Abstract
Malus baccata Borkh., an apple rootstock, is found to be damaged by oxidation at sub-low root-zone temperature. In previous studies, we have found that exogenous sucrose could alleviate oxidative damage and increase the indole acetic acid (IAA) in roots under sub-low temperature (L). However, the role of IAA in sucrose-induced tolerance to L remains unclear. A pot experiment was conducted to evaluate the effects of exogenous sucrose and IAA synthesis/transport inhibitors (2,3,5-triiodobenzoic acid, TIBA; 4-biphenylboronic acid, BBo) on growth, IAA levels, sugars, and the antioxidant system of M. baccata under L. The results showed that the L treatment decreased IAA contents by 23.69% (48 h) and induced significant increases in root contents of malondialdehyde (MDA) and reactive oxygen (ROS), along with increasing catalase (CAT), ascorbate peroxidase (APX), and glucose-6-phosphate dehydrogenase (G6PDH) activities, while superoxide dismutase (SOD) and monodehydroascorbate reductase (MDHAR) activities first increased (24 h) and then decreased (48 h), and glutathione reductase (GR) and peroxidase (POD) activities significantly decreased. The L treatment also decreased ascorbate/oxidized ascorbate (AsA/DHA), glutathione/oxidized glutathione (GSH/GSSG), and coenzyme II/oxidized coenzyme II (NADPH/NADP+) ratios. Furthermore, the L treatment increased the contents of sucrose, fructose, glucose and sorbitol in the roots and suppressed plant growth. Sucrose pretreatment significantly increased IAA contents (12.42%, 24 h and 14.44%, 48 h) and decreased MDA and ROS contents, which improved the activities of antioxidant enzymes other than APX and increased the contents of AsA, GSH, and NADPH, and increased sucrose, fructose, and sorbitol contents and promoted plant growth. However, the sucrose + TIBA or BBo treatments decreased IAA contents and attenuated or almost abolished the positive effects of exogenous sucrose under sub-low temperature. Our findings indicate that IAA is involved in the sucrose-induced regulation of the antioxidant system in M. baccata roots under sub-low temperature and we provided theoretical references for further study on the adaptability of apple roots to low temperature.
1. Introduction
Low temperature is an environmental stress that inhibits plant growth and seriously threatens global agricultural production [1]. Low temperature stress is generally classified by freezing injury and chilling injury; the occurrence of ice formation in tissues within the plant below 0 °C is referred to as freezing injury, and those adverse effects on the physiological metabolism and growth of the plant above 0 °C are called chilling injuries [2,3]. A sub-low temperature is distinguished from a chilling temperature as being above 0 °C but below the optimum temperature for plant growth and development [4]. Sub-low temperature can lead to an increase in reactive oxygen species (ROS) in plants, and excessive ROS may cause membranous phase transition, protein oxidation, and DNA damage, even leading to unrecoverable metabolic disorders and cell death [4,5,6]. To quickly remove excessive ROS, plants produce ROS scavengers, including superoxide dismutase (SOD), catalase (CAT), peroxidase (POD) and enzymes related to the ascorbate-glutathione (AsA-GSH) cycle [7,8,9]. When plants are subjected to chilling stress, the activities of SOD, POD, CAT, and ascorbate peroxidase (APX) all change significantly, effectively alleviating the cell damage caused by higher ROS in plants [10]. Some studies have found that the CAT, POD, APX, and glutathione reductase (GR) activities of Avena sativa L. roots increased under low temperature, and the malondialdehyde (MDA) content decreased, alleviating oxidative damage caused by low temperatures [11]. The air temperature in early spring rises rapidly in cool regions of northern China, but the change in soil temperature is less dramatic, and the temperature is lower. The sub-low temperature in the root zone affects root metabolism and inhibits root growth and functional expression, which is not conducive to the process of sprouting and leaf expansion of the aerial parts of fruit trees. Malus baccata (L.) Borkh., an apple rootstock, is widely grown in the cool fruit-growing area of northern China, where the problem of low soil temperature stress occurs. Our previous study found that when the root-zone temperature was about 5 °C, the roots of M. baccata underwent membrane lipid peroxidation, hydrogen peroxide (H2O2) and MDA contents increased, and the activities of APX and monodehydroascorbate reductase (MDHAR) increased, but dehydroascorbate reductase (DHAR) and GR activities decreased, thereby inhibiting the rate of aboveground leaf expansion and plant growth [12].
Under chilling stress, there are changes to sucrose metabolism and an increase in the level of soluble sugars (glucose, fructose, and sucrose) in plants [13,14]. In addition to regulating cellular water potential, soluble sugars are widely believed to be related to ROS scavenging [15]. Exogenous application of sucrose can significantly improve plant morphology and regulate plant resistance to environmental stress, which is mainly reflected in the regulation of antioxidant levels as well as osmoregulation [16,17,18,19]. Under low temperature, sucrose pretreatment increased the activity of ROS-scavenging enzymes, including SOD, glutathione peroxidase (GPX), APX, DHAR, MDHAR, and GR in cucumber seedling leaves, thus decreasing the superoxide anions (O2.−), H2O2, and MDA contents, as well as reducing membrane peroxidation and improving chilling tolerance in cucumbers [17]. It has been shown that high levels of GSH and AsA in plants help to maintain a suitable redox condition and reduce damage caused by abiotic stress [20]. The exogenous application of sucrose has been shown to induce AsA and GSH accumulation and rise ratios of AsA/DHA and GSH/GSSG in maize seedlings under salt stress, thus improving the salt tolerance of maize seedlings [21]. Endogenous sugars can provide feedstock for the pentose phosphate oxidation pathway (PPP), promote the reduction of GSH, and contribute to the elimination of H2O2 [15]. Glucose-6-phosphate dehydrogenase (G6PDH) is a key enzyme in the PPP pathway, and its activity is related to the induction of high anti-oxidative capacity by exogenous sugars. For example, exogenous sucrose can slow the decrease rate of G6PDH activity in maize leaves under salt stress, positively affect the nicotinamide adenine dinucleotide phosphate (NADPH) accumulation, and maintain intracellular redox balance [21]. The above studies indicate that exogenous sucrose can participate in the elimination of excessive ROS by affecting antioxidant enzyme activity and antioxidant content. In addition to physiological and biochemical regulation, sugars can also participate in the chilling stress response through interactions with hormones [22].
Sucrose plays a role in regulating IAA levels in plants, and its effect on IAA levels is more obvious in plant roots than in shoots, indicating that sugar may also affect the synthesis and transport of IAA in plants [23]. Sucrose promoted higher IAA levels in the roots of Arabidopsis, and the transport of IAA to roots increased, while the addition of an IAA inhibitor inhibited the transport of IAA and completely prevented the growth induced by sucrose [23]. In Arabidopsis, sugar−regulated IAA biosynthesis and degradation by altering gene expression and metabolites related to IAA biosynthesis pathways [24]. Wingler et al. found that sucrose treatment can increase IAA content in Arabidopsis grown on agar medium at low temperature, indicating that sucrose may be closely related to the level of IAA [22]. As a very important hormone in plants, IAA can significantly reduce ROS and lipid peroxidation by promoting an increase in antioxidant enzyme activity in plants under abiotic stress, thus improving cell viability [25,26]. Studies have shown that endogenous IAA may reduce O2.− and H2O2 contents by increasing the SOD, CAT, POD and GR activities to increase the drought tolerance of Arabidopsis [27]. Khan et al. found that pretreatment with TIBA further decreased the APX, MDHAR, DHAR and GR activities in tomato (Lycopersicum esculentum) roots under cadmium stress, and O2.− and H2O2 accumulation increased, indicating that endogenous IAA can alleviate the toxic damage of cadmium stress by increasing antioxidant enzyme activity and scavenging ROS [28].
There have been some reports on the effects of exogenous sucrose or IAA on the antioxidant system in plants under abiotic stress. In addition, it has been confirmed that sucrose can regulate the content of endogenous IAA in plant roots. Hence, we propose a hypothesis that exogenous sucrose can alleviate plant oxidative damage by inducing endogenous IAA under sub-low temperature stress. The results obtained from this study will clarify the physiological mechanism of IAA in sucrose regulation of M. baccata roots response to sub-low temperature and provide a theoretical basis for the application of sucrose in alleviating low-temperature damage to apple roots.
2. Materials and Methods
2.1. Plant Materials and Experimental Treatments
Seeds of M. baccata were collected from the scientific research base of Shenyang Agricultural University, Liaoning Province. In March 2020, the seeds were stratified at 4 °C for about 30 days. Then, they were sown in a tray with drainage holes after germination. When the seedlings grew to five leaves, they were planted into plastic basins (13 × 12 cm) containing the same volume of river sand, garden soil and seedling substrate (v:v:v = 1:2:1). Each pot contained 0.8 kg homogenized soil (pH, 6.52; available nitrogen, 192.5 mg·kg−1; available phosphorus, 20.1 mg·kg−1; and available potassium, 193.1 mg·kg−1) and received a single seedling. All seedlings were cultivated in a transparent rain shelter and irrigated using an optimal quantity of water under the same environmental conditions (24 ± 3 °C day and 14 ± 3 °C night). After two months, healthy plants with fifteen leaves and that were similar in height were selected for our treatments.
The experiment was conducted in an artificial climate room with a light/dark cycle of 14 h/10 h (day, 20 °C/night, 10 °C), a light intensity of 300 μmol·m−2·s−1, and 70% relative humidity. See Table 1 for a description of each treatment method. After the seedlings were treated with the second root application solution for 24 h, the control was not treated with a sub-low temperature in the root zone, and this was defined as sub-low temperature for 0 h. The sub-low temperature (L) treatment involved keeping the plant roots at approximately 5 °C as described in our previous study [12]. The L + S treatment was watered with 90 mmol·L−1 sucrose solution 24 h before the L treatment. The L + S + TIBA and L + S + BBo treatments were pretreated with 500 μmol·L−1 2,3,5-triiodobenzoic acid or 300 μmol·L−1 4-biphenylboronic acid, and watered with 90 mmol·L−1 sucrose solution 8 h later. About 100 mL of each solution was used for watering once before L treatment. After 0, 24, and 48 h of L treatment, the seedling roots were collected, washed with distilled water, and treated with liquid nitrogen, then kept in the refrigerator at −80 °C. Each treatment was biologically repeated 3 times, with 5 seedlings per repetition. The growth and root morphology were measured after 7 days of treatment.

Table 1.
Treatment methods for exogenous sucrose and IAA inhibitor.
2.2. Measurement of Endogenous IAA Contents
IAA extraction was assayed according to the method of Qi et al. [29]. IAA in roots was extracted using 0.1 g samples dissolved in 1 mL of cold phosphate buffer (pH 7.4). The mixture was then centrifuged at 3000× g (4 °C, 20 min), and the supernatant was collected for testing. IAA concentrations were then measured using a plant IAA kit (ELISA, Shanghai, China).
2.3. Measurement of Sucrose, Glucose, Fructose, and Sorbitol Contents
Sugar (sucrose, glucose, fructose, sorbitol) contents of M. baccata roots were analyzed as described by Cao et al. with minor modifications [17]. The root powders (0.2 g) were dissolved in 80% ethanol (3 mL), heated at 80 °C (30 min), and then centrifuged at 6000× g for 10 min. The pellet was reextracted with 80% ethanol, and the supernatant was collected. Sugar content determination was performed using an Agilent 1260 high-performance liquid chromatograph (Agilent, Santa Clara, CA, USA) equipped with a quaternary pump, autosampler, and differential refractive detector (RID detector). The chromatographic column was a Carbomix Ca-NP (7.8 × 300 mm, 10 μm), and the column temperature was 80 °C.
2.4. Measurement of MDA and ROS Contents and ROS Staining of Roots
The MDA contents were measured according to Du et al. [30]. Root samples (0.3 g) were homogenized intrichloroacetic acid (TCA, 3 mL, 10%) and centrifuged for 10 min at 4000× g. Two milliliters of supernatant were mixed with TCA (2 mL, 10%) containing 0.6% thiobarbituric acid (TBA), and then heated at 100 °C for 15 min. The mixture centrifuged for 10 min at 4000× g and determined at 532 and 600 nm.
The H2O2 and O2.− contents were determined with reference to the methods of Li et al. [12] and Farooq et al. [31], respectively.
In situ O2.− and H2O2 accumulation was assayed by histochemical staining of roots with nitrotetrazolium blue chloride (NBT) and 3,3-diaminobenzidine (DAB), respectively [25,32]. The fresh roots (1 g) were placed into a 50 mL centrifuge tube containing 30 mL dye liquor (0.2% NBT or 10% DAB), and the centrifuge tube was oscillated at 800 rpm for 20 min on a shaker. Then, the roots were boiled in 90% ethanol for 10 min for bleaching. Subsequently, the stained sections were imaged with a microscope.
2.5. Measurement of Nonenzymatic Metabolites and Antioxidative Enzyme Activities
The enzyme was extracted according to a previously described method [12]. Samples (0.1 g) were homogenized in potassium phosphate buffer (2 mL, pH 7.8) containing 0.5% Triton X-100 and 0.1 g polyvinylpyrrolidone (PVPP). The mixture was shaken at 4 °C for 15 min and extracted for 10–12 h. The mixture was centrifuged at 8000× g (4 °C, 15 min), and the supernatant was collected to determine the enzyme activities.
The enzyme activities of SOD, POD [33], CAT [34], APX [35], MDHAR, DHAR, and GR [36] were quantified by spectrophotometry. SOD activity was measured at 560 nm in a reaction mixture (3 mL) consisting of sodium phosphate buffer (50 mM), methionine (Met, 130 mM), NBT (750 μM), EDTA (100 μM), riboflavin (20 µM), and 0.05 mL enzyme extract. POD was assayed at 470 nm in a 4 mL buffer containing sodium phosphate buffer (50 mM), H2O2 (0.3%), guaiacol (0.2%), and 1 mL enzyme extract. CAT activity was determined at 240 nm in a 2.8 mL volume of Tris-HCl (50 mM) and enzyme extract (0.1 mL). H2O2 was added to initiate the reaction. APX activity was tested at 290 nm in a 1 mL reaction mixture (50 mM phosphate buffer, 0.2 mM AsA, 0.2 mM H2O2, enzyme extract). MDHAR activity was determined in a 3 mL volume of 2 mM ascorbate, 2 mM NADPH, 0.06 mL AsA oxidase, and 0.3 mL enzyme extract at 340 nm. DHAR was assayed at 265 nm in a reaction mixture (1 mL) containing phosphate buffer (50 mM), DHA (1 mM), EDTA (0.1 mM), GSH (0.2 mM), and 0.2 mL enzyme extract. GR activity was assayed at 340 nm for 5 min in 2 mL 0.1 M 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPE) containing NADPH (0.2 mM) and GSSG (0.5 mM).
Frozen root powders (0.2 g) were added into 2 mL pre-cooled TCA (5%), the homogenate was then centrifuged at 13,000× g (15 min, 4 °C) and the supernatant was collected for antioxidant detection. AsA and DHA contents determination was performed at 525 nm according to Yu et al. [37]. GSH and GSSG contents were assayed at 412 nm according to Li et al. [38].
2.6. Measurement of G6PDH Activity
G6PDH extraction and activity assays were performed according to the methods of Castiglia et al. and Tian et al. with appropriate modifications [39,40]. Root powders (0.1 g) were homogenized in 1 mL Tris-HCl buffer (50 mM, pH 8.0) containing EDTA (1 mM), phenylmethylsulfonyl fluoride (PMSF, 1 mM), dithiothreitol (1 mM), and MgCl2 (3 mM), and then centrifuged at 10,000× g (20 min, 4 °C). A total of 100 µL supernatant was added to a 2.9 mL buffer containing Tris-HCl (50 mM, pH 8.0), MgCl2 (3 mM), glucose-6-phosphate (0.5 mM), and then mixed with 1.5 mM disodium oxidized coenzyme II (NADPNa2). Immediately, the mixture was measured spectrophotometrically at 340 nm within 3 min.
2.7. Measurement of NADPH and NADP+ Contents
NADPH and NADP+ contents were determined at 570 nm by the method of Jung et al. [41]. NADPH was extracted with HCl (0.1 M), and NADP+ was extracted with NaOH (0.1 M). Fifty milligrams of root sample were homogenized in 0.9 mL extraction solution, and the mixture was centrifuged at 10,000× g (10 min, 4 °C). About 500 μL supernatant was pipetted into 500 μL of HCI (0.1 M) or NaOH (0.1 M), which was centrifuged again. Fifty microliters of supernatant were used for the reaction, and the assay reaction media included 250 μL HEPES, 16.6 mM phenazine methosulfate (PMS), 4.2 mM thiazolyl blue tetrazolium bromide (MTT), 25 mM G6P, and 14 U·mL−1 G6PDH.
2.8. Morphometric Index Determination
The height of M. baccata was measured by a ruler, and stem thickness was measured by a 50 degrees Vernier caliper (precision 0.02 mm). The roots were washed with distilled water and then scanned with the WinRHIZO Root Analysis System (WinRHIZO 2009; Regent Instruments Canada Inc., Montreal, PQ, Canada). The roots and aerial plant parts were dried for 72 h (75 °C) to determine the dry weights.
2.9. Data Statistics and Analysis
In this study, a total of 150 seedlings were designed in a completely randomized block at every time point during the experimental treatment. Data analysis in this study was performed using Statgraphics 18 (STN; St. Louis, MO, USA). The mean with standard error (SE) are used to represent the results. Differences between the treatments were regarded as significant when the p value less than 0.05 by analysis of variance (ANOVA) using the Duncan’s multiple range test. The formula used to calculate the percentage change (PC) of an indicator between treatments is PC = (B − A)/A × 100%, and the formula refers to the percentage increase or decrease of B when compared to A. All figures were mapped by Origin 9.0.
3. Results
3.1. Changes of Growth Parameters
In this study, the L treatment significantly suppressed plant height, total biomass, and shoot biomass (Table 2). Compared with the L treatment, the application of sucrose significantly increased the plant height, total biomass, and shoot biomass by 14.03%, 11.17%, and 13.82% of M. baccata, respectively. However, compared with sucrose alone, pretreatment with TIBA and BBo significantly reduced the subspecies height, total biomass, and shoot biomass under sub-low root-zone temperature by 9.69%, 19.8%, 29.14%, and 8.6%, 15.72%, 18.32%, respectively. There were no significant effects of each treatment on stem diameter and root biomass. Thus, exogenous sucrose has a positive effect on the growth of plants, whereas pretreatment with TIBA and BBo obviously inhibited the enhancing effect of exogenous sucrose on the growth of plants. In addition, the total biomass and shoot biomass under the L + S + TIBA and L + S + BBo treatments were significantly lower than that under the L treatment.

Table 2.
Effects of exogenous sucrose and IAA inhibitors on the growth parameters of M. baccata under sub-low temperature.
Table 3 shows that the L treatment caused a significant decrease in root surface area by 16.76% compared to CK, and it also reduced total root length, root volume, and root mean diameter, but not at significant levels. Compared with the L treatment, the L + S treatment significantly increased the total root length of M. baccata by 20.61%. Moreover, the L + S treatment effectively alleviated the inhibition of sub-low root-zone temperature on total root length and root surface area, which did not differ significantly from that of CK. When compared with the L + S treatment, the L + S + TIBA and L + S + BBo treatments significantly decreased the total root length (by 26.66% and 26.74%) and root surface area (by 34.49% and 41.84%) of M. baccata, with no significant difference in root average diameter, and the L + S + BBo treatment significantly decreased root volume when compared with the L + S treatment and the L treatment. The root surface area under the L + S + TIBA and L + S + BBo treatments was significantly lower than that under the L treatment.

Table 3.
Effects of exogenous sucrose and IAA inhibitors on root morphology of M. baccata under sub-low temperature.
3.2. Changes of Endogenous IAA Levels
The L treatment significantly decreased the IAA contents in roots by 23.69% compared with the control (CK) at 48 h. Compared with the L treatment, the L + S treatment significantly increased the endogenous IAA contents in roots by 12.42% and 14.44% at 24 and 48 h, respectively. Compared with the L + S treatment, pretreatment with TIBA and BBo significantly decreased the IAA contents of roots. However, pretreatment with TIBA and BBo also significantly decreased the IAA contents compared with the L treatment and CK (Figure 1).
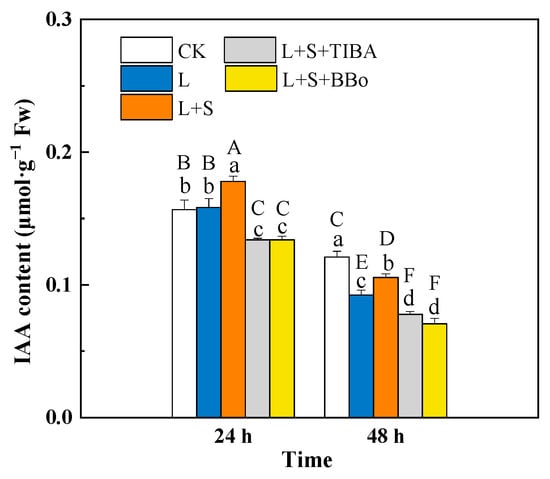
Figure 1.
Effects of exogenous sucrose and IAA inhibitors on endogenous IAA content of M. baccata under sub-low temperature. Bars indicate means ± standard error (n = 3). The lowercase letters represent significant differences between treatments (p < 0.05). The capital letters represent significant differences between full data (p < 0.05). The comparison of means is based on the Duncan’s multiple range test. CK, control treatment; L, sub-low root-zone temperature (5 °C); L + S, 5 °C + 90 mmol·L−1 sucrose; L + S + TIBA, 5 °C + 90 mmol·L−1 sucrose + 500 μmol·L−1 2,3,5-triiodobenzoic acid; L + S + BBo, 5 °C + 90 mmol·L−1 sucrose + 300 μmol·L−1 4-biphenylboronic acid. (See Supplementary Materials).
3.3. Changes of Sucrose, Glucose, Fructose, and Sorbitol Contents
As shown in Figure 2, compared with CK, the L treatment significantly increased the sucrose, glucose, fructose, and sorbitol contents by 52.87%, 35.48%, 12.5%, and 17.49%, respectively, at 24 h, by 35.68%, 27.5%, 31.11%, and 42.86%, respectively, at 48 h. Exogenous sucrose alone significantly increased sucrose, fructose, and sorbitol contents in roots and significantly increased the glucose content by 7.84% at 48 h of the L treatment. Sugar contents increased by L + S treatment did not exceed 20% when compared with L treatment. Compared with the L + S treatment, the L + S + TIBA treatment significantly decreased the sucrose and sorbitol contents, with no significant change in glucose content, and the fructose contents increased by 16.67% at 24 h but then decreased by 7.69% at 48 h. L + S + BBo treatment produced a significant decrease in sucrose, glucose and sorbitol contents in roots, and the fructose contents increased at first, but then decreased when compared with the L + S treatment group. Compared with the L treatment, the L + S + TIBA and L + S + BBo treatments had significantly decreased sucrose (48 h) and sorbitol contents (24 h, 48 h), and significantly increased fructose content at 24 h.
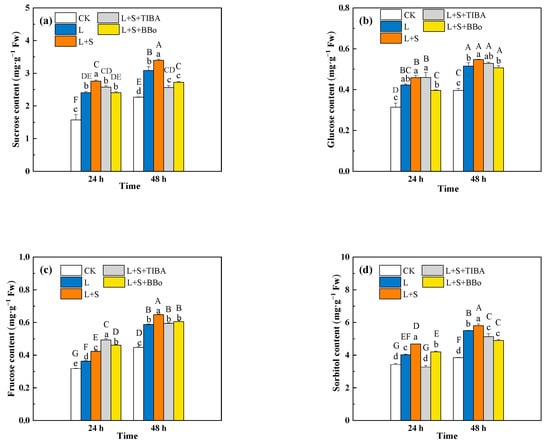
Figure 2.
Effects of exogenous sucrose and IAA inhibitors on sucrose (a), glucose (b), fructose (c) and sorbitol (d) contents of M. baccata under sub-low temperature. Bars indicate means ± standard error (n = 3). The lowercase letters represent significant differences between treatments (p < 0.05). The capital letters represent significant differences between full data (p < 0.05). The comparison of means is based on the Duncan’s multiple range test. CK, control treatment; L, sub-low root-zone temperature (5 °C); L + S, 5 °C + 90 mmol·L−1 sucrose; L + S + TIBA, 5 °C + 90 mmol·L−1 sucrose + 500 μmol·L−1 2,3,5-triiodobenzoic acid; L + S + BBo, 5 °C + 90 mmol·L−1 sucrose + 300 μmol·L−1 4-biphenylboronic acid. (See Supplementary Materials).
3.4. Changes of MDA Contents
To evaluate the degree of damage to the cell membrane under sub-low temperature, we determined the content of MDA, the end product of lipid peroxidation, in roots (Figure 3). Compared with CK, the L treatment significantly increased the MDA contents in roots by 28.48% and 30.26% at 24 and 48 h, respectively. Compared with the L treatment, the L + S treatment significantly decreased the MDA contents by 22.80% and 19.45% at 24 and 48 h. In contrast, the MDA contents in roots significantly increased after pretreating roots with TIBA and BBo, and there was no obvious difference compared with the L treatment group.
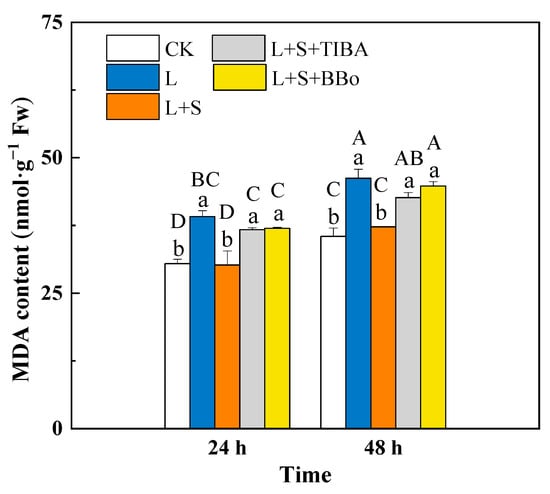
Figure 3.
Effects of exogenous sucrose and IAA inhibitors on MDA contents of M. baccata under sub-low temperature. Bars indicate means ± standard error (n = 3). The lowercase letters represent significant differences between treatments (p < 0.05). The capital letters represent significant differences between full data (p < 0.05). The comparison of means is based on the Duncan’s multiple range test. CK, control treatment; L, sub-low root-zone temperature (5 °C); L + S, 5 °C + 90 mmol·L−1 sucrose; L + S + TIBA, 5 °C + 90 mmol·L−1 sucrose + 500 μmol·L−1 2,3,5-triiodobenzoic acid; L + S + BBo, 5 °C + 90 mmol·L−1 sucrose + 300 μmol·L−1 4-biphenylboronic acid. (See Supplementary Materials).
3.5. Changes of ROS Contents
As shown in Figure 4a,c, the L treatment significantly increased the O2.− and H2O2 contents in roots. The L + S treatment resulted in significantly lower O2.− and H2O2 contents in roots than did the L treatment. The O2.− and H2O2 contents under the L + S + TIBA and L + S + BBo treatments were significantly higher than that under the L + S treatment, and much lower than that under the L treatment. However, the H2O2 content under the L + S + TIBA treatment was higher than that under the L treatment at 48 h. The O2.− and H2O2-generated sites of white roots were bluish purple and brown in color, respectively. The deeper the root color was, the higher the ROS contents in the roots, and the root staining results were consistent with the changing trends of root ROS contents (Figure 4b,d).
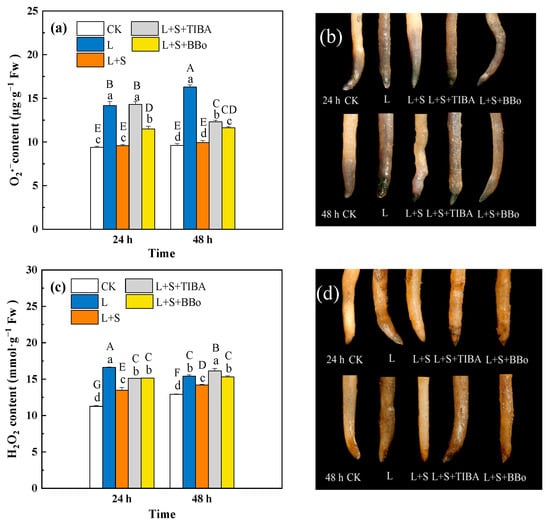
Figure 4.
Effects of exogenous sucrose and IAA inhibitors on O2.− (a) and H2O2 (c) contents and histochemical staining (b,d) of M. baccata roots under sub-low temperature. Bars indicate means ± standard error (n = 3). The lowercase letters represent significant differences between treatments (p < 0.05). The capital letters represent significant differences between full data (p < 0.05). The comparison of means is based on the Duncan’s multiple range test. CK, control treatment; L, sub-low root-zone temperature (5 °C); L + S, 5 °C + 90 mmol·L−1 sucrose; L + S + TIBA, 5 °C + 90 mmol·L−1 sucrose + 500 μmol·L−1 2,3,5-triiodobenzoic acid; L + S + BBo, 5 °C + 90 mmol·L−1 sucrose + 300 μmol·L−1 4-biphenylboronic acid. (See Supplementary Materials).
3.6. Changes of SOD, POD, and CAT Activities
As shown in Figure 5, the L treatment significantly increased SOD and CAT activities in roots of M. baccata at 24 h by 18.47% and 48.45%, respectively, when compared with CK, with no significant change in POD activity; the L treatment significantly increased CAT activity by 56.85% and decreased SOD and POD activities at 48 h by 14.39% and 5.18%, respectively. Sucrose significantly increased the SOD and POD activities in roots compared to the L treatment, whereas CAT activity showed no obvious change at 24 h; SOD, CAT, and POD activities in roots were significantly increased after 48 h of L + S treatment. Compared to the L + S treatment, the L + S + TIBA and L + S + BBo treatments significantly decreased SOD, CAT, and POD activities in the roots. In addition, CAT activity under L + S + TIBA and L + S + BBo treatments was significantly decreased than that under L treatment, and after 24 h of treatment, POD activity under L + S + BBo treatment was significantly decreased compared with that under L treatment.
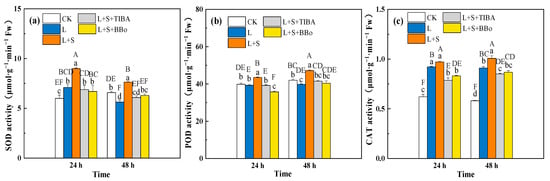
Figure 5.
Effects of exogenous sucrose and IAA inhibitors on SOD (a), POD (b) and CAT (c) activities of M. baccata under sub-low temperature. Bars indicate means ± standard error (n = 3). The lowercase letters represent significant differences between treatments (p < 0.05). The capital letters represent significant differences between full data (p < 0.05).The comparison of means is based on the Duncan’s multiple range test. CK, control treatment; L, sub-low root-zone temperature (5 °C); L + S, 5 °C + 90 mmol·L−1 sucrose; L + S + TIBA, 5 °C + 90 mmol·L−1 sucrose + 500 μmol·L−1 2,3,5-triiodobenzoic acid; L + S + BBo, 5 °C + 90 mmol·L−1 sucrose + 300 μmol·L−1 4-biphenylboronic acid. (See Supplementary Materials).
3.7. Changes of the AsA-GSH Cycle
Compared with CK, APX activity significantly increased, GR activity significantly decreased, MDHAR activity first increased and then decreased, and DHAR activity significantly decreased at 24 h and showed no obvious change at 48 h under the L treatment. Compared with the L treatment, the L + S treatment significantly increased DHAR, MDHAR and GR activities, but no significant changes were observed in APX in roots. When compared with those under the L + S treatment, the L + S + TIBA and L + S + BBo treatments significantly suppressed APX, DHAR, MDHAR, and GR activities. Compared with the L treatment, the L + S + TIBA and L + S + BBo treatments showed significantly decreased APX, DHAR (48 h), GR (24 h) activities and increased DHAR activity (24 h), while no significant changes were observed for MDHAR (Figure 6).
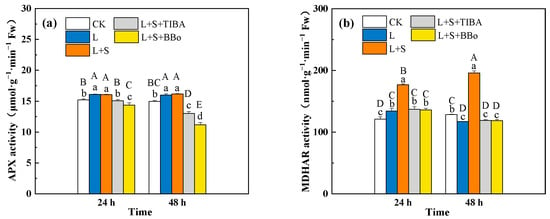
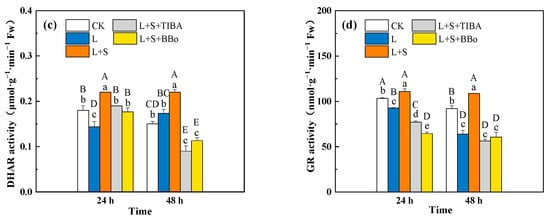
Figure 6.
Effects of exogenous sucrose and IAA inhibitors on APX (a), MDHAR (b), DHAR (c), and GR (d) activities of M. baccata under sub-low temperature. Bars indicate means ± standard error (n = 3). The lowercase letters represent significant differences between treatments (p < 0.05). The capital letters represent significant differences between full data (p < 0.05). The comparison of means is based on the Duncan’s multiple range test. CK, control treatment; L, sub-low root-zone temperature (5 °C); L + S, 5 °C + 90 mmol·L−1 sucrose; L + S + TIBA, 5 °C + 90 mmol·L−1 sucrose + 500 μmol·L−1 2,3,5-triiodobenzoic acid; L + S + BBo, 5 °C + 90 mmol·L−1 sucrose + 300 μmol·L−1 4-biphenylboronic acid. (See Supplementary Materials).
AsA and GSH are products of the AsA-GSH cycle and can participate in scavenging excessive ROS as nonenzymatic metabolites. The L treatment significantly increased the AsA contents and AsA/DHA ratio at 24 h compared with CK (Figure 7a,c), it also significantly increased the DHA contents by 16.84% and decreased the AsA/DHA ratio by 19.25% at 48 h of treatment (Figure 7b). The L + S treatment caused a significant increase in both AsA contents and the AsA/DHA ratio, and a significant decrease in DHA compared with the L treatment. However, when the roots of M. baccata were pretreated with TIBA and BBo and then treated with sucrose, the effect of sucrose on AsA, DHA, and the AsA/DHA ratio was completely abolished (Figure 7a–c).
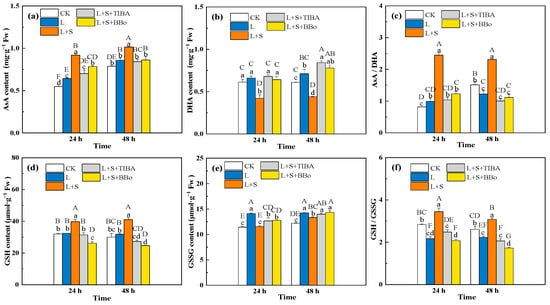
Figure 7.
Effects of exogenous sucrose and IAA inhibitors on AsA (a), DHA (b), AsA/DHA ratio (c), GSH (d), GSSG (e), and GSH/GSSG ratio (f) of M. baccata under sub-low temperature. Bars indicate means ± standard error (n = 3). The lowercase letters represent significant differences between treatments (p < 0.05). The capital letters represent significant differences between full data (p < 0.05). The comparison of means is based on the Duncan’s multiple range test. CK, control treatment; L, sub-low root-zone temperature (5 °C); L + S, 5 °C + 90 mmol·L−1 sucrose; L + S + TIBA, 5 °C + 90 mmol·L−1 sucrose + 500 μmol·L−1 2,3,5-triiodobenzoic acid; L + S + BBo, 5 °C + 90 mmol·L−1 sucrose + 300 μmol·L−1 4-biphenylboronic acid. (See Supplementary Materials).
The L treatment significantly increased the GSSG contents in roots of M. baccata without a significant effect on the GSH contents and, relative to those in the control plants, it significantly reduced the GSH/GSSG. When compared with the L treatment, the L + S treatment significantly increased GSH contents and decreased the GSSG contents in the roots of M. baccata, resulting in increases in GSH/GSSG by 59.10% and 37.94% at 24 and 48 h of treatment, respectively. Compared with the L + S treatment, the L + S + TIBA and L + S + BBo treatments significantly decreased GSH contents and increased GSSG contents, resulting in a decreased GSH/GSSG ratio (Figure 7d–f). However, the GSH/GSSG ratio under L + S + BBo treatment was significantly lower than that under the L treatment.
3.8. Changes of G6PDH Activity and NADPH Contents
G6PDH is a key enzyme in the pentose phosphate pathway that can maintain plant redox balance by generating NADPH. The L treatment significantly increased G6PDH activity in roots by 35.61% and 53.17% at 24 and 48 h of treatment, respectively, compared to those of the unstressed plants (Figure 8). When compared with the L treatment, the L + S treatment significantly increased G6PDH activity in roots. However, compared with L + S treatment, pretreatment with TIBA and BBo significantly decreased G6PDH activity under a sub-low root-zone temperature at 48 h. Therefore, we assume that endogenous IAA is involved in the regulation of G6PDH activity in roots under sub-low temperature by exogenous sucrose. However, pretreatment with TIBA and BBo significantly decreased the G6PDH activity compared with the L treatment.
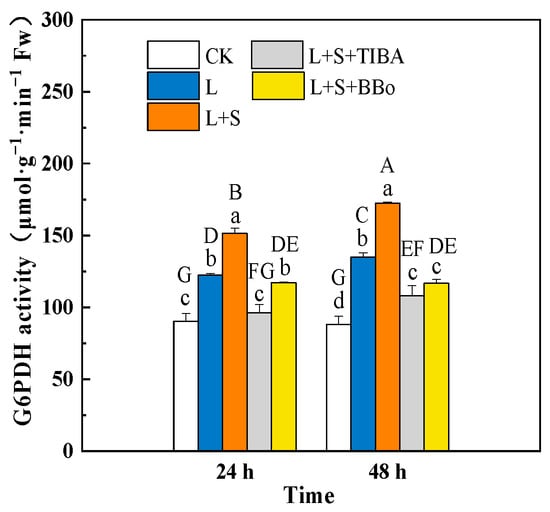
Figure 8.
Effects of exogenous sucrose and IAA inhibitors on G6PDH activity of M. baccata under sub-low temperature. Bars indicate means ± standard error (n = 3). The lowercase letters represent significant differences between treatments (p < 0.05). The capital letters represent significant differences between full data (p < 0.05). The comparison of means is based on the Duncan’s multiple range test. CK, control treatment; L, sub-low root-zone temperature (5 °C); L + S, 5 °C + 90 mmol·L−1 sucrose; L + S + TIBA, 5 °C + 90 mmol·L−1 sucrose + 500 μmol·L−1 2,3,5-triiodobenzoic acid; L + S + BBo, 5 °C + 90 mmol·L−1 sucrose + 300 μmol·L−1 4-biphenylboronic acid. (See Supplementary Materials).
NADPH/NADP+ is an interdependent redox couple in the AsA-GSH cycle that plays a crucial role in regulating the levels of ROS. As shown in Figure 9b, the L treatment significantly decreased the NADPH/NADP+ ratio in the roots at 48 h. However, there was no obvious change in NADPH contents (Figure 9a). Compared with the L treatment, the L + S treatment significantly increased NADPH contents and the NADPH/NADP+ ratio in roots. However, compared with the L + S treatment, pretreatment with TIBA and BBo significantly decreased NADPH contents and the NADPH/NADP+ ratio under sub-low temperature. Therefore, endogenous IAA could participate in the exogenous sucrose regulation of the conversion process of NADPH in roots under sub-low temperature. However, pretreatment with TIBA and BBo significantly decreased the NADPH contents at 48 h and decreased the NADPH/NADP+ ratio at 24 h when compared with the L treatment.
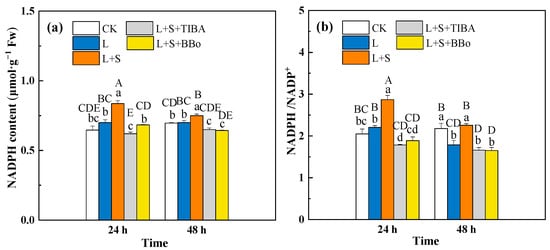
Figure 9.
Effects of exogenous sucrose and IAA inhibitors on the NADPH contents (a) and NADPH/NADP+ (b) of M. baccata under sub-low temperature. Bars indicate means ± standard error (n = 3). The lowercase letters represent significant differences between treatments (p < 0.05). The capital letters represent significant differences between full data (p < 0.05). The comparison of means is based on the Duncan’s multiple range test. CK, control treatment; L, sub-low root-zone temperature (5 °C); L + S, 5 °C + 90 mmol·L−1 sucrose; L + S + TIBA, 5 °C + 90 mmol·L−1 sucrose + 500 μmol·L−1 2,3,5-triiodobenzoic acid; L + S + BBo, 5 °C + 90 mmol·L−1 sucrose + 300 μmol·L−1 4-biphenylboronic acid. (See Supplementary Materials).
3.9. Principal Component Analysis
A principal component analysis (PCA) was performed on the IAA levels, sugar contents (sucrose, glucose, fructose, and sorbitol), ROS contents, anti-oxidative enzymes, and antioxidant contents to evaluate the role of IAA in sucrose regulation of M. baccata roots is response to LRZT (Figure 10). We found that the L and L + S treatments were located in different PCA quadrants, and the L + S treatment was separated from the L + S + TIBA and L + S + BBo treatments. The roots responded similarly under the L, L + S + TIBA, and L + S + BBo treatments. PC1 showed the effect of L + S treatment on the anti-oxidative enzymes and IAA contents, and accounted for 43.2% of the total variation. APX, GR, POD, DHAR, MDHAR (48 h) activity and IAA content (24 h) were key positive contributors to PC1. PC2 showed the effects of L + S + TIBA and L + S + BBo treatments on the ROS levels and MDA contents and accounted for 34.9% of the total variation. MDA, O2.−, and H2O2 (48 h) contents were main factors for PC2.
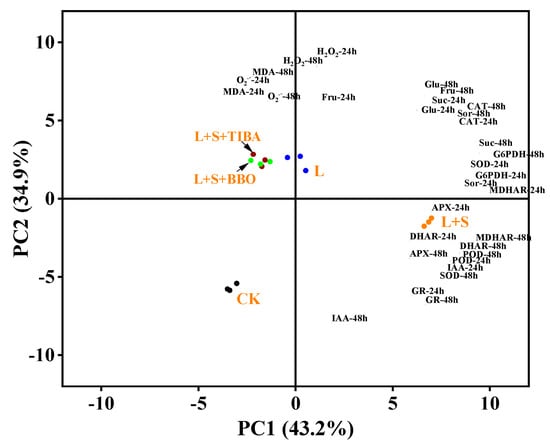
Figure 10.
Principal component analysis of IAA levels, sugar contents, ROS contents, anti-oxidative enzymes, and antioxidants contents of M. baccata under sub-low temperature with exogenous sucrose and IAA inhibitors application. CK, control treatment; L, sub-low root-zone temperature (5 °C); L + S, 5 °C + 90 mmol·L−1 sucrose; L + S + TIBA, 5 °C + 90 mmol·L−1 sucrose + 500 μmol·L−1 2,3,5-triiodobenzoic acid; L + S + BBo, 5 °C + 90 mmol·L−1 sucrose + 300 μmol·L−1 4-biphenylboronic acid.
4. Discussion
Auxin plays a key role in plant responses to abiotic stress. Low temperature decrease auxin level in the roots, possibly through the repressed expression of genes related to auxin biosynthesis and the attenuation of auxin transport (PINs) [42,43]. In this study, we found that the application of sucrose increased the IAA content and sugars in roots (Figure 1 and Figure 2). Previous studies showed that soluble sugars can regulate auxin biosynthesis, signaling, and transport [44,45]. In addition, sugar acts as a signal that can regulate the auxin concentration by promoting the auxin biosynthesis gene expression [45]. Therefore, the expression of auxin biosynthesis and transport -related genes needs further study. Endogenous IAA also participated in promoting the activities of antioxidant enzymes, reducing the degree of lipid peroxidation, and improving cell viability [46]. Therefore, we speculate that endogenous IAA was involved in sucrose regulation of the antioxidant system in roots at sub-low temperature; we applied an IAA synthesis and transport inhibitor (TIBA and BBo) treatment, and pretreatment with the inhibitors was found to reduce IAA contents in roots under sub-low temperature when compared to those under sucrose treatment. At the same time, the ROS and MDA contents in the roots of M. baccata were significantly elevated (Figure 3 and Figure 4). Therefore, it is speculated that exogenous sucrose might decrease the MDA and ROS contents of M. baccata roots under sub-low temperature by increasing the endogenous IAA content.
Soluble sugars play a vital role in maintaining osmotic potential and are accumulated in plants in response to low temperature. In this study, the application of sucrose increased the soluble sugar (sucrose, glucose, fructose and sorbitol) content under a sub-low temperature (Figure 2). The higher content of soluble sugars may be caused by the enhanced activities of neutral invertase and soluble acid invertase [17]. Moreover, auxin is directly involved in the regulation of sugar homeostasis. In this study, pretreatment of roots with TIBA and BBo led to decreased sucrose, glucose, fructose, and sorbitol contents. Therefore, auxin was thought to be involved in the accumulation of soluble sugars. However, there is a complicated interaction between auxin and sugars, and other phytohormones are also involved in this process.
Low temperature can increase the ROS level in cell, and induce the establishment of the plant defense system at the same time. In this study, the contents of ROS and the activities of SOD, CAT, APX and MDHAR increased rapidly under sub-low temperature. However, with prolonged stress time, the ROS level continuously increased in the roots, which suppressed the SOD, POD, MDHAR, and GR activities and reduced the plant’s protection abilities. With the increase in APX activity, more AsA was consumed, and the content of DHA increased continuously. These results indicate that antioxidant enzymes and antioxidants act together to reduce the damage caused by sub-low temperature in the roots of M. baccata. Studies have shown that exogenous sucrose treatment could improve stress resistance in plants by increasing antioxidant enzyme activities. In this work, sucrose treatment led to higher contents of soluble sugars under sub-low temperature and stimulated antioxidant enzymes, which scavenge ROS and reduce lipid peroxidation. In addition, ROS can be quenched directly by soluble sugars [15]. In the AsA-GSH cycle, AsA and GSH are also involved in ROS detoxification, and low temperature severely affects the plant redox balance, leading to higher accumulation of DHA and GSSG [47]. In this study, the increase in the AsA content and AsA/DHA ratio was related to the enhancement of MDHAR and DHAR activities. Some studies showed that under low temperature, sodium hydrosulfide (NaHS) treatment promoted the accumulation of endogenous IAA, significantly increased the activities of SOD, POD, APX, and GR, the contents of AsA and GSH, and significantly reduced ROS accumulation in cucumber seedlings. However, 1-naphthalene phthalic acid (NPA) treatment reduced the content of endogenous IAA in cucumber seedlings, and then reduced the improvement of the antioxidant capacity of seedlings by NaHS. Therefore, it is speculated that IAA could be a downstream signal of NaHS in alleviating the oxidative damage caused by low temperature stress [48]. In this study, the L + S + TIBA and L + S + BBo treatments significantly reduced the endogenous IAA content of M. baccata roots under a sub-low temperature, significantly inhibited the antioxidant enzyme activity and antioxidant contents and led to a significant increase in MDA and ROS contents. Therefore, we speculate that IAA could act as a downstream signal of sucrose to regulate the antioxidant system at sub-low root-zone temperature.
A sub-low root-zone temperature induced a significant increase in G6PDH activity in the roots (Figure 8), suggesting that the whole oxidative phase of the PPP may be involved in the stress response. However, the NADPH content did not change significantly under sub-low temperature, which was probably due to the reduction of AsA by MDHAR using NADPH as the electron donor. Studies have shown that endogenous sugars can provide raw materials for the PPP, supply reducing activity for GSH production and contribute to H2O2 scavenging [15]. In this study, exogenous sucrose increased G6PDH activity and NADPH, as well as the NADPH/NADP+ ratio in M. baccata roots under sub-low temperature, possibly by inducing an increase in endogenous sucrose, fructose, glucose, and sorbitol contents. Pretreatment of roots with TIBA and BBo almost diminished the positive effect of sucrose on G6PDH activity, the NADPH content, and the NADPH/NADP+ ratio (Figure 8 and Figure 9), which suggests that endogenous IAA potentially participated in the regulation of the PPP as influenced by sucrose application under sub-low temperature.
The most easily observed symptoms of chilling injury are plant growth changes. Sub-low temperature significantly inhibited the growth and development of M. baccata, as indicated by varying degrees of reduction in plant height, aboveground biomass, and root surface area (Table 1 and Table 2), and the inhibition may be due to the reduction in IAA accumulation in the roots. Exogenous sucrose treatment alleviated the inhibitory effect of sub-low temperature on the growth of M. baccata to some extent; however, the effects of sucrose treatment on root surface area, root volume, and root mean diameter were not significant (Table 2), so we speculated that sucrose could preferentially regulate the uptake function of M. baccata seedlings and promote the aboveground growth of plants rather than root growth under sub-low temperature. Moreover, sucrose may have an indirect effect on growth by affecting IAA synthesis and transport, since this effect can be simulated by the direct addition of IAA [23]. The alleviating effect of sucrose against sub-low temperature on M. baccata roots was significantly weakened after pretreatment with TIBA and BBo, which could have been due to the decreased IAA content in the roots of M. baccata. The lower IAA content inhibited the root growth and caused abnormalities in aboveground growth. Therefore, we consider the promoting effect of exogenous sucrose on the growth of M. baccata under sub-low temperature to be related to the regulation of IAA levels.
5. Conclusions
This study clarified the physiological role of IAA in exogenous sucrose regulation of M. baccata roots in response to sub-low temperature. Under sub-low root-zone temperature, exogenous sucrose increased the antioxidant enzyme activities, antioxidant contents, and soluble sugars, then, reduced the degree of ROS and lipid peroxidation. In addition, application of sucrose alleviated the inhibitory effect of sub-low temperature on the growth of M. baccata to some extent. However, the positive effects of sucrose application on the growth and biochemical parameters of M. baccata were largely abolished by pretreatment with BBo or TIBA. Hence, application of sucrose could enhance the antioxidation and promote growth of M. baccata under sub-low temperature by regulating IAA accumulation. However, the molecular mechanism of auxin and sucrose regulating the low temperature response in roots needs further study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9030297/s1.
Author Contributions
Conceptualization, L.L. and S.Q.; methodology, B.Y. and P.W.; software, B.Y. and X.Z.; validation, L.L., P.W. and X.Z.; formal analysis, B.Y.; investigation, P.W.; resources, D.L.; data curation, B.Y.; writing—original draft preparation, B.Y.; writing—review and editing, L.L.; visualization, S.Q.; supervision, S.Q. and D.L.; project administration, L.L.; funding acquisition, L.L. and D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Basic Scientific Research Foundation of Liaoning Province Education Department-Youth Project [grant number LJKZ0634], the Doctoral Scientific Research Foundation of Liaoning Province [grant number 2019-BS-209], and the China Agriculture Research System of MOF and MARA [grant number CARS-27].
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jha, U.C.; Bohra, A.; Jha, R. Breeding approaches and genomics technologies to increase crop yield under low-temperature stress. Plant Cell Rep. 2017, 36, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.R.; Knight, H. Low-temperature perception leading to gene expression and cold tolerance in higher plants. New Phytol. 2012, 195, 737–751. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Jia, F.F.; Zhang, X.M.; Qiao, Y.X.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Temperature effects on the reactive oxygen species formation and antioxidant defence in roots of two cucurbit species with contrasting root zone temperature optima. Acta Physiol. Plant. 2012, 34, 713–720. [Google Scholar] [CrossRef]
- Jan, N.; Majeed, U.; Andrabi, K.I.; John, R. Cold stress modulates osmolytes and antioxidant system in Calendula officinalis. Acta Physiol. Plant. 2018, 40, 73. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Deng, F.; Yuan, R.; Shen, F. Genome-wide characterization and expression analyses of superoxide dismutase (SOD) genes in Gossypium hirsutum. BMC Genom. 2017, 18, 376. [Google Scholar] [CrossRef]
- Hosseini, M.; Maali-Amiri, R.; Mahfoozi, S.; Fowler, D.B.; Mohammadi, R. Developmental regulation of metabolites and low temperature tolerance in lines of crosses between spring and winter wheat. Acta Physiol. Plant. 2016, 38, 87. [Google Scholar] [CrossRef]
- Ren, R.; Li, Z.; Zhang, L.; Zhou, H.; Jiang, X.; Liu, Y. Enzymatic and nonenzymatic antioxidant systems impact the viability of cryopreserved Paeonia suffruticosa pollen. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 144, 233–246. [Google Scholar] [CrossRef]
- Shan, C.; Zhou, Y.; Liu, M. Nitric oxide participates in the regulation of the ascorbate-glutathione cycle by exogenous jasmonic acid in the leaves of wheat seedlings under drought stress. Protoplasma 2015, 252, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zuo, T.; Ni, W. Important roles of glycinebetaine in stabilizing the structure and function of the photosystem II complex under abiotic stresses. Planta 2020, 251, 36. [Google Scholar] [CrossRef]
- Goyal, M.; Kaur, N. Low temperature induced oxidative stress tolerance in oats (Avena sativa L.) genotypes. Indian J. Plant Physiol. 2018, 23, 316–324. [Google Scholar] [CrossRef]
- Li, L.; Lu, X.; Ma, H.; Lyu, D. Comparative proteomic analysis reveals the roots response to low root-zone temperature in Malus baccata. J. Plant Res. 2018, 131, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Meguro-Maoka, A.; Yoshida, M. Analysis of sugar content and expression of sucrose transporter genes (OsSUTs) in rice tissues in response to a chilling temperature. Jpn. Agric. Res. Q. 2017, 51, 137–146. [Google Scholar] [CrossRef]
- Wang, H.; Gong, M.; Xin, H.; Tang, L.; Dai, D.; Gao, Y.; Liu, C. Effects of chilling stress on the accumulation of soluble sugars and their key enzymes in Jatropha curcas seedlings. Physiol. Mol. Biol. Plants 2018, 24, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Bolouri-Moghaddam, M.R.; Le Roy, K.; Xiang, L.; Rolland, F.; Van den Ende, W. Sugar signalling and antioxidant network connections in plant cells. FEBS J. 2010, 277, 2022–2037. [Google Scholar] [CrossRef]
- Van den Ende, W.; Valluru, R. Sucrose, sucrosyl oligosaccharides, and oxidative stress: Scavenging and salvaging? J. Exp. Bot. 2009, 60, 9–18. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Yang, M.T.; Li, X.; Zhou, Z.Q.; Wang, X.J.; Bai, J.G. Exogenous sucrose increases chilling tolerance in cucumber seedlings by modulating antioxidant enzyme activity and regulating proline and soluble sugar contents. Sci. Hortic. 2014, 179, 67–77. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Yang, M.T.; Chen, S.Y.; Zhou, Z.Q.; Li, X.; Wang, X.J.; Bai, J.G. Exogenous sucrose influences antioxidant enzyme activities and reduces lipid peroxidation in water-stressed cucumber leaves. Biol. Plant. 2015, 59, 147–153. [Google Scholar] [CrossRef]
- Gong, H.L.; Chen, Q.Q. Exogenous sucrose protects potato seedlings against heat stress by enhancing the antioxidant defense system. J. Soil Sci. Plant Nutr. 2021, 21, 1511–1519. [Google Scholar] [CrossRef]
- Wu, P.; Xiao, C.; Cui, J.; Hao, B.; Zhang, W.; Yang, Z.; Ahammed, G.J.; Liu, H.; Cui, H. Nitric oxide and its interaction with hydrogen peroxide enhance plant yolerance to low temperatures by improving the efficiency of the calvin cycle and the ascorbate–glutathione cycle in cucumber seedlings. J. Plant Growth Regul. 2021, 40, 2390–2408. [Google Scholar] [CrossRef]
- Ying, Z. Sugar-induced tolerance to the salt stress in maize seedlings by balancing redox homeostasis. Agric. For. Fish. 2016, 5, 126. [Google Scholar] [CrossRef]
- Wingler, A.; Tijero, V.; Müller, M.; Yuan, B.; Munné-Bosch, S. Interactions between sucrose and jasmonate signalling in the response to cold stress. BMC Plant Biol. 2020, 20, 176. [Google Scholar] [CrossRef]
- Lilley, J.L.; Gee, C.W.; Sairanen, I.; Ljung, K.; Nemhauser, J.L. An endogenous carbon-sensing pathway triggers increased auxin flux and hypocotyl elongation. Plant Physiol. 2012, 160, 2261–2270. [Google Scholar] [CrossRef]
- Sairanen, I.; Novák, O.; Pěnčík, A.; Ikeda, Y.; Jones, B.; Sandberg, G.; Ljung, K. Soluble carbohydrates regulate auxin biosynthesis via PIF proteins in Arabidopsis. Plant Cell 2012, 24, 4907–4916. [Google Scholar]
- Mir, A.R.; Siddiqui, H.; Alam, P.; Hayat, S. Foliar spray of Auxin/IAA modulates photosynthesis, elemental composition, ROS localization and antioxidant machinery to promote growth of Brassica juncea. Physiol. Mol. Biol. Plants 2020, 26, 2503–2520. [Google Scholar] [CrossRef] [PubMed]
- Yemelyanov, V.; Lastochkin, V.; Chirkova, T.; Lindberg, S.; Shishova, M. Indoleacetic acid levels in wheat and rice seedlings under oxygen deficiency and subsequent reoxygenation. Biomolecules 2020, 10, 276. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Chen, L.; Ye, T.; Liu, X.; Ding, K.; Chan, Z. Modulation of auxin content in Arabidopsis confers improved drought stress resistance. Plant Physiol. Biochem. 2014, 82, 209–217. [Google Scholar] [CrossRef]
- Khan, M.Y.; Prakash, V.; Yadav, V.; Chauhan, D.K.; Prasad, S.M.; Ramawat, N.; Singh, V.P.; Tripathi, D.K.; Sharma, S. Regulation of cadmium toxicity in roots of tomato by indole acetic acid with special emphasis on reactive oxygen species production and their scavenging. Plant Physiol. Biochem. 2019, 142, 193–201. [Google Scholar] [CrossRef]
- Qi, X.; Li, Q.; Shen, J.; Qian, C.; Xu, X.; Xu, Q.; Chen, X. Sugar enhances waterlogging-induced adventitious root formation in cucumber by promoting auxin transport and signalling. Plant Cell Environ. 2020, 43, 1545–1557. [Google Scholar] [CrossRef]
- Du, F.; Xu, J.N.; Li, D.; Wang, X.Y. The identification of novel and differentially expressed apple-tree genes under low-temperature stress using high-throughput Illumina sequencing. Mol. Biol. Rep. 2015, 42, 569–580. [Google Scholar] [CrossRef]
- Farooq, M.A.; Li, L.; Ali, B.; Gill, R.A.; Wang, J.; Ali, S.; Gill, M.B.; Zhou, W. Oxidative injury and antioxidant enzymes regulation in arsenic-exposed seedlings of four Brassica napus L. cultivars. Environ. Sci. Pollut. Res. 2015, 22, 10699–10712. [Google Scholar] [CrossRef]
- Juszczak, I.; Cvetkovic, J.; Zuther, E.; Hincha, D.K.; Baier, M. Natural variation of cold deacclimation correlates with variation of cold-acclimation of the plastid antioxidant system in Arabidopsis thaliana accessions. Front. Plant Sci. 2016, 7, 305. [Google Scholar] [CrossRef]
- Afzal, J.; Hu, C.; Imtiaz, M.; Elyamine, A.M.; Rana, M.S.; Imran, M.; Farag, M.A. Cadmium tolerance in rice cultivars associated with antioxidant enzymes activities and Fe/Zn concentrations. Int. J. Environ. Sci. Technol. 2019, 16, 4241–4252. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y.; Zheng, X.; Wang, Y. Silicon dioxide nanoparticles improve plant growth by enhancing antioxidant enzyme capacity in bamboo (Pleioblastus pygmaeus) under lead toxicity. Trees 2020, 34, 469–481. [Google Scholar] [CrossRef]
- Maksimović, J.D.; Zhang, J.; Zeng, F.; Živanović, B.D.; Shabala, L.; Zhou, M.; Shabala, S. Linking oxidative and salinity stress tolerance in barley: Can root antioxidant enzyme activity be used as a measure of stress tolerance? Plant Soil 2013, 365, 141–155. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, Q.; Zhu, J.; Liu, G.; Tang, H. Dissimilarity of ascorbate–glutathione (AsA–GSH) cycle mechanism in two rice (Oryza sativa L.) cultivars under experimental free-air ozone exposure. Agric. Ecosyst. Environ. 2013, 165, 39–49. [Google Scholar] [CrossRef]
- Yu, J.; Cang, J.; Li, Y.; Huang, R.; Lu, Q.; Wang, X.; Liu, L.; Xu, Q.; Zhang, K. Salicylic acid-induced antioxidant protection against low temperature in cold-hardy winter wheat. Acta Physiol. Plant. 2016, 38, 261. [Google Scholar] [CrossRef]
- Li, L.; Lu, X.; Ma, H.; Lyu, D. Jasmonic acid regulates the ascorbate–glutathione cycle in Malus baccata Borkh. roots under low root-zone temperature. Acta Physiol. Plant. 2017, 39, 174. [Google Scholar] [CrossRef]
- Castiglia, D.; Cardi, M.; Landi, S.; Cafasso, D.; Esposito, S. Expression and characterization of a cytosolic glucose 6 phosphate dehydrogenase isoform from barley (Hordeum vulgare) roots. Protein Expr. Purif. 2015, 112, 8–14. [Google Scholar] [CrossRef]
- Tian, Y.; Peng, K.; Bao, Y.; Zhang, D.; Meng, J.; Wang, D.; Wang, X.; Cang, J. Glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase genes of winter wheat enhance the cold tolerance of transgenic Arabidopsis. Plant Physiol. Biochem. 2021, 161, 86–97. [Google Scholar] [CrossRef]
- Jung, H.i.; Lee, B.R.; Chae, M.J.; Lee, E.J.; Lee, T.G.; Jung, G.B.; Kim, M.S.; Lee, J. Ascorbate-mediated modulation of cadmium stress responses: Reactive oxygen species and redox status in brassica napus. Front. Plant Sci. 2020, 11, 586547. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A. Auxin: A regulator of cold stress response. Physiol Plant. 2013, 147, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, K.; Wang, W.; Gong, W.; Liu, W.; Chen, H.; Xu, H.; Lu, Y. Low temperature inhibits root growth by reducing auxin accumulation via ARR1/12. Plant Cell Physiol. 2015, 56, 727–736. [Google Scholar] [CrossRef]
- Barbier, F.; Peron, T.; Lecerf, M.; Perez-Garcia, M.D.; Barriere, Q.; Rolcik, J.; Boutet-Mercey, S.; Citerne, S.; Lemoine, R.; Porcheron, B.; et al. Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida. J. Exp. Bot. 2015, 66, 2569–2582. [Google Scholar] [CrossRef] [PubMed]
- Meitzel, T.; Radchuk, R.; McAdam, E.; Thormählen, I.; Feil, R.; Munz, E.; Hilo, A.; Geigenberger, P.; Ross, J.; Lunn, J. Trehalose 6-phosphate promotes seed filling by activating auxin biosynthesis. New Phytol. 2021, 229, 1553–1565. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Dalal, M.; Verma, R.K.; Kumar, S.V.V.; Yadav, S.K.; Pushkar, S.; Kushwaha, S.R.; Bhowmik, A.; Chinnusamy, V. Auxin protects spikelet fertility and grain yield under drought and heat stresses in rice. Environ. Exp. Bot. 2018, 150, 9–24. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, F.; Xiao, Y.; Wang, W.; Wu, X. Peach PpSnRK1 participates in sucrose-mediated root growth through auxin signaling. Front. Plant Sci. 2020, 11, 409. [Google Scholar] [CrossRef]
- Zhang, X.W.; Liu, F.J.; Zhai, J.; Li, F.D.; Bi, H.G.; Ai, X.Z. Auxin acts as a downstream signaling molecule involved in hydrogen sulfide-induced chilling tolerance in cucumber. Planta 2020, 251, 69. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).