Physiological and Transcriptomic Analyses Reveal the Response of Medicinal Plant Bletilla striata (Thunb. ex A. Murray) Rchb. f. via Regulating Genes Involved in the ABA Signaling Pathway, Photosynthesis, and ROS Scavenging under Drought Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth and Drought Treatment
2.2. Leaf Photosynthesis Measurement
2.3. RNA Extraction, Library Construction, and Sequencing
2.4. De Novo Assembly and Sequence Annotation
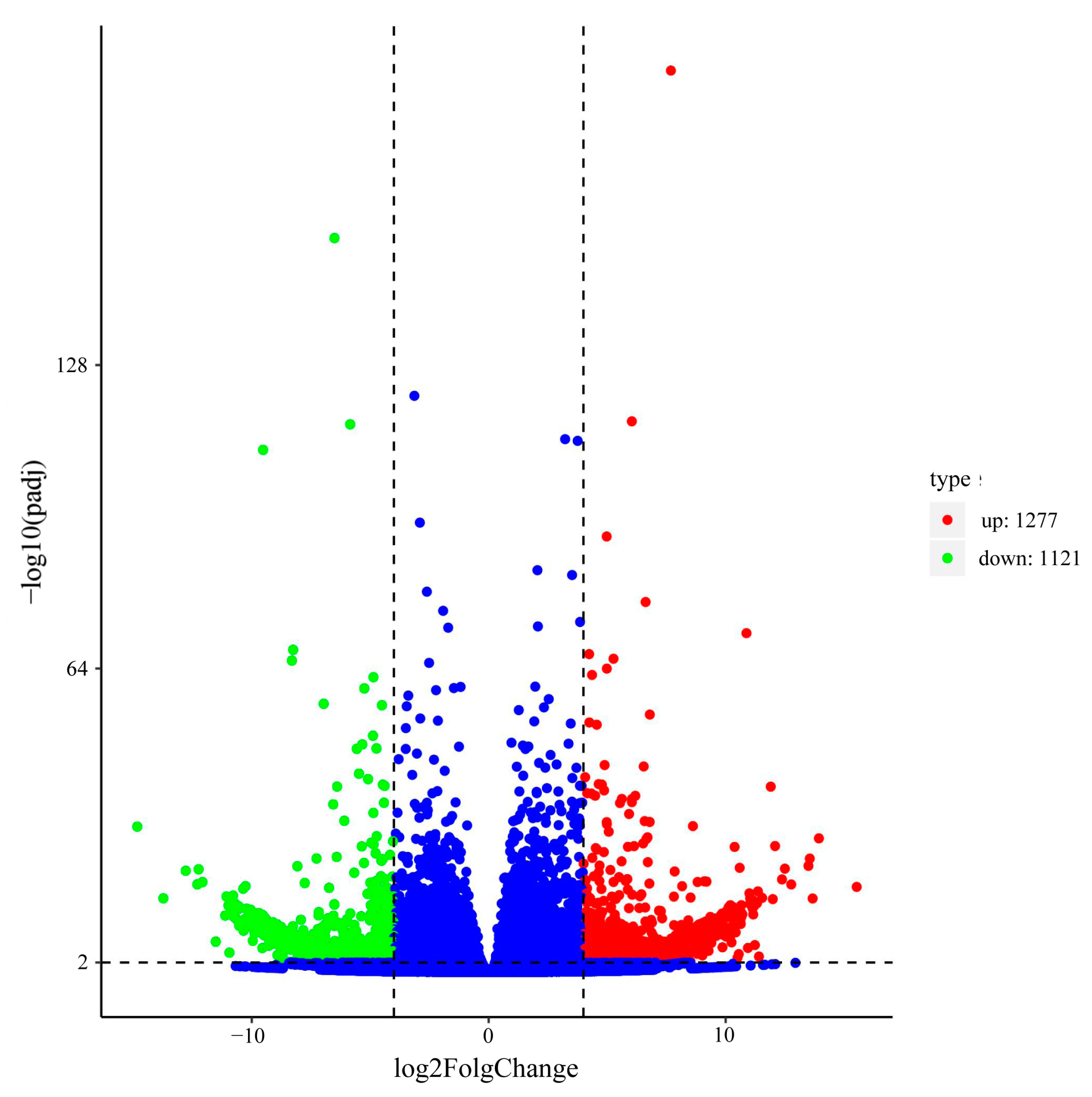
2.5. Analyses of Differentially Expressed Genes (DEGs)
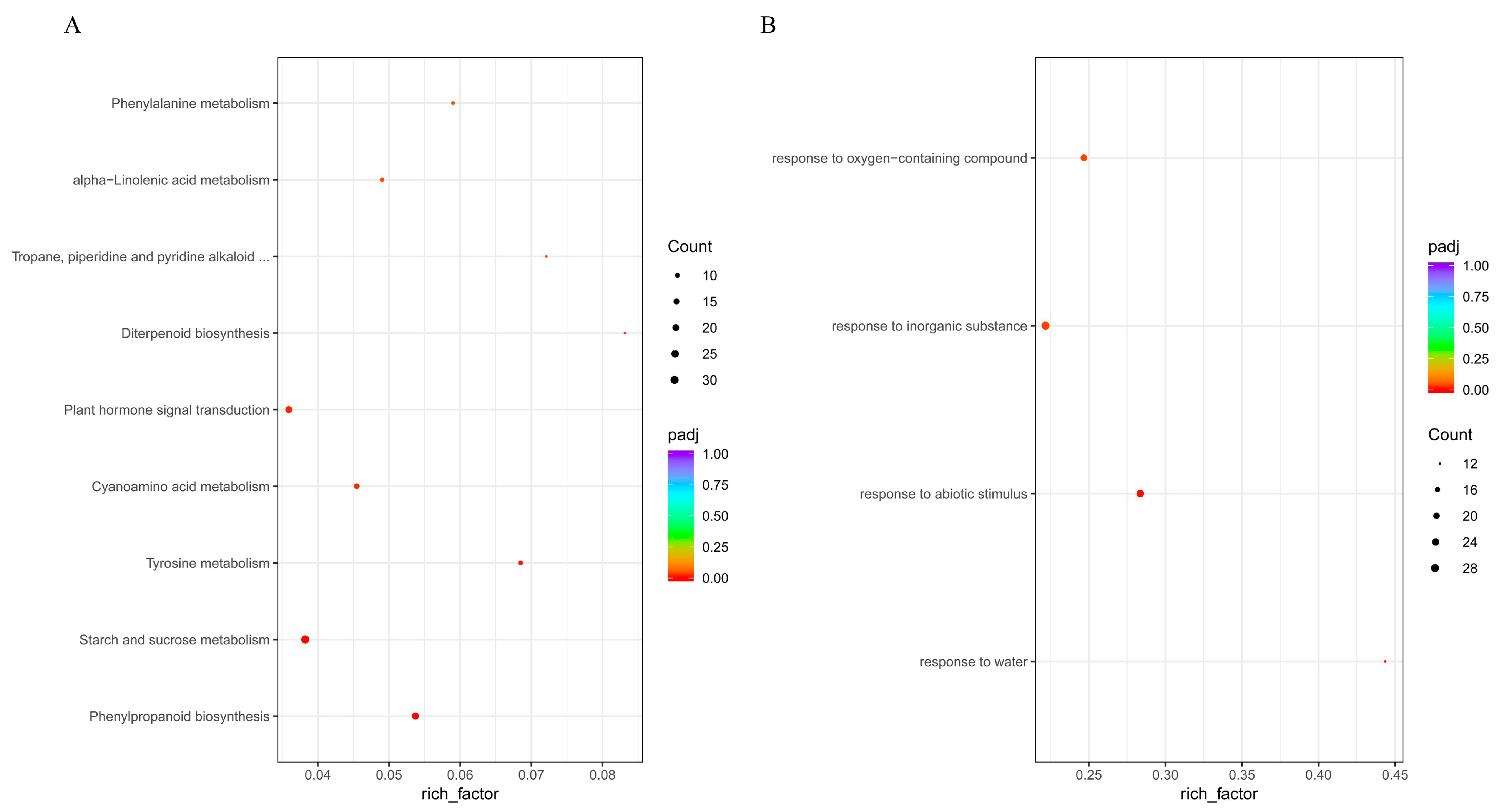
2.6. KEGG Enrichment Analysis of Differentially Expressed Genes
2.7. Statistical Analysis
3. Results
3.1. The Photosynthesis of B. striata under Drought Stress
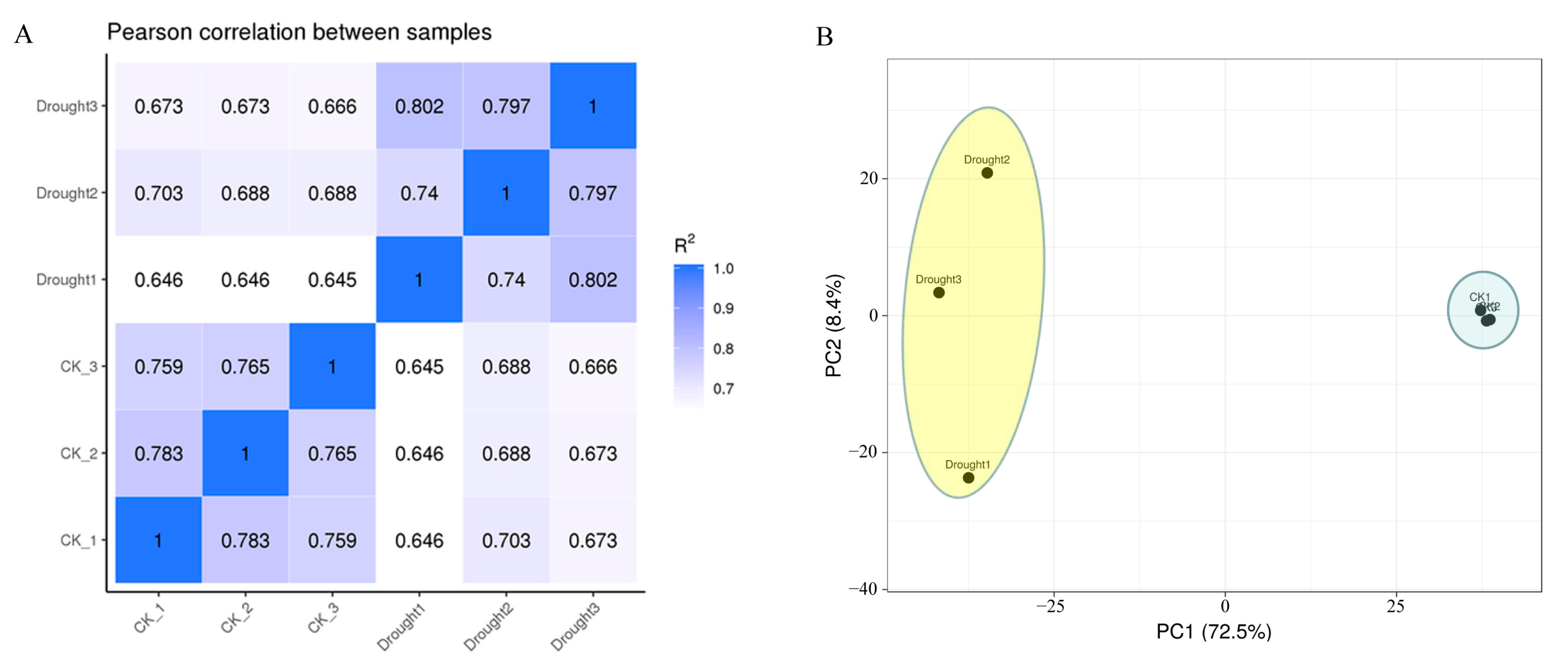
3.2. De Novo Assembly, Quality Assessment, and Annotation of Transcriptome
3.3. KEGG and GO Enrichment Analysis of Differentially Expressed Genes (DEGs)
4. Discussion
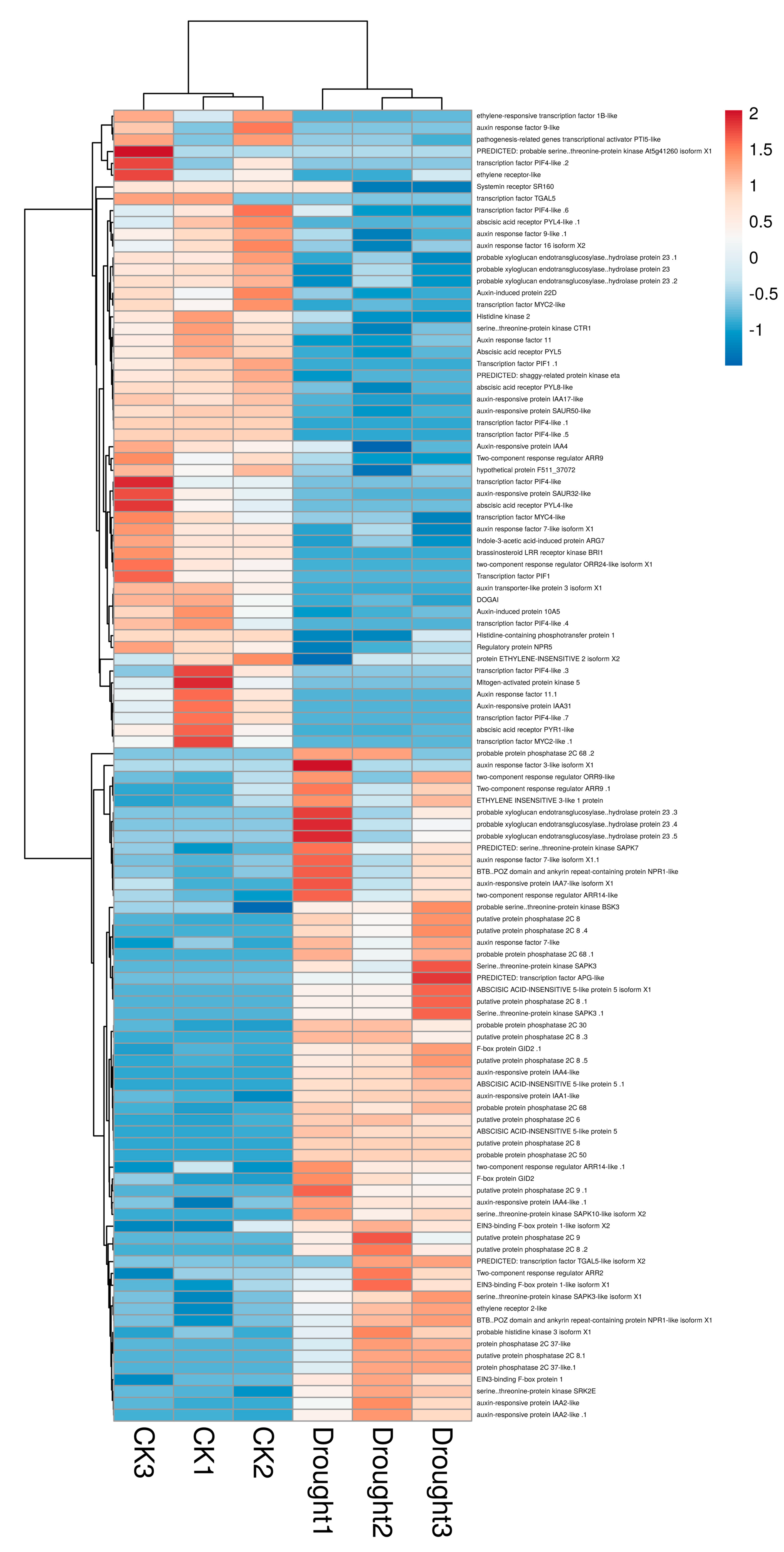
4.1. ABA Signal Transduction in B. striata Leaves under Drought Stress
4.2. Effect of Drought Stress on DEGs Involved in Photosynthesis
4.3. Drought-Induced Gene Expression of Stress-Response Protein
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Malik, M.A.; Farooq, M.; Ashraf, M.Y.; Cheema, M.A. Improving Drought Tolerance by Exogenous Application of Glycinebetaine and Salicylic Acid in Sunflower. J. Agron. Crop. Sci. 2008, 194, 193–199. [Google Scholar] [CrossRef]
- Cattivelli, L.; Rizza, F.; Badeck, F.-W.; Mazzucotelli, E.; Mastrangelo, A.M.; Francia, E.; Marè, C.; Tondelli, A.; Stanca, A.M. Drought tolerance improvement in crop plants: An integrated view from breeding to genomics. Field Crop. Res. 2008, 105, 1–14. [Google Scholar] [CrossRef]
- Mckay, J.K.; Richards, J.H.; Mitchell-Olds, T. Genetics of drought adaptation in Arabidopsis thaliana: I. Pleiotropy contributes to genetic correlations among ecological traits. Mol. Ecol. 2003, 12, 1137–1151. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Staggenborg, S.; Ristic, Z. Impacts of drought and/or heat stress on physiological, developmental, growth, and yield processes of crop plants. Adv. Agric. Syst. Model. Ser. 2008, 1, 301–355. [Google Scholar]
- Shao, H.-B.; Chu, L.-Y.; Jaleel, C.A.; Zhao, C.-X. Water-deficit stress-induced anatomical changes in higher plants. Comptes Rendus Biol. 2008, 331, 215–225. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The Role of the Plant Antioxidant System in Drought Tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Corpas, F.J.; González-Gordo, S.; Palma, J.M. Plant Peroxisomes: A Factory of Reactive Species. Front. Plant Sci. 2020, 11, 853. [Google Scholar] [CrossRef]
- Lukatkin, A.S.; Anjum, N.A. Control of cucumber (Cucumis sativus L.) tolerance to chilling stress—evaluating the role of ascorbic acid and glutathione. Front. Environ. Sci. 2014, 2, 62. [Google Scholar] [CrossRef]
- Mukarram, M.; Choudhary, S.; Kurjak, D.; Petek, A.; Khan, M.M.A. Drought: Sensing, signalling, effects and tolerance in higher plants. Physiol. Plant. 2021, 172, 1291–1300. [Google Scholar] [CrossRef]
- Quan, L.-J.; Zhang, B.; Shi, W.-W.; Li, H.-Y. Hydrogen Peroxide in Plants: A Versatile Molecule of the Reactive Oxygen Species Network. J. Integr. Plant Biol. 2008, 50, 2–18. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Modulation of Antioxidant Defense System Is Associated with Combined Drought and Heat Stress Tolerance in Citrus. Front. Plant Sci. 2017, 8, 953. [Google Scholar] [CrossRef]
- Chen, Z.; Cheng, L.; He, Y.; Wei, X. Extraction, characterization, utilization as wound dressing and drug delivery of Bletilla striata polysaccharide: A review. Int. J. Biol. Macromol. 2018, 120, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gao, F.; Gan, S.; He, Y.; Chen, Z.; Liu, X.; Fu, C.; Qu, Y.; Zhang, J. Chemical characterization and gastroprotective effect of an isolated polysaccharide fraction from Bletilla striata against ethanol-induced acute gastric ulcer. Food Chem. Toxicol. 2019, 131, 110539. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, L.; Li, C.; Huang, C.; ShangGuan, Y.; Chen, R.; Xiao, S.; Wen, W.; Xu, D. Identification and bioinformatic analysis of Aux/IAA family based on transcriptome data of Bletilla striata. Bioengineered 2019, 10, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Zhao, G.; Mi, Z.; Chen, L.; Liu, S.; Wang, S.; Wang, D.; Wang, Z. De novo sequencing of Bletilla striata (Orchidaceae) transcriptome and identification of genes involved in polysaccharide biosynthesis. Genet. Mol. Biol. 2020, 43, e20190417. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Lin, M.; Wang, H.; Song, H.; Zhang, L.; Huang, Q.; Chen, R.; Song, C.; Li, G.; Cao, Y. Haplotype-resolved genome assembly of Bletilla striata (Thunb.) Reichb.f. to elucidate medicinal value. Plant J. 2022, 111, 1340–1353. [Google Scholar] [CrossRef]
- Ding, L.; Shan, X.; Zhao, X.; Zha, H.; Chen, X.; Wang, J.; Cai, C.; Wang, X.; Li, G.; Hao, J.; et al. Spongy bilayer dressing composed of chitosan–Ag nanoparticles and chitosan–Bletilla striata polysaccharide for wound healing applications. Carbohydr. Polym. 2017, 157, 1538–1547. [Google Scholar] [CrossRef]
- Wang, C.X.; Tian, M.; Li, Q.J.; Liu, F. Floral syndrome and breeding system of Bletilla striata. Acta Hortic. Sin. 2012, 39, 1159–1166. [Google Scholar]
- Zhang, M.; Shao, Q.; Xu, E.; Wang, Z.; Wang, Z.; Yin, L. Bletilla striata: A review of seedling propagation and cultivation modes. Physiol. Mol. Biol. Plants 2019, 25, 601–609. [Google Scholar] [CrossRef]
- Liu, S.; Lu, C.; Jiang, G.; Zhou, R.; Chang, Y.; Wang, S.; Wang, D.; Niu, J.; Wang, Z. Comprehensive functional analysis of the PYL-PP2C-SnRK2s family in Bletilla striata reveals that BsPP2C22 and BsPP2C38 interact with BsPYLs and BsSnRK2s in response to multiple abiotic stresses. Front. Plant Sci. 2022, 13, 963069. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Cai, C.; Yang, Q.; Quan, W.; Li, C.; Wu, Y. Response of Bletilla striata to Drought: Effects on Biochemical and Physiological Parameter Also with Electric Measurements. Plants 2022, 11, 2313. [Google Scholar] [CrossRef]
- Larocque, G.R. Coupling a detailed photosynthetic model with foliage distribution and light attenuation functions to compute daily gross photosynthesis in sugar maple (Acer saccharum Marsh.) stands. Ecol. Model. 2002, 148, 213–232. [Google Scholar] [CrossRef]
- Ramanjulu, S.; Sreenivasulu, N.; Sudhakar, C. Effect of Water Stress on Photosynthesis in Two Mulberry Genotypes with Different drought Tolerance. Photosynthetica 1998, 35, 279–283. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef]
- Weyers, J.D.B.; Paterson, N.W. Plant hormones and the control of physiological processes. New Phytol. 2001, 152, 375–407. [Google Scholar] [CrossRef]
- Kazan, K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015, 20, 219–229. [Google Scholar] [CrossRef]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. Res. 2018, 25, 33103–33118. [Google Scholar] [CrossRef]
- Danquah, A.; de Zelicourt, A.; Colcombet, J.; Hirt, H. The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol. Adv. 2014, 32, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Sun, H.; Yang, X.; Zhang, X. Drought coping strategies in cotton: Increased crop per drop. Plant Biotechnol. J. 2017, 15, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Hobo, T.; Ichimura, K.; Mizoguchi, T.; Takahashi, F.; Aronso, J.; Ecker, J.; Shinozaki, K. ABA-Activated SnRK2 Protein Kinase is Required for Dehydration Stress Signaling in Arabidopsis. Plant Cell Physiol. 2002, 43, 1473–1483. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic Acid-Induced Stomatal Closure: An Important Component of Plant Defense Against Abiotic and Biotic Stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef]
- Mori, I.C.; Murata, Y.; Yang, Y.; Munemasa, S.; Wang, Y.-F.; Andreoli, S.; Tiriac, H.; Alonso, J.M.; Harper, J.F.; Ecker, J.R.; et al. CDPKs CPK6 and CPK3 Function in ABA Regulation of Guard Cell S-Type Anion- and Ca2+- Permeable Channels and Stomatal Closure. PLoS Biol. 2006, 4, 1749–1762. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [PubMed]
- de Ollas, C.; Dodd, I.C. Physiological impacts of ABA–JA interactions under water-limitation. Plant Mol. Biol. 2016, 91, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Fujita, M.; Satoh, R.; Maruyama, K.; Parvez, M.M.; Seki, M.; Hiratsu, K.; Ohme-Takagi, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1 Is a Transcription Activator of Novel ABRE-Dependent ABA Signaling That Enhances Drought Stress Tolerance in Arabidopsis. Plant Cell 2005, 17, 3470–3488. [Google Scholar] [CrossRef]
- Stoppel, R.; Lezhneva, L.; Schwenkert, S.; Torabi, S.; Felder, S.; Meierhoff, K.; Westhoff, P.; Meurer, J. Recruitment of a Ribosomal Release Factor for Light- and Stress-Dependent Regulation of petB Transcript Stability in Arabidopsis Chloroplasts. Plant Cell 2011, 23, 2680–2695. [Google Scholar] [CrossRef]
- Haldrup, A.; Simpson, D.J.; Scheller, H.V. Down-regulation of the PSI-F Subunit of Photosystem I (PSI) in Arabidopsis thaliana—The PSI-F subunit is essential for photoautotrophic growth and contributes the antenna function. J. Biol. Chem. 2000, 275, 31211–31218. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Shimakawa, G.; Sétif, P. Role of the two PsaE isoforms on O2 reduction at photosystem I in Arabidopsis thaliana. Biochim. Biophys. Acta (BBA)—Bioenerg. 2019, 1861, 148089. [Google Scholar] [CrossRef] [PubMed]
- Bricker, T.M.; Roose, J.L.; Zhang, P.; Frankel, L.K. The PsbP family of proteins. Photosynth. Res. 2013, 116, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Kakiuchi, S.; Uno, C.; Ido, K.; Nishimura, T.; Noguchi, T.; Ifuku, K.; Sato, F. The PsbQ protein stabilizes the functional binding of the PsbP protein to photosystem II in higher plants. Biochim. Biophys. Acta (BBA)—Bioenerg. 2012, 1817, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Żelisko, A.; García-Lorenzo, M.; Jackowski, G.; Jansson, S.; Funk, C. AtFtsH6 is involved in the degradation of the light-harvesting complex II during high-light acclimation and senescence. Proc. Natl. Acad. Sci. USA 2005, 102, 13699–13704. [Google Scholar] [CrossRef]
- Close, T.J.; Kortt, A.A.; Chandler, P.M. A cDNA-based comparison of dehydration-induced proteins (dehydrins) in barley and corn. Plant Mol. Biol. 1989, 13, 95–108. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Nisha, N.; Singh, K.; Verma, R.; Gupta, R. Involvement of dehydrin proteins in mitigating the negative effects of drought stress in plants. Plant Cell Rep. 2021, 41, 519–533. [Google Scholar] [CrossRef]
- Yang, Z.; Sheng, J.; Lv, K.; Ren, L.; Zhang, D. Y2SK2 and SK3 type dehydrins from Agapanthus praecox can improve plant stress tolerance and act as multifunctional protectants. Plant Sci. 2019, 284, 143–160. [Google Scholar] [CrossRef]
- Verma, G.; Dhar, Y.V.; Srivastava, D.; Kidwai, M.; Chauhan, P.S.; Bag, S.K.; Asif, M.H.; Chakrabarty, D. Genome-wide analysis of rice dehydrin gene family: Its evolutionary conservedness and expression pattern in response to PEG induced degydration stress. PLoS ONE 2017, 12, e0176399. [Google Scholar] [CrossRef]



| Sample | CK1 | CK2 | CK3 | Drought1 | Drought2 | Drought3 |
|---|---|---|---|---|---|---|
| Raw Data | 51,068,510 | 47,394,690 | 49,121,326 | 45,204,114 | 56,709,020 | 55,046,454 |
| Clean Data | 49,902,994 | 46,355,072 | 48,119,710 | 44,225,174 | 55,207,808 | 53,905,350 |
| Q30 (%) | 92.56 | 90.14 | 92.24 | 92.34 | 92.40 | 92.03 |
| GC Content (%) | 47.71 | 47.61 | 47.65 | 46.99 | 47.30 | 46.97 |
| Database | Number of Genes | Percentage (%) |
|---|---|---|
| Annotated in NR | 81,958 | 67.09 |
| Annotated in NT | 45,174 | 36.97 |
| Annotated in KO | 29,847 | 24.43 |
| Annotated in SwissProt | 57,293 | 46.89 |
| Annotated in PFAM | 56,063 | 45.89 |
| Annotated in GO | 56,063 | 45.89 |
| Annotated in KOG | 21,869 | 17.9 |
| Annotated in At Least One Database | 86,618 | 70.9 |
| Gene ID | NR ID | NR Description | log2FC | q-Value |
|---|---|---|---|---|
| Photosynthesis | ||||
| Cluster-6724.91253 | YP_009347733.1 | Cytochrome b6, chloroplast | −2.2224 | 0.009795 |
| Cluster-6724.49822 | XP_020586841.1 | Photosystem I reaction center subunit III, chloroplastic | −1.2025 | 0.015406 |
| Cluster-6724.49802 | XP_020584337.1 | Photosystem I reaction center subunit V, chloroplastic | −1.2516 | 0.000058 |
| Cluster-6724.50075 | XP_020592430.1 | Photosystem I reaction center subunit psaK, chloroplastic | −1.2483 | 0.00262 |
| Cluster-6724.44868 | XP_020674733.1 | psbQ-like protein 3, chloroplastic | 7.5757 | 0.000007 |
| Cluster-6724.68113 | PKU73612.1 | PsbP domain-containing protein 3, chloroplastic | 7.6345 | 0.026749 |
| Cluster-6724.86842 | XP_020701207.1 | psbP domain-containing protein 1, chloroplastic isoform X1 | 1.1728 | 0.000497 |
| Cluster-6724.55459 | PKU68001.1 | ATP-dependent zinc metalloprotease FTSH 11, chloroplastic | 6.1654 | 0.012827 |
| Cluster-6724.47025 | XP_020682163.1 | ATP-dependent zinc metalloprotease FTSH 11, chloroplastic | 7.0383 | 0.018672 |
| Cluster-6724.49015 | AVI16663.1 | Photosystem I reaction center subunit psaK | 3.4885 | 0.020947 |
| Cluster-6724.74411 | XP_020682631.1 | Oxygen-evolving enhancer protein 2, chloroplastic-like | 1.7217 | 0.005108 |
| Cluster-2398.0 | XP_018676095.1 | PREDICTED: photosynthetic NDH subunit of lumenal location 3, chloroplastic-like | 5.4806 | 0.041334 |
| Cluster-6724.11910 | XP_020600282.1 | Oxygen-evolving enhancer protein 3-2, chloroplastic-like | 1.1112 | 0.030315 |
| Cluster-6724.49786 | XP_020672629.1 | Photosystem II core complex proteins psbY, chloroplastic isoform X2 | 1.3762 | 0.000000 |
| ABA Signal Transduction | ||||
| Cluster-6724.40260 | XP_020672595.1 | Abscisic-acid receptor PYL4-like | −6.9396 | 0.000001 |
| Cluster-6724.91783 | XP_020673631.1 | Abscisic-acid receptor PYR1-like | −3.4996 | 0.000000 |
| Cluster-6724.61155 | XP_020587854.1 | Abscisic-acid receptor PYL8-like | −1.4327 | 0.000000 |
| Cluster-6724.38513 | PKU64533.1 | Abscisic-acid receptor PYL5 | −2.9087 | 0.000000 |
| Cluster-6724.40259 | XP_020672595.1 | Abscisic-acid receptor PYL4-like | −4.046 | 0.000000 |
| Cluster-6724.61497 | KZV54161.1 | Hypothetical protein F511_37072 | −1.5431 | 0.046313 |
| Cluster-6724.3652 | PKU68005.1 | Putative protein phosphatase 2C 8 | 2.1736 | 0.032413 |
| Cluster-6724.16415 | PKU68005.1 | Putative protein phosphatase 2C 8 | 3.9737 | 0.000260 |
| Cluster-6724.91192 | XP_020597478.1 | Protein phosphatase 2C 37-like | 4.6738 | 0.000000 |
| Cluster-6724.6728 | XP_020698862.1 | Probable protein phosphatase 2C 68 | 4.1538 | 0.000000 |
| Cluster-6724.46469 | PKA58960.1 | Putative protein phosphatase 2C 8 | 3.0705 | 0.000000 |
| Cluster-6724.86228 | PKA58960.1 | Putative protein phosphatase 2C 8 | 4.132 | 0.01917 |
| Cluster-6724.93558 | PKU74952.1 | Putative protein phosphatase 2C 9 | 5.5698 | 0.000110 |
| Cluster-6724.93557 | PKU74952.1 | Putative protein phosphatase 2C 9 | 5.4473 | 0.000036 |
| Cluster-6724.15036 | XP_020682015.1 | Probable protein phosphatase 2C 30 | 2.1485 | 0.000000 |
| Cluster-6724.22798 | XP_020698862.1 | Probable protein phosphatase 2C 68 | 8.7029 | 0.000000 |
| Cluster-6724.22799 | XP_020698862.1 | Probable protein phosphatase 2C 68 | 7.5918 | 0.000025 |
| Cluster-6724.5916 | PKU68005.1 | Putative protein phosphatase 2C 8 | 2.9511 | 0.000000 |
| Cluster-6724.5917 | PKU68005.1 | Putative protein phosphatase 2C 8 | 3.25 | 0.000000 |
| Cluster-6724.5919 | PKU68005.1 | Putative protein phosphatase 2C 8 | 3.9231 | 0.000000 |
| Cluster-6724.90331 | XP_020597478.1 | Protein phosphatase 2C 37-like | 6.6068 | 0.000000 |
| Cluster-6724.48042 | PKU76292.1 | Putative protein phosphatase 2C 6 | 1.9241 | 0.000000 |
| Cluster-6724.69799 | XP_020693557.1 | Probable protein phosphatase 2C 50 | 5.1397 | 0.000052 |
| Cluster-6724.5918 | PKU68005.1 | Putative protein phosphatase 2C 8 | 3.8178 | 0.000000 |
| Cluster-6724.65584 | XP_020573821.1 | Serine/threonine protein kinase SAPK3-like isoform X1 | 1.0795 | 0.041358 |
| Cluster-6724.50267 | XP_020705528.1 | Serine/threonine protein kinase SAPK10-like isoform X2 | 2.5198 | 0.000000 |
| Cluster-6724.56769 | XP_015636932.1 | PREDICTED: serine/threonine protein kinase SAPK7 | 1.9686 | 0.000100 |
| Cluster-6724.18821 | PKU80471.1 | Serine/threonine protein kinase SAPK3 | 5.6694 | 0.004812 |
| Cluster-6724.60505 | API65110.1 | Serine/threonine protein kinase SRK2E | 1.0474 | 0.000001 |
| Cluster-6724.52592 | PKU80471.1 | Serine/threonine protein kinase SAPK3 | 6.8354 | 0.000240 |
| Cluster-6724.41934 | PKU79905.1 | ABSCISIC ACID-INSENSITIVE 5-like protein 5 | 1.2712 | 0.000000 |
| Cluster-6724.51613 | XP_020694098.1 | ABSCISIC ACID-INSENSITIVE 5-like protein 5 isoform X1 | 3.9425 | 0.008781 |
| Cluster-6724.93796 | PKU83951.1 | ABSCISIC ACID-INSENSITIVE 5-like protein 5 | 3.2259 | 0.000000 |
| Cluster-7290.0 | PKU75117.1 | S-type anion channel SLAH2 | 4.146 | 0.000001 |
| Antioxidant Metabolism | ||||
| Cluster-6724.52388 | ACN25039.1 | Ascorbate peroxidase | −4.3302 | 0.000005 |
| Cluster-6724.52387 | ACN25039.1 | Ascorbate peroxidase | −9.2054 | 0.000000 |
| Cluster-6724.51106 | XP_020590426.1 | Superoxide dismutase [Cu-Zn] 4A | 7.6369 | 0.000002 |
| Cluster-6724.49968 | XP_020702876.1 | Catalase isozyme A | 7.8841 | 0.000000 |
| Cluster-4051.0 | XP_020585759.1 | Peroxidase P7-like isoform X1 | 4.5187 | 0.000000 |
| Cluster-6724.2897 | PKU65314.1 | Peroxidase 42 | 4.6461 | 0.000000 |
| Cluster-6724.98401 | PKU59654.1 | Cationic peroxidase 1 | 7.797 | 0.000086 |
| Cluster-6724.78777 | XP_020679253.1 | Probable glutathione S-transferase parA | 9.8673 | 0.000006 |
| Cluster-6724.95492 | PKU87189.1 | Putative glutathione S-transferase parA | 6.9382 | 0.003953 |
| Cluster-6724.45643 | PKU87189.1 | Putative glutathione S-transferase parA | 4.1034 | 0.002935 |
| Cluster-15974.0 | XP_020573757.1 | Glutathione S-transferase F8, chloroplastic-like | 4.5425 | 0.000004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Chen, K.; Yang, L.; Han, X.; Wu, M.; Shen, Z. Physiological and Transcriptomic Analyses Reveal the Response of Medicinal Plant Bletilla striata (Thunb. ex A. Murray) Rchb. f. via Regulating Genes Involved in the ABA Signaling Pathway, Photosynthesis, and ROS Scavenging under Drought Stress. Horticulturae 2023, 9, 307. https://doi.org/10.3390/horticulturae9030307
Liu H, Chen K, Yang L, Han X, Wu M, Shen Z. Physiological and Transcriptomic Analyses Reveal the Response of Medicinal Plant Bletilla striata (Thunb. ex A. Murray) Rchb. f. via Regulating Genes Involved in the ABA Signaling Pathway, Photosynthesis, and ROS Scavenging under Drought Stress. Horticulturae. 2023; 9(3):307. https://doi.org/10.3390/horticulturae9030307
Chicago/Turabian StyleLiu, Hai, Kaizhang Chen, Lin Yang, Xue Han, Mingkai Wu, and Zhijun Shen. 2023. "Physiological and Transcriptomic Analyses Reveal the Response of Medicinal Plant Bletilla striata (Thunb. ex A. Murray) Rchb. f. via Regulating Genes Involved in the ABA Signaling Pathway, Photosynthesis, and ROS Scavenging under Drought Stress" Horticulturae 9, no. 3: 307. https://doi.org/10.3390/horticulturae9030307
APA StyleLiu, H., Chen, K., Yang, L., Han, X., Wu, M., & Shen, Z. (2023). Physiological and Transcriptomic Analyses Reveal the Response of Medicinal Plant Bletilla striata (Thunb. ex A. Murray) Rchb. f. via Regulating Genes Involved in the ABA Signaling Pathway, Photosynthesis, and ROS Scavenging under Drought Stress. Horticulturae, 9(3), 307. https://doi.org/10.3390/horticulturae9030307









