.; Ma, G.H. Shoot organogenesis and somatic embryogenesis from leaf and petiole explants of endangered Euryodendron excelsum. Sci. Rep. 2022, 12, 20506. [Google Scholar] [CrossRef]
- Niu, R.Q.; Zhang, Y.; Tong, Y.; Liu, Z.Y.; Wang, Y.H.; Feng, H. Effects of p-chlorophenoxyisobutyric acid, arabinogalactan, and activated charcoal on microspore embryogenesis in kale. Genet. Mol. Res. 2015, 14, 3897–3909. [Google Scholar] [CrossRef]
- Liu, C.H.; Song, G.X.; Zhao, Y.H.; Fang, B.; Liu, Z.Y.; Ren, J.; Feng, H. Trichostatin A induced microspore embryogenesis and promoted plantlet regeneration in ornamental kale (Brassica oleracea var. acephala). Horticulturae 2022, 8, 790. [Google Scholar] [CrossRef]
- Sato, T.; Nishio, T.; Hirai, M. Plant regeneration from isolated microspore cultures of Chinese cabbage (Brassica campestris spp. pekinensis). Plant Cell Rep. 1989, 8, 486–488. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, A.J.; Liu, Y.; Wang, Y.S.; Feng, H. Improved production of doubled haploids in Brassica rapa through microspore culture. Plant Breed. 2012, 131, 164–169. [Google Scholar] [CrossRef]
- Fang, S.Y.; Li, J.; Zheng, W.F.; Liu, Z.Y.; Feng, H.; Zhang, Y. Effects of compound sodium nitrophenol on microspore embryogenesis and plantlet regeneration in flowering Chinese cabbage (Brassica campestris L. ssp. chinensis var. utilis Tsen et Lee). Protoplasma 2023, 260, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Celik, B.O.; Onus, A.N. Effect of genotype on microspore culture of eggplant (Solanum melongena L.). In Proceedings of the XXX International Horticultural Congress IHC2018: II International Symposium on Plant Breeding in Horticulture 1282, Istanbul, Turkey, 12–17 August 2018; pp. 377–382. [Google Scholar]
- Cistué, L.; Soriano, M.; Castillo, A.M.; Vallés, M.P.; Sanz, J.M.; Echávarri, B. Production of doubled haploids in durum wheat (Triticum turgidum L.) through isolated microspore culture. Plant Cell Rep 2006, 25, 257–264. [Google Scholar] [CrossRef]
- Kozar, E.; Domblides, E. Protocol of European Radish (Raphanus sativus L.) Microspore Culture for Doubled Haploid Plant Production. Methods Mol. Biol 2021, 2288, 217–232. [Google Scholar]
- Yi, D.X.; Sun, J.F.; Su, Y.B.; Tong, Z.Y.; Zhang, T.J.; Wang, Z. Retraction Note: Doubled haploid production in alfalfa (Medicago sativa L.) through isolated microspore culture. Sci. Rep. 2019, 9, 13517. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Li, H.X.; Zhang, J.H.; Ouyang, B.; Lu, Y.G.; Ye, Z.B. Initiation and development of microspore embryogenesis in recalcitrant purple flowering stalk (Brassica campestris ssp. chinensis var. purpurea Hort.) genotypes. Sci. Hortic. 2009, 121, 419–424. [Google Scholar] [CrossRef]
- Ahmadi, B.; Ahmadi, M.; Teixeira da Silva, J.A. Microspore embryogenesis in Brassica: Calcium signaling, epigenetic modification, and programmed cell death. Planta 2018, 248, 1339–1350. [Google Scholar] [CrossRef]
- Gland, A.; Lichter, R.; Schweiger, H.G. Genetic and Exogenous Factors Affecting Embryogenesis in Isolated Microspore Cultures of Brassica napus L. J. Plant Physiol. 1988, 132, 613–617. [Google Scholar] [CrossRef]
- Takahata, Y.; Komatsu, H.; Kaizuma, N. Microspore culture of radish (Raphanus sativus L.): Influence of genotype and culture conditions on embryogenesis. Plant Cell Rep. 1996, 16, 163–166. [Google Scholar]
- Aslam, F.N.; Macdonald, M.V.; Loudon, P.T.; Ingram, D.S. Rapid-cycling Brassica Species: Inbreeding and Selection of Brassica napus for Anther Culture Ability and an Assessment of its Potential for Microspore Culture. Ann. Bot. 1990, 3, 331–339. [Google Scholar] [CrossRef]
- Haddadi, P.; Moieni, A.; Karimzadeh, G.; Abdollahi, M.R. Effects of gibberellin, abscisic acid and embryo desiccation on normal plantlet regeneration, secondary embryogenesis and callogenesis in microspore culture of Brassica napus L. cv. PF704. Int. J. Plant Prod. 2012, 2, 153–162. [Google Scholar]
- Ipekci, Z.; Gozukirmizi, N. Direct somatic embryogenesis and synthetic seed production from Paulownia elongata . Plant Cell Rep. 2003, 22, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Kumari, K.G.; Jayabalan, M.G. Somatic organogenesis and plant regeneration in Ricinus communis . Biol. Plant 2008, 52, 17–25. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, J.; Henny, R.J. Direct somatic embryogenesis and plant regeneration from leaf, petiole, and stem explants of Golden Pothos. Plant Cell Rep. 2005, 23, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.C.; Garda, M. Plant tissue culture media and practices: An overview. In Vitro Cell Dev. Biol. Plant 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Tereso, S.; Migue, C.M.; Masarenhas, M.; Roque, A.; Trindade, H.; Maroco, J.; Oliveira, M.M. Improved in vitro rooting of Prunusdulcis Mill. Cultivars. Biologiaplanarum. 2008, 52, 437–444. [Google Scholar]
- Naeem, I.; Munir, I.; Durrett, T.P.; Iqbal, A.; Aulakh, K.S.; Ahmad, M.A.; Khan, H.; Khan, I.A.; Hussain, F.; Shuaib, M.; et al. Feasible regeneration and agro bacterium-mediated transformation of Brassica juncea with Euonymus alatus diacylglycerol acetyltransferase (EaDAcT) gene. Saudi J. Biol. Sci. 2020, 27, 1324–1332. [Google Scholar] [CrossRef]
- Ren, J.W.; Lei, Y.; Li, X.L. Tissue culture of callus and establishment of regeneration system of Tussilago farfara petiole. China J. Chin. Mater. Med. 2017, 42, 3895–3900. [Google Scholar]
- Li, Q.; Jiang, F.Q.; Tan, Y.Y.; Xue, H.Y. Tissue cultivation of Lonicera macranthoides ‘Yuleil’. J. Chin. Med. Mater. 2014, 37, 1121. [Google Scholar]
- Gambhir, G.; Kumar, P.; Srivastava, D. High frequency regeneration of plants from cotyledon and hypocotyl cultures in Brassica oleracea cv. Pride of India. Biotechnol. Rep. 2017, 15, 107–113. [Google Scholar] [CrossRef]


| Genotype | Concentration of TDZ (mg/L) | No. of Embryos Per Bud |
|---|---|---|
| Starbor F2 | 0 | 8.00 ± 0.56 c |
| 0.1 | 12.33 ± 0.33 ab | |
| 0.2 | 14.67 ± 1.45 a | |
| 0.3 | 10.33 ± 0.74 bc | |
| 0.4 | 10.00 ± 0.00 bc | |
| Winterbor F2 | 0 | 2.67 ± 0.33 b |
| 0.1 | 5.33 ± 0.33 a | |
| 0.2 | 4.33 ± 0.88 ab | |
| 0.3 | 3.00 ± 0.58 b | |
| 0.4 | 2.71 ± 0.64 b |
| Genotype | Concentration (mg/L) | Rate of Directly Conversion to Seedlings (%) | Rate of Embryos Conversion to Callus (%) | Rate of Embryos Death (%) |
|---|---|---|---|---|
| Starbor F2 | 0 | 16.83 ± 0.08 b | 36.46 ± 1.23 c | 46.73 ± 0.35 bc |
| 0.1 | 14.36 ± 0.08 c | 15.74 ± 1.31 e | 69.89 ± 0.60 a | |
| 0.2 | 27.10 ± 0.29 a | 55.29 ± 0.16 a | 17.6 ± 0.38 d | |
| 0.3 | 14.13 ± 2.88 c | 39.50 ± 0.96 b | 46.42 ± 0.71 c | |
| 0.4 | 9.85 ± 0.22 d | 32.97 ± 0.36 d | 57.2 ± 0.48 b | |
| Winterbor F2 | 0 | 15.77 ± 2.87 bc | 33.13 ± 2.58 b | 51.11 ± 1.11 a |
| 0.1 | 21.53 ± 2.06 b | 31.27 ± 0.49 b | 47.10 ± 1.92 a | |
| 0.2 | 33.13 ± 1.31 a | 53.08 ± 0.43 a | 13.17 ± 0.74 c | |
| 0.3 | 21.48 ± 0.74 b | 54.82 ± 0.74 a | 23.67 ± 1.67 b | |
| 0.4 | 11.70 ± 1.76 c | 35.15 ± 1.21 b | 52.78 ± 2.78 a |
| NAA (mg/L) | Number of Observed Plants | Rooting Rate | |
|---|---|---|---|
| MS | 1/2 MS | ||
| 0.1 | 90 | 60.00 ± 0.00 a | 83.33 ± 0.33 ab |
| 0.2 | 90 | 63.33 ± 0.12 a | 93.33 ± 0.33 b |
| 0.3 | 90 | 71.67 ± 0.83 a | 76.67 ± 0.67 a |
| Genotype | Number of Observed Plants | Number of Haploid Plants | Number of Double Haploid Plants | Number of Polyploid Plants | Doubling Efficiency (%) |
|---|---|---|---|---|---|
| Starbor F2 | 61 | 41 | 18 | 2 | 32.78 |
| Winterbor F2 | 61 | 34 | 24 | 3 | 39.34 |
| DH Lines | Variety Source | Plant | The Maximum Leaf | Petiole | Leaf | ||||
|---|---|---|---|---|---|---|---|---|---|
| Height (cm) | Width (cm) | Length (cm) | Width (cm) | Length (cm) | Width (cm) | Shape | Color | ||
| S1 | Starbor F2 | 24.00 | 33.00 | 13.50 | 9.20 | 8.50 | 0.70 | highly curled | green |
| S2 | Starbor F2 | 24.00 | 34.00 | 12.70 | 7.50 | 8.00 | 0.80 | highly curled | dark green |
| S3 | Starbor F2 | 23.50 | 37.00 | 12.00 | 8.00 | 9.00 | 0.70 | highly curled | green |
| S4 | Starbor F2 | 22.00 | 33.00 | 10.50 | 7.80 | 9.50 | 0.50 | highly curled | green |
| S5 | Starbor F2 | 17.00 | 33.50 | 10.00 | 8.50 | 8.50 | 0.60 | highly curled | dark green |
| S6 | Starbor F2 | 26.00 | 37.00 | 14.00 | 9.00 | 7.50 | 0.60 | highly curled | dark green |
| S7 | Starbor F2 | 17.50 | 33.00 | 11.50 | 8.50 | 8.00 | 0.70 | Moderately curled margin | dark green |
| S8 | Starbor F2 | 16.00 | 32.40 | 9.50 | 8.50 | 8.00 | 0.50 | Moderately curled margin | dark green |
| S9 | Starbor F2 | 20.00 | 37.00 | 14.00 | 9.00 | 8.00 | 0.70 | highly curled | dark green |
| Mean | 21.11 | 34.43 | 11.97 | 8.44 | 8.33 | 0.64 | |||
| Min | 16.00 | 32.40 | 9.50 | 7.50 | 7.50 | 0.50 | |||
| Max | 26.00 | 37.00 | 14.00 | 9.20 | 9.50 | 0.80 | |||
| SD | 3.61 | 1.97 | 1.71 | 0.58 | 0.61 | 0.10 | |||
| D1 | Winterbor F2 | 17.00 | 33.00 | 10.50 | 7.50 | 11.00 | 0.60 | Circular edge curly | green |
| D2 | Winterbor F2 | 26.00 | 37.00 | 14.00 | 6.50 | 11.00 | 0.60 | Slender curly | green |
| D3 | Winterbor F2 | 26.00 | 35.00 | 11.50 | 6.50 | 11.00 | 0.70 | Circular edge curly | green |
| D4 | Winterbor F2 | 21.00 | 40.50 | 13.50 | 7.50 | 12.00 | 0.80 | Slender curly | yellow green |
| D5 | Winterbor F2 | 16.00 | 37.00 | 13.50 | 6.50 | 11.00 | 0.70 | Circular edge curly | yellow green |
| D6 | Winterbor F2 | 30.00 | 42.00 | 15.00 | 6.50 | 13.00 | 0.60 | Slender curly | yellow green |
| D7 | Winterbor F2 | 26.00 | 40.00 | 14.00 | 6.50 | 12.00 | 0.60 | Slender curly | yellow green |
| D8 | Winterbor F2 | 20.00 | 36.00 | 11.00 | 7.50 | 13.00 | 0.70 | Circular edge curly | green |
| D9 | Winterbor F2 | 15.00 | 38.00 | 14.50 | 6.50 | 10.50 | 0.60 | Slender curly | yellow green |
| Mean | 21.89 | 37.61 | 13.06 | 6.83 | 11.61 | 0.66 | |||
| Min | 15.00 | 33.00 | 10.50 | 6.50 | 10.50 | 0.60 | |||
| Max | 30.00 | 42.00 | 15.00 | 7.50 | 13.00 | 0.80 | |||
| SD | 5.33 | 2.85 | 1.63 | 0.50 | 0.93 | 0.07 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, J.; Zou, X.; Gong, Z.; Song, G.; Ren, J.; Feng, H. Thidiazuron Promoted Microspore Embryogenesis and Plant Regeneration in Curly Kale (Brassica oleracea L. convar. acephala var. sabellica). Horticulturae 2023, 9, 327. https://doi.org/10.3390/horticulturae9030327
Zou J, Zou X, Gong Z, Song G, Ren J, Feng H. Thidiazuron Promoted Microspore Embryogenesis and Plant Regeneration in Curly Kale (Brassica oleracea L. convar. acephala var. sabellica). Horticulturae. 2023; 9(3):327. https://doi.org/10.3390/horticulturae9030327
Chicago/Turabian StyleZou, Jiaqi, Xiao Zou, Zhichao Gong, Gengxing Song, Jie Ren, and Hui Feng. 2023. "Thidiazuron Promoted Microspore Embryogenesis and Plant Regeneration in Curly Kale (Brassica oleracea L. convar. acephala var. sabellica)" Horticulturae 9, no. 3: 327. https://doi.org/10.3390/horticulturae9030327
APA StyleZou, J., Zou, X., Gong, Z., Song, G., Ren, J., & Feng, H. (2023). Thidiazuron Promoted Microspore Embryogenesis and Plant Regeneration in Curly Kale (Brassica oleracea L. convar. acephala var. sabellica). Horticulturae, 9(3), 327. https://doi.org/10.3390/horticulturae9030327





