Influence of the Culture System and Harvest Time on the Specialized Metabolite Composition of Rocket Salad (Eruca sativa) Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant material, Preparation, and Experimental Design
2.2. Growth Conditions in the Three Culture Systems
2.2.1. Hydroponic Culture System
2.2.2. Aquaponic Culture System
2.2.3. Soil Culture System
2.3. Metabolite Extraction
2.4. Liquid Chromatography Coupled with Mass Spectrometry Analyses
2.5. Statistical Analyses
2.6. Annotation of Statistically Selected, Top-Ranked Metabolites by Culture System and Harvest Time Effect
3. Results and Discussion
3.1. Extraction and Chemical Composition of Eruca Sativa Leaves
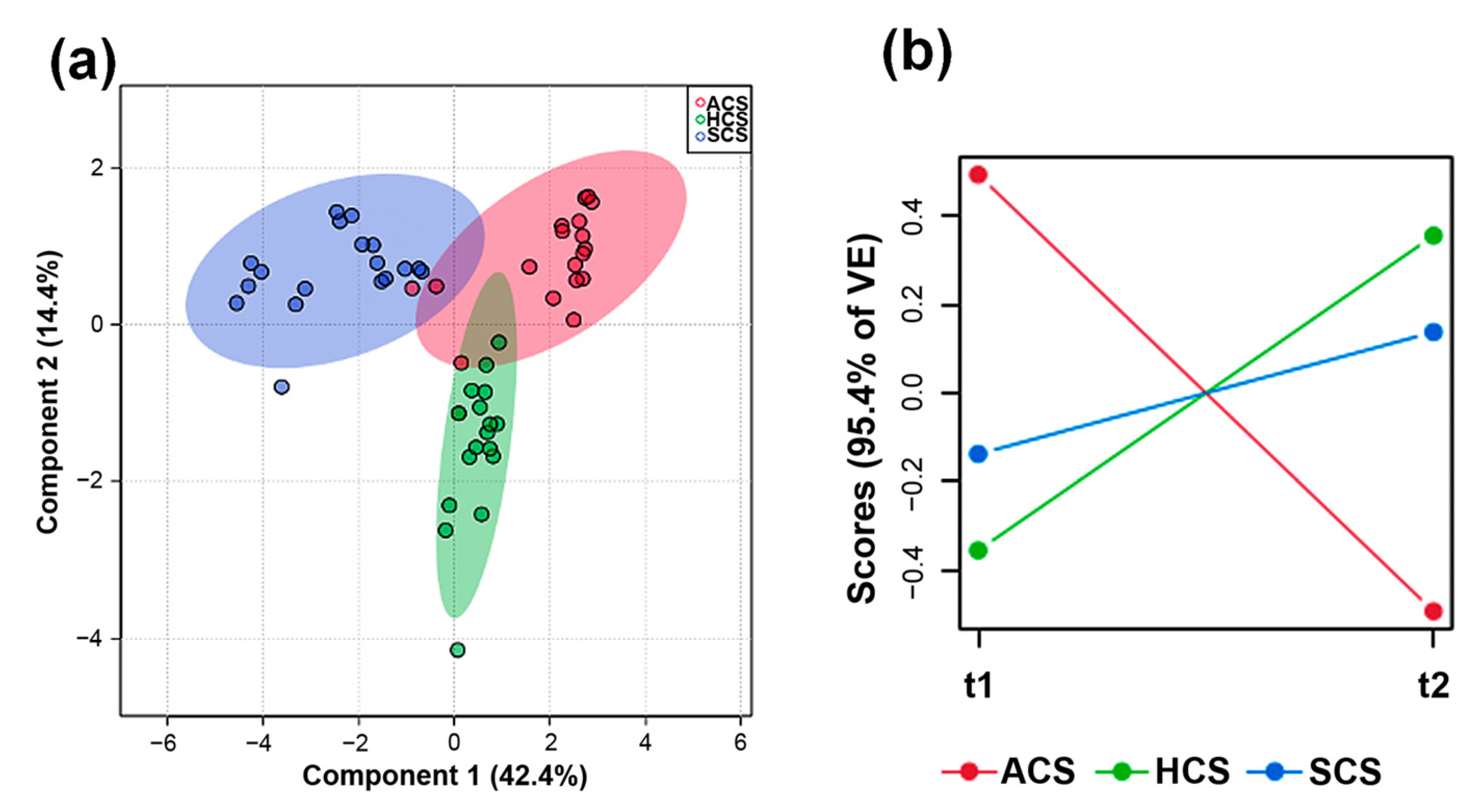
3.2. Multivariate Statistical Analysis on Chemical Data of E. sativa Leaves
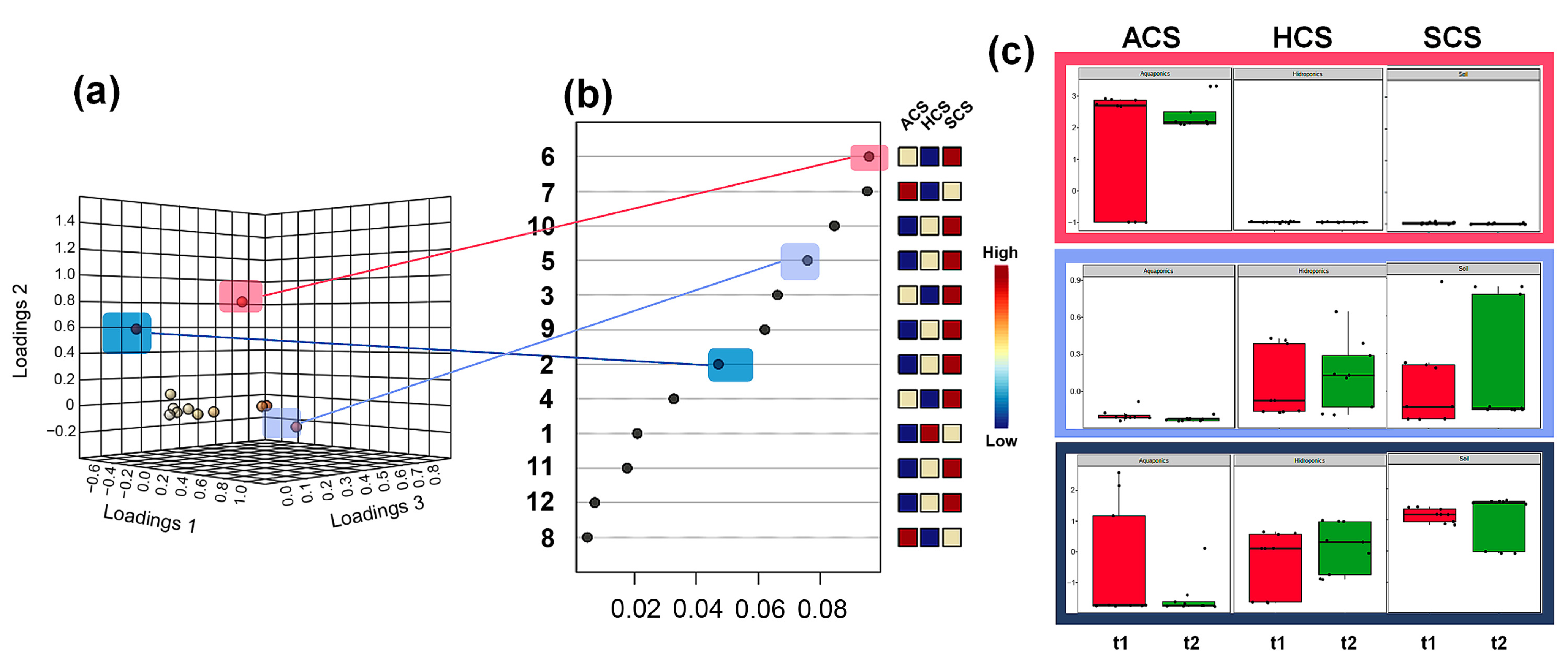
3.3. Identification of the Most Important Metabolites per Culture System and Harvest Time
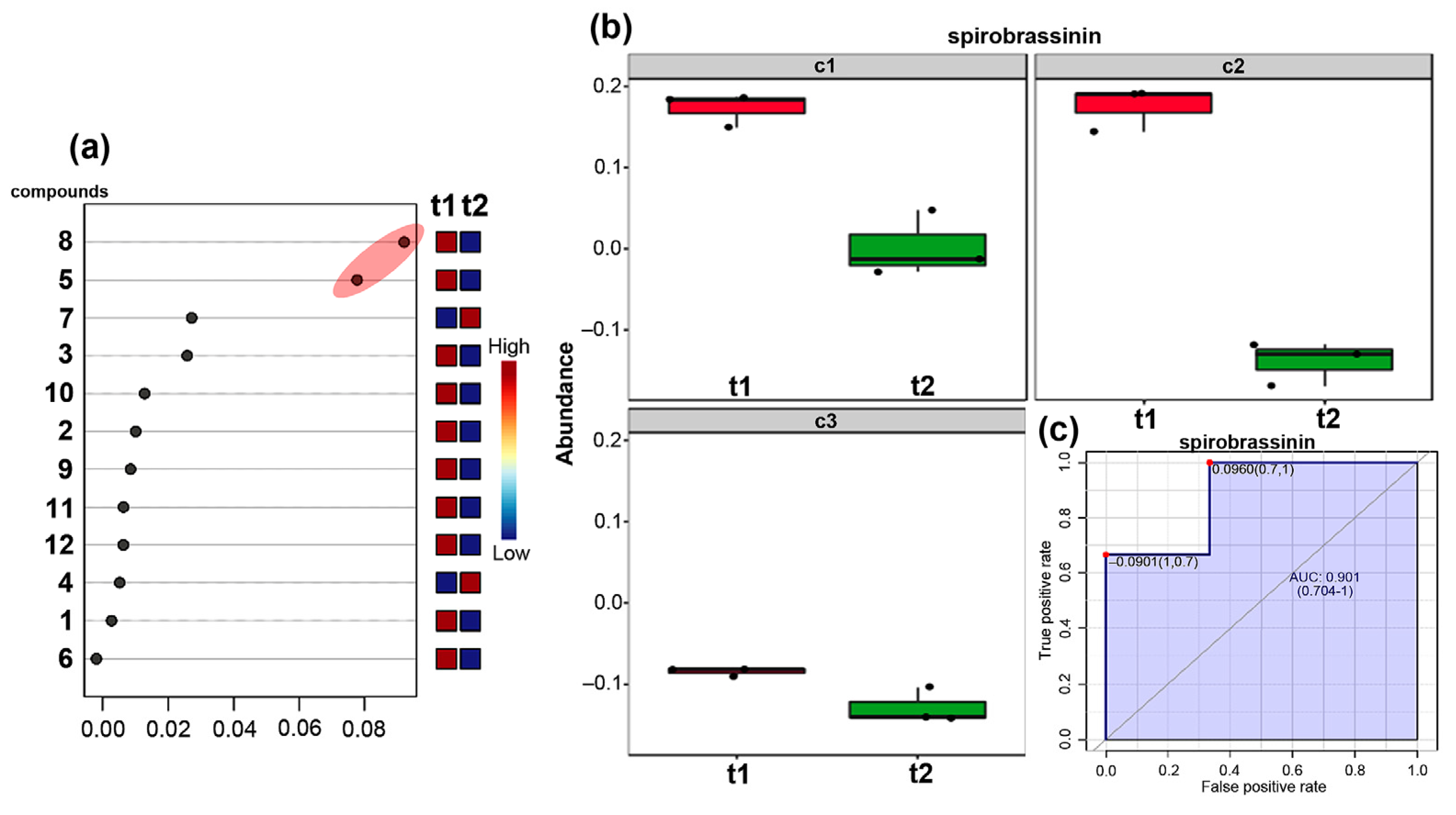
3.4. Analysis of Metabolite-Based Markers Associated with the Culture Type and Harvest Time
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Filho, A.B.C.; Maia, M.M.; Mendoza-Cortez, J.W.; Rodrigues, M.A.; Nowaki, R.H.D. Seasons of cultivation and splitting of nitrogen fertilization for arugula. Comun. Sci. 2014, 5, 252–258. [Google Scholar]
- Garg, G.; Sharma, V. Eruca sativa (L.): Botanical description, crop improvement, and medicinal properties. J. Herbs Spices Med. Plants 2014, 20, 171–182. [Google Scholar]
- Abdul, W.M.; Razvi, S.S. Eruca sativa L.—A promising source of drug lead for antimicrobial, neuroprotective and anticancer treatment regimens: Pharmacological properties of medicinal plant “Eruca sativa”. Eur. J. Cell Sci. 2019, 1, 17–21. [Google Scholar] [CrossRef]
- Ramazzina, I.; Lolli, V.; Lacey, K.; Tappi, S.; Rocculi, P.; Rinaldi, M. Fresh-cut Eruca Sativa treated with plasma activated water (PAW): Evaluation of antioxidant capacity, polyphenolic profile and redox status in Caco2 cells. Nutrients 2022, 14, 5337. [Google Scholar]
- Kim, J.; Jung, Y.; Song, B.; Bong, Y.S.; Ryu, D.H.; Lee, K.S.; Hwang, G.S. Discrimination of cabbage (Brassica rapa ssp. pekinensis) cultivars grown in different geographical areas using 1H NMR-based metabolomics. Food Chem. 2013, 137, 68–75. [Google Scholar]
- Kim, S.J.; Ishii, G. Glucosinolate profiles in the seeds, leaves and roots of rocket salad (Eruca sativa Mill.) and anti-oxidative activities of intact plant powder and purified 4-methoxyglucobrassicin. Soil Sci. Plant Nutr. 2006, 52, 394–400. [Google Scholar]
- Bell, L.; Oruna-Concha, M.J.; Wagstaff, C. Identification and quantification of glucosinolate and flavonol compounds in rocket salad (Eruca sativa, Eruca vesicaria and Diplotaxis tenuifolia) by LC-MS: Highlighting the potential for improving nutritional value of rocket crops. Food Chem. 2015, 172, 852–861. [Google Scholar] [CrossRef]
- Hall, M.K.D.; Jobling, J.J.; Rogers, G.S. Variations in the most abundant types of glucosinolates found in the leaves of baby leaf rocket under typical commercial conditions. J. Sci. Food Agric. 2014, 95, 552–559. [Google Scholar]
- Pasini, F.; Verardo, V.; Cerretani, L.; Caboni, M.F.; D’Antuono, L.F. Rocket salad (Diplotaxis and Eruca spp.) sensory analysis and relation with glucosinolate and phenolic content. J. Sci. Food Agric. 2011, 91, 2858–2864. [Google Scholar]
- Sarwar Alam, M.; Kaur, G.; Jabbar, Z.; Javed, K.; Athar, M. Eruca sativa seeds possess antioxidant activity and exert a protective effect on mercuric chloride induced renal toxicity. Food Chem. Toxicol. 2007, 45, 910–920. [Google Scholar] [CrossRef]
- Kim, B.; Choi, Y.E.; Kim, H.S. Eruca sativa and its flavonoid components, quercetin and isorhamnetin, improveskin barrier function by activation of peroxisome proliferator-activated receptor (PPAR)-α and suppression of inflammatory cytokines. Phyther. Res. 2014, 28, 1359–1366. [Google Scholar]
- Doležalová, I.; Duchoslav, M.; Dušek, K. Biology and yield of rocket (Eruca sativa Mill.) under field conditions of the Czech Republic (Central Europe). Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 530–537. [Google Scholar]
- Nicola, S.; Hoeberechts, J.; Fontana, E. Comparison between traditional and soilless culture systems to produce rocket (Eruca sativa) with low nitrate content. Acta Hortic. 2005, 697, 549–555. [Google Scholar] [CrossRef]
- Ronzón, M.; Hernández, M.P.; Pérez, C.I. Aquaponic production of three vegetables in systems associated to semi-intensive growth of grey tilapia (Oreochromis niloticus). Agroproductividad 2015, 8, 26–32. [Google Scholar]
- Bennett, R.N.; Rosa, E.A.S.; Mellon, F.A.; Kroon, P.A. Ontogenic profiling of glucosinolates, flavonoids, and other secondary metabolites in Eruca sativa (salad rocket), Diplotaxis erucoides (wall rocket), Diplotaxis tenuifolia (wild rocket), and Bunias orientalis (Turkish rocket). J. Agric. Food Chem. 2006, 54, 4005–4015. [Google Scholar]
- Chun, J.H.; Kim, S.; Arasu, M.V.; Al-Dhabi, N.A.; Chung, D.Y.; Kim, S.J. Combined effect of nitrogen, phosphorus and potassium fertilizers on the contents of glucosinolates in rocket salad (Eruca sativa Mill.). Saudi J. Biol. Sci. 2017, 24, 436–443. [Google Scholar] [CrossRef]
- Somerville, C.; Cohen, M.; Pantanella, E.; Stankus, A.; Lovatelli, A. Small-Scale Aquaponic Food Production, 1st ed.; FAO—Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; ISBN 9789251085325. [Google Scholar]
- Rodríguez-Delfí n, A. Advances of hydroponics in Latin America. Acta Hortic. 2012, 947, 23–32. [Google Scholar]
- Rodríguez-Delfín, A.; Hoyos, M.; Chang, M.; Castro, G.; Barreda, E.; Tamo, J. Evaluation of growth and yield of ‘roja arequipeña’ onion grown in two natural substrates. Acta Hortic. 2005, 697, 505–510. [Google Scholar]
- Rodríguez-Delfín, A.; de Jesus López-Herrera, A.; Rojas-Rojas, V.; García-Galindo, B.F. Yield evaluation of lettuce grown in three modified NFT systems in pandemic times. Curr. Top. Agron. Sci. 2022, 2. [Google Scholar]
- Cárdenas-Laverde, D.; Rincón-Aceldas, S.; Coy-Barrera, E. Identification of antifungal compounds from Piper plants against Fusarium oxysporum: An untargeted metabolite profiling-based approach. Nat. Prod. Commun. 2022, 17, 1934578X221089995. [Google Scholar]
- Chong, J.; Wishart, D.S.; Xia, J. Using metaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinforma. 2019, 68, e86. [Google Scholar] [CrossRef]
- Zheng, Y.; Cai, T.; Feng, Z. Application of the time-dependent ROC curves for prognostic accuracy with multiple biomarkers. Biometrics 2006, 62, 279–287. [Google Scholar]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Molecular Formula Finder. Available online: https://www.chemcalc.org (accessed on 12 December 2022).
- Dictionary of Natural Products. Available online: https://dnp.chemnetbase.com/ (accessed on 15 December 2022).
- KNApSAcK Core System. Available online: http://kanaya.naist.jp/knapsack_jsp/top.html (accessed on 13 December 2022).
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 17 December 2022).
- Roosta, H.R.; Hamidpour, M. Effects of foliar application of some macro- and micro-nutrients on tomato plants in aquaponic and hydroponic systems. Sci. Hortic. 2011, 129, 396–402. [Google Scholar] [CrossRef]
- Yildiz, H.Y.; Robaina, L.; Pirhonen, J.; Mente, E.; Domínguez, D.; Parisi, G. Fish welfare in aquaponic systems: Its relation to water quality with an emphasis on feed and faeces-A review. Water 2017, 9, 13. [Google Scholar] [CrossRef]
- Creek, D.J.; Dunn, W.B.; Fiehn, O.; Griffin, J.L.; Hall, R.D.; Lei, Z.; Mistrik, R.; Neumann, S.; Schymanski, E.L.; Sumner, L.W.; et al. Metabolite identification: Are you sure? And how do your peers gauge your confidence? Metabolomics 2014, 10, 350–353. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Okanga, F.I.; Zaharia, I.L.; Khan, A.Q. Phytoalexins from crucifers: Synthesis, biosynthesis, and biotransformation. Phytochemistry 2000, 53, 161–176. [Google Scholar]
- Huber, A.M.; Mamyrova, G.; Lachenbruch, P.A.; Lee, J.A.; Katz, J.D.; Targoff, I.N.; Miller, F.W.; Rider, L.G. Early illness features associated with mortality in the juvenile idiopathic inflammatory myopathies. Arthritis Care Res. 2014, 66, 732–740. [Google Scholar]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Gupta, S.K. Biotechnology of Crucifers; Gupta, S.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 9781461477952. [Google Scholar]
- Goddek, S.; Joyce, A.; Wuertz, S.; Körner, O.; Bläser, I.; Reuter, M.; Keesman, K.J. Decoupled Aquaponics Systems. In Aquaponics Food Production Systems; Goddek, S., JoyceBenz, A., Kotzen, G., Burnell, G.M., Eds.; Springer Open: Wageningen, The Netherlands, 2019; pp. 201–229. ISBN 9783030159429. [Google Scholar]


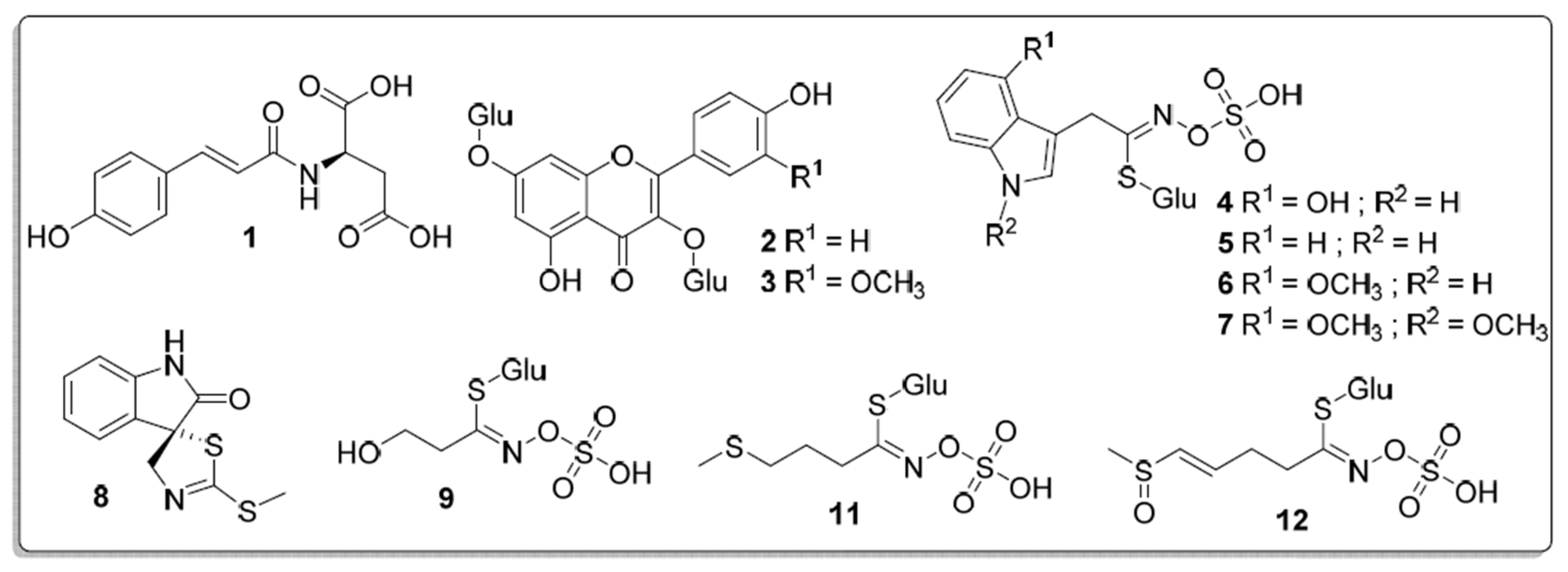
| No | Rt (min) | [M+H]+ m/z | [M-H]− m/z | Metabolite | Accurate Mass [M-H]− | Type |
|---|---|---|---|---|---|---|
| 1 | 8.5 | 280 | 278 | N-coumaroylaspartate | 278.0677 | APA |
| 2 | 18.1 | 611 | 609 | kaempferol diglucopyranoside | 609.1466 | F |
| 3 | 18.4 | 641 | 639 | isorhamnetin diglucopyranoside | 639.1573 | F |
| 4 | 20.2 | 465 | 463 | 4-hydroxyglucobrassicin | 463.0471 | ICG |
| 5 | 21.5 | 449 | 447 | glucobrassicin | 447.0541 | ICG |
| 6 | 21.9 | 479 | 477 | 4-methoxyglucobrassicin | 477.0649 | ICG |
| 7 | 25.9 | 297 | 295 | N,4-dimethoxybrassinin | 295.0584 | IP |
| 8 | 26.8 | 251 | 249 | spirobrassinin | 249.0142 | IP |
| 9 | 33.0 | 364 | 362 | hydroxyethylglucosinolate | 362.0222 | ABG |
| 10 | 36.4 | 504 | 502 | unidentified | 502.1563 | - |
| 11 | 37.4 | 408 | 406 | glucoiberverin | 407.0382 | SCG |
| 12 | 42.5 | 436 | 434 | glucoraphenin | 434.0261 | SCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buitrago-Villanueva, I.; Barbosa-Cornelio, R.; Coy-Barrera, E. Influence of the Culture System and Harvest Time on the Specialized Metabolite Composition of Rocket Salad (Eruca sativa) Leaves. Horticulturae 2023, 9, 235. https://doi.org/10.3390/horticulturae9020235
Buitrago-Villanueva I, Barbosa-Cornelio R, Coy-Barrera E. Influence of the Culture System and Harvest Time on the Specialized Metabolite Composition of Rocket Salad (Eruca sativa) Leaves. Horticulturae. 2023; 9(2):235. https://doi.org/10.3390/horticulturae9020235
Chicago/Turabian StyleBuitrago-Villanueva, Ivon, Ricardo Barbosa-Cornelio, and Ericsson Coy-Barrera. 2023. "Influence of the Culture System and Harvest Time on the Specialized Metabolite Composition of Rocket Salad (Eruca sativa) Leaves" Horticulturae 9, no. 2: 235. https://doi.org/10.3390/horticulturae9020235
APA StyleBuitrago-Villanueva, I., Barbosa-Cornelio, R., & Coy-Barrera, E. (2023). Influence of the Culture System and Harvest Time on the Specialized Metabolite Composition of Rocket Salad (Eruca sativa) Leaves. Horticulturae, 9(2), 235. https://doi.org/10.3390/horticulturae9020235