Abstract
Studies conducted in controlled environments showed that seed priming and foliar treatments with various bioactive substances can stimulate germination, uniform seedling emergence, photosynthesis, and nutrient uptake efficiency and can lead to increased productivity, crop stand, and quality. Only a few studies provide a comparative experimental outlook about the use of the bioactive substances in open-field cultivated canola. The aim of this study was to investigate the effects of seed priming and foliar treatment with ascorbate (AsA), cysteine (Cys), and triacontanol (Tria) on the growth and yield parameters of two canola cultivars rapeseed cultivars (“Factor” and “Hybrirock”) under open-field conditions for three growing seasons under temperate conditions from Central Transylvania. Plant growth and yield parameters were recorded at different plant development stages: in autumn (early vegetative period), in spring, and at harvest. Not all substances that have undergone laboratory testing were successful in the field. Seed priming with 10 ppm Cys and AsA had beneficial effects on the development of seedlings, whereas their foliar application enhanced the number of silique, seed yield, and the oil content of the studied canola cultivars. The effects of Tria were visible on the biometrics parameters only in autumn and spring, and also on the root parameters, but in some cases, 10 ppm Tria had no effect on plants, or even a negative effect could be observed on important parameters for agriculture such as estimated seed yield, seed yield/plant, and oil content. Optimizing treatment and application by determination of the lowest effective concentration, together with the frequency of treatments and persistence effects are crucial for cost-effectiveness.
1. Introduction
On a global scale, canola is the second most important oil crop after soybean [1]. It can be utilized as animal forage, as human food, or as a source of biofuel. Furthermore, its cultivation is an important element of good agricultural practice, incorporating canola in the crop rotation and having beneficial effects on soil fertility. In Europe, the winter-sown cultivar is more widespread, with the highest yields, of 3.3 t ha−1 and 4.3 t ha−1, being achieved in Central and Western Europe [2].
Until 2019, there was a global, constant increase in the area utilized for canola cultivation, the average yields, and overall production. Compared to the data of 2004, in 2018, a 61% increase was achieved in overall production. In 2019, a 7% decrease was observable, with worldwide production reaching 70.51 million tons; Canada and the E.U. were the most important regions of cultivation [1]. Global climate change causes more and more periods of droughts with severe consequences, and the decrease in canola yields in several parts of the globe can be attributed to this phenomenon [3]. Low temperature is another significant environmental factor which limits the productivity of agricultural crops, especially at higher elevations, and it is especially harmful during early developmental stages and during flower induction [4,5].
In terms of agricultural production, it is important to increase the tolerance of economic plants to abiotic stress [6]. There are several methods for enhancing stress tolerance in the case of cultivated plants, which include the traditional breeding methods and also techniques of modern gene engineering. Both paths have their limitations, such as high human resource and time demand, or financial requirements. In addition, the use of genetic engineering is not permitted in several countries. There are various approaches in use for seed preconditioning, such as hydro-, osmo-, halo-, hormonal-, nutri-, bio-, or redox-priming, depending on the plant species and on the stress factor(s) [7]. Seed priming (applied to seeds or seedlings) can be considered a cost-effective, low-input, environmentally friendly, sustainable method that can be easily implemented in farm-scale crop production [8,9].
The list of substances used in pretreatment of seeds and seedlings are long [10,11,12]. From the list, one such substance is L-ascorbic acid or ascorbate (AsA), also known as vitamin C; vitamin C is a natural antioxidant, and its metabolites are found in different plants. These metabolites help plants to acclimate to environmental conditions that may induce oxidative stress [13]. Vitamin C is frequently applied to plants, and the physiological and developmental processes of plants are positively impacted by its exogenous application [14]. AsA may improve drought tolerance by reducing oxidative stress and enhancing photosynthesis, seed germination, leaf, and root development, and lipid peroxidation [15].
Species belonging to the Brassicaceae family (such as canola) have a high sulfur demand [16,17]. Cysteine (Cys) is one of the 20 naturally occurring proteinogenic amino acids. Structurally, it belongs to the sulfur-containing amino acids. As an antioxidant, Cys can inactivate reactive oxygen species and protect enzymes from oxidative inactivation and, thus, protect cells from oxidative damage [18]. Cysteine has reductant properties, enhances photosynthetic pigments and growth characteristics, and reduces oxidative stress caused by NaCl [19]. The antioxidant enzymes’ activity can be increased by cysteine in small concentrations in seed treatment and foliar application [20].
Triacontanol (Tria) is a natural wax constituent of the epicuticular layer of the epidermal cell wall, which has the attributes of plant growth regulators. The positive effect of Tria consists of enhancement of plant growth, stress tolerance, and yield [21,22,23].
Numerous studies examine how abiotic stress affects crops. These stresses could cause a variety of problems, including the production of reactive oxygen species, membrane deterioration, loss of photosynthetic efficiency, altered nutrient uptake, etc., which could alter crop growth and development by affecting biochemical, physiological, and molecular processes, resulting in a significant loss in productivity [24,25,26,27].
When seeds are primed, a physiological situation results that stimulates germination and improves uniform seedling emergence (via causing modulation in hormones, metabolic activities, dormancy, and membrane permeability) [7,8,28,29].
The exogenous application of various bioactive substances to the leaf area and seed priming boost biotic and abiotic resistance in plants by improving antioxidant defense system activity and osmoprotection, as well as by controlling stress-related proteins. In addition, the bioactive substances improve photosynthesis, nutrient uptake efficiency, hormonal changes in crops, productivity, crop stand, and quality [30,31].
According to Farooq’s study [32], seed priming with AsA increased seedling emergence, early growth, root length, and seedling dry weight in both drought and well-watered environments. In addition to these, AsA improved antioxidant potential and osmotic adjustment, which increased the ability of the wheat to withstand drought. By modulating antioxidant mechanisms, priming with AsA is an efficient management method that can be used to reduce the negative effects of salt stress and improve the salt tolerance of tomato plants [33].
Currently available research shows that Tria application in the form of foliar spray considerably reduced the negative effects of drought, salinity, and Cd-induced oxidative stress by increasing photosynthetic pigments and proline content and by reducing the reactive oxygen species (ROS) content, lipoxygenase (LOX) activity, and lipid peroxidation level. In addition, Tria, in some cases, increased plant biomass and enhanced growth and essential oil content [34,35,36]. In particular, under drought stress conditions, foliar application of AsA improved the morphological parameters (shoot and root fresh weight and root dry weight) and chlorophyll fluorescence properties (photochemical quenching (PQ), non-photochemical quenching (NPQ)), as well as the activity of the peroxidase (POD) enzyme of canola [37]. Cysteine reduced oxidative damage and growth inhibition caused by salt stress by activating antioxidant enzymes, increasing glutathione content, and reducing ion toxicity [38,39].
Studies have confirmed the positive effects of seed priming on germination processes, as well as on the early development of plants. The majority of these studies were conducted in a controlled environment, such as growth chambers or greenhouses [40,41].
The most frequent cause of crop risk in open-field production is the combination of several abiotic stressors occurring at once. Future research initiatives with the aim of creating crops and plants with improved tolerance to naturally occurring environmental conditions should focus on tolerance to a variety of stress conditions (especially those that reflect the field environment conditions) [42,43].
The aim of the present study was to investigate the effects of seed priming and plant foliar treatment with ascorbate, cysteine, and triacontanol on the growth and yield parameters of two canola cultivars under open-field conditions in order to assess their growth and yield enhancing potential.
2. Materials and Methods
2.1. Location and Conditions
The experiment took place at the Experimental and Didactic Field of Sapientia Hungarian University of Transylvania, Faculty of Technical and Human Sciences, Târgu Mureș (46.5217° N, 24.5989° E), Romania, during three vegetation periods (2017–2020) under open-field conditions.
2.1.1. Climate
The climate is temperate continental; therefore, there can occur weather fluctuations from one year to another, which are characteristic of this climate. During the experiment, the meteorological data were recorded at the location. The air temperature and precipitation were measured with an automatic weather station (type WS-GP1) situated in the proximity of the experimental field. Climatic data are presented in Table 1, Table 2 and Table 3, Tables S1 and S2.

Table 1.
Temperature trends during the canola growing seasons (2017–2020) and comparison with the multiannual (1981–2010) mean values of the region.

Table 2.
Precipitation trends during the canola growing seasons (2017–2020) and comparison with the multiannual (1981–2010) mean values of the region.
In all three growing seasons, average annual temperature was higher than the long-term average (1981–2010) by 1.4 °C in 2017–2018, 1.2 °C in 2018–2019, and by 1.5 °C in 2019–2020. The mean temperatures recorded during winter dormancy (December to February) and in the growing season (March to July) differed from the long-term average. The deviation from the long-term average monthly temperature during winter dormancy was higher, with 2.6 °C in 2017–2018, 1.5 °C in 2018–2019, and 2.2 °C in 2019–2020 (Table 1). There were also frigid nights in March and April, as seen by the daily minimum and maximum temperatures (Table S1). In the first and second seasons, mean monthly temperatures were higher than the long-term average, in particular in May and July. In the third season, mean monthly temperatures approximated the long-term average (1981–2010). Mean monthly precipitation reached 55.1 mm in 2017–2018, 44 mm in 2018–2019, and 49.2 mm in 2019–2020. Long-term average of monthly precipitation (1981–2010) in the analyzed area was 48.5 mm. Total precipitations from sowing to the end of autumn growth differed from the long-term average. In the first growing season, the deviation from long-term average was −3 mm; in the second, the deviation was −89 mm; and in the third, the deviation was −22 mm (Table 2).
In order to analyze meteorological conditions on canola developmental stages, Sielianinov’s hydrothermal index (K) was used (Table 3) [44,45]. The index is computed as follows:
where P is sum of monthly precipitation in mm, and ∑t is sum of daily mean air temperatures > 0 °C.
K = P/0.1 ∑t,
Based on the calculated Sielianinov hydrothermal coefficient, the first growing season was quite wet, the second and the third were quite dry (Table 3).

Table 3.
Hydrothermal conditions during canola growing period.
Table 3.
Hydrothermal conditions during canola growing period.
| Sielianinov’s Hydrothermal Index * | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Years | Aug. | Sept. | Oct. | Mar. | Apr. | May | June | July | Mean |
| 2017–2018 | 0.60 | 0.90 | 1.70 | 4.80 | 0.40 | 1.30 | 2.20 | 1.04 | 1.61 |
| 2018–2019 | 0.20 | 0.70 | 0.70 | 0.70 | 0.80 | 3.00 | 0.70 | 1.34 | 1.02 |
| 2019–2020 | 0.80 | 1.10 | 1.00 | 1.40 | 0.40 | 1.50 | 2.20 | 1.41 | 1.23 |
* Hydrothermal index value [45]: up to 0.4 extremely dry; 0.41–0.7 very dry; 0.71–1.0 dry; 1.01–1.3 quite dry; 1.31–1.6 optimal; 1.61–2.0 quite wet; 2.01–2.5 wet; 2.51–3.0 very wet; >3 extremely wet.
2.1.2. Soil and Seeding Bed Preparation
The field experiments were carried out on stagnic preluvosoil, in which the soil-forming rock is clayey marl, slope sediment with a high content of montmorillonite and illite-type clay minerals (swelling clay minerals). When it dries out, it compacts strongly and cracks (root rupture may occur); when it becomes excessively wet, it swells strongly, and aeration conditions decrease. The measurements of the soil horizons are as follows: Ap 0–20 cm, Auw 20–40 cm, A/B 41–56 cm, Btwy1 56–70 cm, Btwy2 70–95 cm, B/C 95–120 cm, C 120→. The clay content of the subsoil exceeds the clay content of the topsoil by 1:1.2 part. Clay migration (lessivage) can be recognized on the site by the darker colored and waxy clay membranes that can be seen on the structural elements of the accumulation level (subsoil).
Due to the B level (Bt) with a large amount of leaching clay accumulation, a stagnant water effect develops in the soil, as a result of which iron and manganese oxides are released in large quantities and, in the form of plaque and agglomerates (iron/manganese “peas”), they contribute to the increase in soil acidity.
The agrochemical characteristics of the Ap horizon (0–20 cm) are as follows: pH in KCl—6.47, content of total nitrogen—0.110%, exchangeable phosphorus—58 mg P kg−1, potassium—354 mg K kg−1, humus—2.46 mg kg−1. The soil was sampled before fertilization, and the chemical properties were determined at the Mureș Office of Pedological and Agrochemical Studies.
The field was prepared for sowing by a 25 cm deep plowing followed by seedbed preparation. Complex NPK fertilizer with a 16-16-6% active ingredient content and NH4NO3 with 3.5%N content (50–50%) were incorporated into the soil in a 600 kg ha−1 rate in two equal batches: one prior to sowing (August) and the other in early spring (February). Sowing was executed with a manual machine on the following dates: 20 August 2017, 24 August 2018, and 30 August 2019.
The sowing rate was 60 seeds per m2; row spacing was 25 cm. To achieve a more uniform emergence, a single dose of irrigation was carried out after sowing. For the control of weeds, manual labor was used, whereas for disease and pest control, chemicals were applied with a 10 L capacity manual sprayer.
2.2. Biologic Material
In this study, the seeds of the two canola (Brassica napus ssp. oleifera (DC.) Metzg., syn. Brassica napus L.) cultivars, namely “Factor”, and “Hybrirock”, were used. These seeds were obtained from KWS, and they were chosen because they are the most widely used by farmers in the region. The characteristics of the two genotypes are presented in Table 4.

Table 4.
Characteristics of canola cultivars [46].
2.3. Experimental Design
This was a bi-factorial experiment with the following factors:
Factor H: the cultivar with two levels
- H1—”Factor”;
- H2—”Hybrirock”.
Factor T: the treatment with four levels
- Cys—seed treatment with 10 mM + foliar application at BBCH 16 and BBCH 30;
- AsA—seed treatment with 10 mM + foliar application at BBCH 16 and BBCH 30;
- Tria—seed treatment with 10 ppm + foliar application at BBCH 16 and BBCH 30;
- Tap water (Control).
From the combination of the two factors (H × T) resulted eight experimental variants, and the experiment was repeated in three consecutive years to obtain statistical assurance. The field experiment was established in a Randomized Block Design (RBD); 12 m2 (6 × 2 m) plots were designated for each treatment plus the control group, using four replications. Additional 1.5 m-wide isolation strips were left between the plots for a better control (Figure 1).
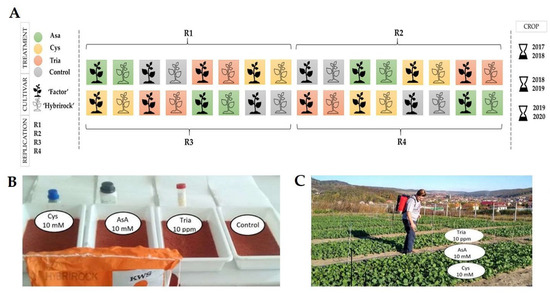
Figure 1.
The experimental treatments and design. (A) Experiment layout: two canola cultivars under four treatments during three-year study. (B,C) Aspects of the seed treatments and the canola crop (original).
Three natural bioactive compounds were used in the experiments: L-ascorbate (AsA, the reduced form of vitamin C in aqueous solution, purchased from VWR); L-cysteine (Cys, purchased from Merck); and triacontanol (Tria, provided by Nutri-Tech Solution in the form of 2.5% stock solution).
The effective seed-priming concentrations of the three bioactive compounds (AsA, Cys, and Tria) were established in a previous experiment [47].
The priming solutions were applied three times. At first, the application was executed before direct sowing as seed priming. The seeds of the two canola cultivars were soaked in aqueous solutions of 10 mM ascorbate, 10 mM cysteine, and 10 ppm triacontanol, respectively, in tap water for the control. The seeds were sowed after 12 h of drying. The second and third applications took place in autumn at BBCH 16 and in spring at BBCH 30 stages of the canola plants by spraying the solutions on their leaves as described by Meier [48].
The canola was harvested in July. Harvest took place at full maturity of seeds (5 plants/treatments/repetitions (80 plants)). Seed yield was expressed in t ha−1 at a moisture content of 10%, based on data from 20 plants per treatment.
2.4. Measurement of Plant Growth Parameters and Yield
- Data about plant growth were collected at different development stages:
- In autumn (in the early vegetation period)—plant height (PHA-cm), number of leaves per plant (NL);
- In spring—plant height (PHS-cm) and the number of branches per plant (NBS);
- At harvest time—plant height (PHH-cm) and plant fresh weight (PFW-g), number of branches per plant (NBH), root length (RL-cm), root neck diameter (RND-mm), and root fresh weight (RFW-g). Furthermore, data regarding the productivity of the plants were collected: number and weight of siliquae (NS and WS-g) per plant, thousand seed weight (TSW-g), seed yield per plant (SYP-g/plant), and estimated seed yield (ESY-t/ha). The oil content of the seed (OC-%) was also examined.
For measurements of the height of the plant, number of leaves, and number of branches, 20 plants were collected for each treatments/repetitions (320 plants); the plants were collected in autumn and also in spring.
The number of plants per 1 m2 was counted before harvesting. For biometric measurements at harvest time and yield parameters, 5 plants/treatments/repetitions (80 plants) were collected. Plant height was measured in centimeters from the surface of the soil to the position of the highest standing bud. After being carefully taken from the ground, the length of the root was also measured in centimeters.
The mass parameters were measured with a KERN PNS 600-3 (620 g/0.001 g) type digital laboratory scale, whereas root neck diameter measurements were performed with the help of a digital caliper.
Seeds used for the thousand grain weight were numbered with a Pfeuffer Contador2 seed counter. The determination of oil content of seeds was made with a Mininfra SmarT SW grain analyzer (Infracont Instruments) [49].
2.5. Statistical Analysis
Normally distributed data were analyzed with one-way ANOVA and the Tukey’s pairwise test for the identification of significant differences among the medians. For datasets with non-normal distribution, the Kruskal–Wallis and the Mann–Whitney pairwise test were used. In order to understand whether there is an interaction between treatment and year or between treatment and cultivar on the measured parameters among canola, factorial analyses (two-way ANOVA) were carried out. For these analyses, the data for PHH and OC were used because other parameters did not meet the assumptions required for a two-way ANOVA (normal distribution and homogeneity of variances for each combination of the groups of the two independent variables). The assumptions were tested using Levene’s test for homogeneity of variances and the Shapiro–Wilk test for normality. The statistical analyses were performed with SPSS software (version 27).
PCA was performed to correlate hydrothermal index (calculated from the monthly precipitation and the daily air temperatures over 0 °C) and mean monthly precipitation (average rainfall), as environmental variables, with the parameters assessed (plant height, number of leaves, number of branches, root length, root neck diameter, above-ground and underground plant biomass, parameters of yield, and oil content of the seeds) during the three growing seasons. Before analyses, all data were first averaged and log10 transformed. The PCA analyses were performed in Past (version 3.06).
3. Results
A two-way ANOVA was conducted that examined the effect of treatment and year on PHH. The results of the two-way ANOVA indicate that there was a statistically significant interaction between the effect of treatment and year, F = 3.339, p = 0.003, on PHH (Plant Height at Harvest). Thus, the effect of treatment on PHH was influenced significantly by the weather conditions from the growing seasons. Cys significantly enhanced PHH compared to Tria and control plants in the first year (Table 5), when average rainfall values were higher and the hydrothermal index indicated quite wet conditions (Table 2 and Table 3). There were no differences between treatments in the second and third growing seasons (Table 5), when the values of the hydrothermal index indicated quite dry conditions (Table 3).

Table 5.
Effects of seed priming and foliar treatment with bioactive substances on canola plant height at harvest (PHH).
Another two-way ANOVA that examined the effect of treatment and cultivar on PHH was conducted. No significant interaction was observed between the effect of treatment and cultivar (F = 0.339, p = 0.823). This means that the effect of treatment on PHH was not influenced significantly by the cultivar. The H2 had higher values for PHH than the H1, regardless of the treatment. Generally, the bioactive substances had a positive effect on the PHH compared to the control plants (means in the last column of Table 5), and in addition, the PHH had higher values in the first and third years (means in the last row of Table 5), when the average rainfall was higher (Table 2). PHH depended more on the weather conditions and cultivar than the applied treatments.
According to the factorial analysis, the effect of treatment on OC (Oil Content) was not influenced significantly either by the year (F = 0.843, p = 0.539) or by the cultivar (F = 2.316, p = 0.080). AsA and Cys had significantly higher values for the OC than control plants in the first year (Table 6). Generally, AsA and Cys had a significantly positive effect compared to the control plants (means in the last column of Table 6) and Tria. OC had significantly higher values in the second year (means in the last row of Table 6). The H2 had higher values for OC than the H1, regardless of the treatment. OC depended also on the weather conditions, cultivar, and applied treatments.

Table 6.
Effects of seed priming and foliar treatment with bioactive substances on canola oil content (OC).
The results of the PCA analysis for the H1 (Figure 2A) showed that 71.78% of the total variance could be explained with the first two components (PC1—50.54%, PC2—21.24%). Measurements from the first growing season presented positive correlation with axis 1 (PC1) and negative correlation with axis 2 (PC2). Mainly the hydrothermal index (Hydtherm) and average rainfall (Prec) but also PHH, SYP (Seed Yield/Plant), ESY (Estimated Seed Yield), WS (Weight of Siliquae/plant), PFW (Plant Fresh Weight), RND (Root Neck Diameter), and RFW (Root Fresh Weight) were correlated positively with component 1. Therefore, the quite wet conditions favored the above mentioned parameters. Negative correlations could be observed between component 1, weather conditions, and the following parameters: mainly OC, PHA, and NL but also TSW (Thousand Seed Weight) and NS. In the first growing season, in the cases of both cultivars, compared to the control plants, AsA significantly increased the following parameters: PHA, NL, NBS, and RFW (Table 7, Table 8 and Table 9). Similarly, Cys increased the values for PHA, NBS, and RFW, whereas Tria increased only the value of NBS (Number of Branches/plant at Spring). Therefore, the increase in PHA in the first growing season can be attributed to AsA or Cys, the values being significantly higher as compared to Tria and the control group, whereas the increase in NBS to AsA, Cys, or Tria and the increase in NL (Number of Leaves/plants at Autumn) can be attributed to AsA. The H1 cultivars treated with AsA had significantly higher values for PFW, RL, and NS than control plants (Table 8 and Table 9). The same cultivars treated with Cys had significantly higher values for RL and OC. Tria also increased RL significantly. Weather conditions correlated positively with PFW and negatively with RL (Root Length), NS (Number of Siliquae/plant), and OC (Figure 2A). The cultivar-dependent positive effects of the treatments were AsA on NS, Cys on RL and OC, and Tria on RL.
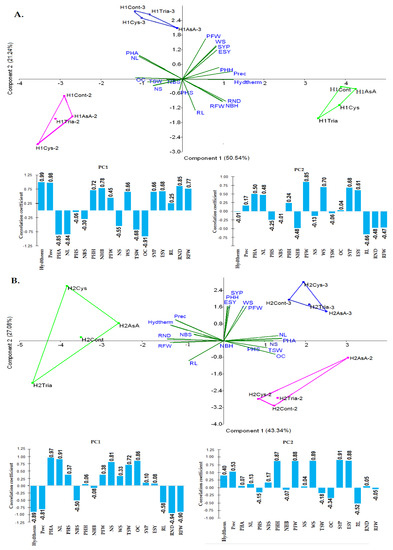
Figure 2.
Principal Components Analyses (PCA) was performed to correlate the hydrothermal index (Hydtherm) and average rainfall (Prec), as environmental variables, with the assessed parameters and growing seasons (AsA-, Cys-, Tria-, Cont-I. growing season; AsA_2, Cys_2, Tria_2, Cont_2-II. grooving season); (AsA_3, Cys_3, Tria_3, Cont_3-III. grooving season) at H1 (A) and H2 canola cultivars (B). In the case of H1, hydrothermal index and average rainfall positively correlated with PHH, SYP, ESY, WS, PFW, RND, and RFW. Negative correlations could be observed between weather conditions and the following parameters: OC, PHA, NL, TSW, and NS. In the case of H2, the negatively correlated parameters with the average rainfall and the hydrothermal index were RND, RFW, RL, and NBS, whereas the positively correlated ones were SYP, ESY, WS, PHH, and PFW.

Table 7.
Effects of seed priming and foliar treatment with bioactive substances on biometrics parameters of canola cultivars in autumn and spring (between the years 2017–2020).

Table 8.
Effects of seed priming and foliar treatment with bioactive substances on biometrics parameters of canola cultivars at harvest time (between the years 2017–2020).

Table 9.
Effects of seed priming and foliar treatment with bioactive substances on yield parameters of canola at harvest time (between the years 2017–2020).
Measurements from the second growing season presented negative correlations with both axes 1 (PC1) and 2 (PC2). The negatively correlated parameters with component 1 were mainly PHA, NL, and OC but also TSW and NS. The quite dry conditions from the second year favored these parameters. Component 2 presented negative correlations mainly with RL but also with RND, RFW, and NBH. AsA significantly increased PHA in the second growing season compared with the control plants in the cases of both cultivars (Table 7, Table 8 and Table 9). Thus, the increase in PHA in the second growing season can be attributed to AsA and weather conditions.
The H1 cultivars treated with Cys had significantly higher values than control plants for the following parameters: NL, NBH, RFW, and TSW (Table 7, Table 8 and Table 9). AsA and Tria treatments resulted in somewhat comparable results in the case of TSW, both being close to the control groups. In fact, Tria treatment resulted in lower values than the control group.
The same cultivar treated with Tria had significantly higher values for NL and NBH (Table 7 and Table 8). These parameters were influenced positively also by the weather conditions from the second year.
Measurements from the third growing season correlated negatively with axis 1 (PC1) and positively with axis 2 (PC2). Mainly PFW but also WS, SYP, ESY, PHA, and NL were positively correlated with axis 2. Negative correlations were found with OC. In this growing season, Cys and Tria significantly increased the NL compared to control plants, in the cases of both cultivars (Table 7). Precipitation and hydrothermal index were not correlated with axis 2. Therefore, Cys and Tria contributed to the increase in NL in the third growing season, and plants treated with AsA had lower values as compared to the control group.
Significantly higher values were obtained compared to control plants in the case of the H1 cultivars for RL when the H1 cultivars were treated with AsA, Cys, or Tria (Table 8). The H1 cultivars treated with Cys had significantly higher values for SYP and ESY compared with controls (Table 9). These parameters were not influenced by weather conditions and depended on treatment and cultivar.
The results of the PCA analysis for the H2 (Figure 2B) showed that 70.43% of the total variance could be explained with the first two components (PC1—43.34%, PC2—27.08%). In the first growing season, the treatments with Cys (H2Cys) and AsA (H2AsA) but also control plants (H2Cont) presented negative correlation with axis 1 and positive correlation with axis 2.
The negatively correlated parameters with axis 1 were RND, RFW, RL, the average rainfall (prec), the hydrothermal index (hydtherm), and NBS. Thus, the quite wet conditions from the first year positively influenced RND, RFW, RL, and NBS. The parameters that correlated positively with axis 2 were SYP, ESY, WS, PHH and PFW. In the case of H2Cys, correlation with the annual rainfall could be observed. In the first growing season, in the case of both cultivars, compared to the control plants, AsA significantly increased the following parameters: PHA, NL, NBS, and RFW (Table 7, Table 8 and Table 9). Similarly, Cys increased the values for PHA, NBS, and RFW. Tria increased NBS for both cultivars. Because the quite wet conditions had a negative influence on PHA, NL, OC, NS, and TSW, the increase in PHA in the first growing season can be attributed to treatments with AsA or Cys, whereas NL was increased by AsA. Tria and weather conditions positively influenced NBS.
In the case of the H2 cultivar, Cys produced significantly more leaves (NL), and AsA and Cys increased SYP and ESY. However, the values for the Tria-treated plants remained less than the values of the control group. AsA had higher values for OC (Table 7, Table 8 and Table 9). Tria and weather conditions favored RFW. The positive effect of treatments depended on the cultivar.
Measurements from the second growing season were correlated positively with axis 1 and negatively with axis 2. The parameters that correlated positively with PC1 were PHA, NL, OC, NS, and TSW. PC2 correlated only with RL. AsA significantly increased PHA in the second growing season compared with the control plants in the case of both cultivars (Table 7); in addition, the weather conditions (quite dry) favored PHA. Thus, the increase in PHA in the second growing season can be attributed both to weather conditions and AsA. In the case of the H2 cultivars, the following treatments produced significantly higher values compared to control plants: AsA in the case of PHS, NBS, PFW, WS, NS, SYP, ESY, TSW, and OC; Cys in the case of PHA, ESY, and OC; and Tria in the case of PHA. Tria-treated plants in terms of SYP and ESY values remained lower than those of the control group, and the differences were not statistically significant. Dry weather conditions favored PHA, NL, OC, NS, and TSW (Figure 2B). The increase in PHS, NBS, PFW, and WS in the second growing season can be attributed to AsA and cultivar. PHA was increased by both Cys and Tria, but the positive effect depended on the cultivar.
The third year correlated positively with both axes. PHA, NL, NS, TSW, and OC presented positive correlation with axis 1. Furthermore, a negative correlation could be observed between these parameters and weather conditions (hydtherm and prec) (Figure 2B). Therefore, the higher values in the third season for these parameters can be explained by the lower values of hydrothermal index and average rainfall. In another order, PHH, PFW, WS, SYP, ESY, and average rainfall (prec) were in positive correlation with axis 2. These parameters were favored in the third growing season by the average rainfall. Cys and Tria significantly increased the NL compared to control plants, whereas AsA decreased these values in the cases of both cultivars (Table 7). The weather (quite dry) conditions favored PHA, NL, OC, NS, and TSW (Figure 2B). Therefore, the increase in NL in the third growing season depended on weather conditions and treatment. The positive effect of Cys on PHA depended on weather, cultivar, and treatment.
4. Discussion
4.1. The First Growing Season
In August during seedbed preparation, average temperatures were higher by 1.7 °C, and the sum of precipitation was 40% less than usual. In autumn, there were drought conditions right at the time when canola should have been sown. These factors not only made seedbed preparation difficult but also affected in a negative manner seed emergence and the early development of plants (Table 2). Under these unfavorable conditions present in the experimental field, the results indicate that treatments with low concentrations of AsA and Cys could exert several positive effects on the early development of canola plants (Table 7). The effects of treatments were not influenced by the weather conditions or cultivar. It was demonstrated that exogenously provided ascorbate has an important metabolic role in various stages of plant growth and development [50]. Studies performed on other plant species also support the positive effects of ascorbate on the development of plants subjected to different stress factors: priming with 0.5% ascorbate of sunflower (Helianthus annuus) seeds germinated under cold stress conditions enhanced the vigor of the emerged seedlings [51]. Vitamin C (50 ppm) also had positive effects on the emergence and early growth parameters of wheat (Triticum aestivum L.) in growth chamber plants exposed to salt stress [52]. Sadak et al. [53] investigated the effects of cysteine foliar treatment (20 and 40 mg/L) on soybean (Glycine max (L.) Merr.) plants, and their results show that the treatment increased the quantity of photosynthetic pigments, which resulted in the mitigation of unfavorable effects of salt stress on plant height, number of branches per plant, number of leaves, and seed yield. The experiments were conducted in greenhouse conditions. According to Nasibi et al. [39], seed pretreating of wheat (Triticum aestivum L.) grains with 0.01 µM cysteine can be an effective method for protecting the seedlings from the harmful effects of salt stress when they were growing in controlled laboratory conditions.
Most of the morphological and yield parameters recorded at harvest time (in July, Table 8 and Table 9) showed that the treatments were dependent on the cultivar. Moreover, the hydrothermal and precipitation values had a negative effect on various parameters. According to the factorial analysis (and PCA analyses), the effect of Cys and AsA on OC was not influenced significantly either by the year or by the cultivar. Investigation of the effects of plant growth regulator substances on the oil content of canola drew attention to the fact that along with high nitrogen doses, additional sulfur application increased the seed yield and oil content of seeds [54]. On the other hand, Lucas et al. [55] found that elevated nitrogen and sulfur doses had no effects on canola oil and protein content, although they had a positive effect on the seed yield. In accordance with our findings, studies [56,57] regarding the foliar application of nutrients reported that different weather conditions among the experimental years altered the yield parameters of the canola. In the works of Sikorska et al. [58] and Malhi et al. [59], the plants from field experiments did not react to foliar fertilization with a greater seed yield. In multiple cases [60,61,62] in field and controlled trials, the abiotic conditions influenced the thousand seed weight, seed yield, seed germination, and seedling emergence of canola. In our work, despite the negative effect of the weather on PHA, NL, NS, and OC, the treatments increased these parameters.
4.2. The Second Growing Season
In 2018, the average temperatures (August–September) were higher by 1.5 °C compared to the multiannual means (1981–2010 period), whereas the sum of precipitation was 60.75% less than usual. Dry weather conditions occurred also in June (Table 1 and Table 2). The deficit in precipitation and the higher average temperature in the early growth stage and before harvest time favored PHA, NL, OC, NS, and TSW. The early growth stage parameters showed that the positive effects of treatments depended on the cultivar or the weather conditions. The treatment of canola seeds with triacontanol showed effectiveness in the improvement of several germination parameters in laboratory conditions [47]. When applied to the leaf surface, triacontanol (Niraculan 0.05% diluted in water, 1–2 mL/L) has been shown to increase plant height, leaf number, leaf length, and stem diameter of kohlrabi (Brassica oleracea L. var. gongylodes L.) [63]. According to Chandra et al. [64], several studies show that Tria increased root and shoot fresh weight and chlorophyll content and decreased enzymatic levels. It was found that the foliar application of Tria at concentrations of 10 and 20 μM diminished the salinity-induced adverse effects in wheat cultivars by improving the plant biomass and leaf area in the vegetative stage [65]. Similar results were observed by Perveen et al. [35]. Tria (5 µM) application significantly alleviated salinity-induced toxic impacts in maize (Zea mays L.) plants by increasing the stem length, leaf area, and fresh weight of root and shoot. In our study, although 10 ppm Tria was effective for some biometrics parameters, it had a detrimental impact on several yield parameters.
Data from the literature illustrate the positive effect of ascorbic acid treatment on the seed germination, growth, and yield parameters of canola plants under drought conditions [66,67]. Contrary to our observations, drought reduced siliquae number, fresh weight of seeds per plant, thousand seed weight, and seed yield through the alteration of various morphological and physiological characteristics of the plants. For counteracting the negative effects of drought, certain plant growth regulators and inorganic nutrients supplied to canola plants showed efficiency [68]. In the work of Farooq et al. [32], treating wheat plants that were grown in pots with 2 mM ascorbate solution under drought conditions enhanced germination, improved leaf emergence and elongation, and induced uniform seedling stand and shoot and root growth.
4.3. The Third Growing Season
During August 2019, the higher than usual air temperature values were accompanied by only a moderate precipitation deficit (only 14% less than the multiannual mean values), compared to the previous experimental years. The applied substances significantly increased the NL compared to control plants in the cases of both cultivars (Table 7). Ahmadi et al. [57] reported that foliar application of 300 mg/L ascorbate under mild stress conditions could enhance the seed yield of canola plants under field conditions. Cys (25 and 50 ppm) also increased the number of seeds per plant, the yield, and the thousand seed weight of winter wheat (Triticum aestivum L.) plants [69].
Temperature during wintertime is another crucial factor in canola cultivation. In the study region, the temperature during winter was warmer than average. The frost periods (of magnitude of −8 °C to −10 °C) during early plant development tested the cold tolerance of canola plants (Table S1). It was found that, when applied to Brassica species, 1 mM ascorbate moderated the oxidative damage provoked by osmotic stress and enhanced physiological acclimation to chilling [70]. A recent study suggests that the average temperature values of the winter period have a strong correlation with the yield values of canola. A 1 °C increase in average temperature values may induce a 113 (±21) kg ha−1 yield loss. With milder winter temperatures, the continuously growing above-ground biomass impairs the proper development of root biomass, which is needed by the plants in the drought periods [71]. In addition to a better fixation to the ground for the developing plant, a vigorous root system can also fulfill its function by taking up water and mineral nutrients from greater depths. This uptake of nutrients also contributes to the buildup of yield [72]. According to our results, AsA, Cys, and Tria increased the average values of root length (RL) and root fresh weight (RFW) in several cases.
Altogether, treatments had several positive effects on canola development. The results from autumn indicate that seed priming leads to better seedling development throughout the early growth stage. A larger number of leaves can contribute to a more effective assimilation by increasing the light harvesting and CO2 binding capacity of plants, which can lead to a greater net production. The corresponding leaf number is important because reaching the 8–10 leaf rosette stage is a precondition for good overwintering in canola. The higher number of ramifications (NBS, NBH) may also result in higher seed yield. Treatments had positive effects also on the yield parameters. The oil content and root parameters were also influenced by treatments. In addition to a better fixation to the ground for the developing plant, a vigorous root system can also fulfill its function by taking up water and mineral nutrients from greater depths. This uptake of nutrients also contributes to the buildup of yield [72]. In most of the cases, the effect of the treatments depended on the cultivar.
5. Conclusions
Altogether, the present work shows that seed priming with 10 ppm cysteine (Cys) and ascorbate (AsA) can have beneficial effects on the development of seedlings, whereas their foliar application can enhance the number of silique, seed yield, and the oil content of the studied canola cultivars.
Not all substances that have undergone laboratory testing were successful in the field. In some cases, Tria had no effect on plants, or even a negative effect could be observed on important parameters for agriculture, such as estimated seed yield, seed yield/plant, and oil content. The effects of AsA and Cys could be observed throughout the growing season in both cultivars, whereas the effects of Tria were visible on the biometrics parameters only in autumn and spring, as well as on the root parameters. Generally, the H1 cultivar responded better to treatments than the H2. Additionally, the effectiveness order of the treatments for the H1 is Cys, AsA, and Tria (Triacontanol), whereas for the H2, the order is AsA, Cys, and Tria.
As a result of our research, it can be concluded that the selection of the right treatment and the determination of the lowest effective concentration, together with the frequency of treatments, are crucial for cost-effectiveness. In a time when fertilizer prices are increasing and pesticides are lacking or rapidly changing, farmers need support to succeed in a competitive market and also in protecting their crops with alternative methods that are based on enhancing the defense system of the plants.
More field research is necessary to determine the ideal conditions for the application of the different bioactive substances. It is also recommended to test these elicitors on a diverse range of crops cultivated in the spring and autumn. Drought in autumn crops and cold/wet soil in spring crops may hinder initial germination/emergence and seedling development. The bioactive substances used in our study are natural, technologically simple to use, and environmentally friendly, and their applications support the current forms of cultivation technology.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9020207/s1, Table S1: Maximum and minimum daily temperature values during the early spring vegetation season of canola in 2018–2020 years, Table S2: Daily precipitation values during the vegetation season of canola in 2017–2020 years.
Author Contributions
Conceptualization, K.M.; methodology and formal analysis, I.-I.N., K.M. and M.M.D.; investigation, K.M. and I.-I.N.; data curation, K.M., B.B.-J. and E.D.; writing—original draft preparation, K.M.; writing—review and editing, L.F., B.B.-J., E.D. and A.S.; supervision, I.-I.N., M.M.D., L.F. and B.B.-J.; funding acquisition, M.M.D. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Acknowledgments
This work was supported by the Collegium Talentum Programme of Hungary. The authors are grateful of John R. Akeroyd’s assistance in helping us bring this article together.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations:
Treatments: AsA—L-ascorbic acid; Cys—Cysteine; Tria—Triacontanol. Biometrics parameters: PHA—Plant Height at Autumn; NL—Number of Leaves/plants at Autumn; PHS—Plant Height at Spring; NBS—Number of Branches/plant at Spring; PHH—Plant Height at Harvest; NBH—Number of Branches/plant at Harvest; PFW—Plant Fresh Weight; RL—Root Length; RND—Root Neck Diameter; RFW—Root Fresh Weight; NS—Number of Siliquae/plant; WS—Weight of Siliquae/plant; TSW—Thousand Seed Weight; SYP—Seed Yield/Plant; ESY—Estimated Seed Yield; OC-Oil Content. Cultivars: H1—”Factor”; H2—”Hybrirock”. Growing seasons: 2017–2018-I. growing season (sowing in autumn 2017 and harvest in summer 2018); 2018–2019-II. growing season (sowing in autumn 2018 and harvest in summer 2019); 2019–2020-III. growing season (sowing in autumn 2019 and harvest in summer 2020).
References
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 24 September 2021).
- Friedt, W.; Tu, J.; Fu, T. Academic and Economic Importance of Brassica napus Rapeseed. In The Brassica napus Genome. Compendium of Plant Genomes; Liu, S., Snowdon, R., Chalhoub, B., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–20. [Google Scholar] [CrossRef]
- Wu, W.; Ma, B.; Whalen, J.K. Enhancing Rapeseed Tolerance to Heat and Drought Stresses in a Changing Climate: Perspectives for Stress Adaptation from Root System Architecture. In Advances in Agronomy; Donald, L.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 151, pp. 87–157. [Google Scholar] [CrossRef]
- Thakur, P.; Kumar, S.; Malik, J.A.; Berger, J.D.; Nayyar, H. Cold Stress Effects on Reproductive Development in Grain Crops: An Overview. Environ. Exp. Bot. 2010, 67, 429–443. [Google Scholar] [CrossRef]
- Sanghera, G.S.; Wani, S.; Hussain, W. Engineering Cold Stress Tolerance in Crop Plants. Curr. Genom. 2011, 12, 30–43. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Basra, S.M.A.; Rehman, A.; Siddique, K.H.M. Improving Crop Resistance to Abiotic Stresses through Seed Invigoration. In Handbook of Plant and Crop Stress, 4th ed.; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 775–792. [Google Scholar]
- Bose, B.; Kumar, M.; Singhal, R.K.; Mondal, S. Impact of Seed Priming on the Modulation of Physico-Chemical and Molecular Processes during Germination, Growth, and Development of Crops. In Advances in Seed Priming; Rakshit, A., Singh, H.B., Eds.; Springer: Singapore, 2018; pp. 23–40. ISBN 9789811300325. [Google Scholar]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed Priming for Abiotic Stress Tolerance: An Overview. Acta Physiol. Plant. 2013, 35, 1381–1396. [Google Scholar] [CrossRef]
- Sen, A.; Puthur, J. Seed Priming-Induced Physiochemical and Molecular Events in Plants Coupled to Abiotic Stress Tolerance: An Overview. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Academic Press: Cambridge, MA, USA, 2020; pp. 303–316. ISBN 978-0-12-817892-8. [Google Scholar] [CrossRef]
- Abdelkader, M.; Voronina, L.; Puchkov, M.; Shcherbakova, N.; Pakina, E.; Zargar, M.; Lyashko, M. Seed Priming with Exogenous Amino Acids Improves Germination Rates and Enhances Photosynthetic Pigments of Onion Seedlings (Allium cepa L.). Horticulturae 2023, 9, 80. [Google Scholar] [CrossRef]
- Hernándiz, A.E.; Aucique-Perez, C.E.; Ćavar Zeljković, S.; Štefelová, N.; Salcedo Sarmiento, S.; Spíchal, L.; De Diego, N. Priming with Small Molecule-Based Biostimulants to Improve Abiotic Stress Tolerance in Arabidopsis thaliana. Plants 2022, 11, 1287. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, G.; Cocetta, G.; Prinsi, B.; Ferrante, A.; Espen, L. Biostimulants on Crops: Their Impact under Abiotic Stress Conditions. Horticulturae 2022, 8, 189. [Google Scholar] [CrossRef]
- Yoshimura, K.; Ishikawa, T. Chemistry and Metabolism of Ascorbic Acid in Plants. In Ascorbic Acid in Plant Growth, Development and Stress Tolerance; Hossain, M.A., Munné-Bosch, S., Burritt, D.J., Diaz-Vivancos, P., Fujita, M., Lorence, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–23. ISBN 978-3-319-74057-7. [Google Scholar]
- Baig, Z.; Khan, N.; Sahar, S.; Sattar, S.; Zehra, R. Effects of Seed Priming with Ascorbic Acid to Mitigate Salinity Stress on Three Wheat (Triticum aestivum L.) Cultivars. Acta Ecol. Sin. 2021, 41, 491–498. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic Acid-A Potential Oxidant Scavenger and Its Role in Plant Development and Abiotic Stress Tolerance. Front. Plant Sci. 2017, 8, 613–630. [Google Scholar] [CrossRef]
- Khan, N.; Khan, M.; Asgher, M.; Mehar, F.; Masood, A.; Syeed, S. Salinity Tolerance in Plants: Revisiting the Role of Sulfur Metabolites. J. Plant Biochem. Physiol. 2014, 2, 2. [Google Scholar] [CrossRef]
- Raza, A. Eco-Physiological and Biochemical Responses of Rapeseed (Brassica Napus L.) to Abiotic Stresses: Consequences and Mitigation Strategies. J. Plant Growth Regul. 2021, 40, 1368–1388. [Google Scholar] [CrossRef]
- Zagorchev, L.; Seal, C.E.; Kranner, I.; Odjakova, M. A Central Role for Thiols in Plant Tolerance to Abiotic Stress. Int. J. Mol. Sci. 2013, 14, 7405–7432. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.-A.A.; Alshammari, S.O. Cysteine Mitigates the Effect of NaCl Salt Toxicity in Flax (Linum usitatissimum L.) Plants by Modulating Antioxidant Systems. Sci. Rep. 2022, 12, 11359. [Google Scholar] [CrossRef]
- Teixeira, W.F.; Fagan, E.B.; Soares, L.H.; Umburanas, R.C.; Reichardt, K.; Neto, D.D. Foliar and Seed Application of Amino Acids Affects the Antioxidant Metabolism of the Soybean Crop. Front. Plant Sci. 2017, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Tompa, B.; Jakab, K.; Fodorpataki, L. Triacontanol Compensates for Cadmium Toxicity Effects on Growth and Photosynthesis. Ann. Univ. Oradea Biol. Ser. 2020, 27, 123–128. [Google Scholar]
- Ahmad, J.; Ali, A.A.; Al-Huqail, A.A.; Qureshi, M.I. Triacontanol Attenuates Drought-Induced Oxidative Stress in Brassica juncea L. by Regulating Lignification Genes, Calcium Metabolism and the Antioxidant System. Plant Physiol. Biochem. 2021, 166, 985–998. [Google Scholar] [CrossRef]
- Islam, S.; Zaid, A.; Mohammad, F. Role of Triacontanol in Counteracting the Ill Effects of Salinity in Plants: A Review. J. Plant Growth Regul. 2021, 40, 1–10. [Google Scholar] [CrossRef]
- Dresselhaus, T.; Hückelhoven, R. Biotic and Abiotic Stress Responses in Crop Plants. Agronomy 2018, 8, 267. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Kumari, A.; Harish; Singh, V.K.; Verma, K.K.; Mandzhieva, S.; Sushkova, S.; Srivastava, S.; Keswani, C. Coping with the Challenges of Abiotic Stress in Plants: New Dimensions in the Field Application of Nanoparticles. Plants 2021, 10, 1221. [Google Scholar] [CrossRef]
- Mittler, R. ROS and Redox Signaling in Cell-to-Cell and Systemic Responses of Plants. Free. Radic. Biol. Med. 2022, 189, 1. [Google Scholar] [CrossRef]
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological Responses to Drought, Salinity, and Heat Stress in Plants: A Review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Aboutalebian, M.A.; Nazari, S. Seedling Emergence and Activity of Some Antioxidant Enzymes of Canola (Brassica napus) Can Be Increased by Seed Priming. J. Agric. Sci. 2017, 155, 1541–1552. [Google Scholar] [CrossRef]
- Aymen, E.M. Seed Priming with Plant Growth Regulators to Improve Crop Abiotic Stress Tolerance. In Advances in Seed Priming; Rakshit, A., Singh, H.B., Eds.; Springer: Singapore, 2018; pp. 95–106. ISBN 9789811300325. [Google Scholar]
- Waqas, M.; Korres, N.E.; Khan, M.D.; Nizami, A.-S.; Deeba, F.; Ali, I.; Hussain, H. Advances in the Concept and Methods of Seed Priming. In Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Hasanuzzaman, M., Fotopoulos, V., Eds.; Springer: Singapore, 2019; pp. 11–41. ISBN 9789811386251. [Google Scholar]
- Zulfiqar, F. Effect of Seed Priming on Horticultural Crops. Sci. Hortic. 2021, 286, 110197. [Google Scholar] [CrossRef]
- Farooq, M.; Irfan, M.; Aziz, T.; Ahmad, I.; Alam, S. Seed Priming with Ascorbic Acid Improves Drought Resistance of Wheat. J. Agron. Crop. Sci. 2013, 199, 11–22. [Google Scholar] [CrossRef]
- Alves, R.; Rossatto, D.; Silva, J.; Checchio, M.; Oliveira, K.; Oliveira, F.; Queiroz, S.; Cruz, M.; Gratão, P. Seed Priming with Ascorbic Acid Enhances Salt Tolerance in Micro-Tom Tomato Plants by Modifying the Antioxidant Defense System Components. Biocatal. Agric. Biotechnol. 2021, 31, 101927. [Google Scholar] [CrossRef]
- Shahbaz, M.; Noreen, N.; Perveen, S. Triacontanol Modulates Photosynthesis and Osmoprotectants in Canola (Brassica napus L.) under Saline Stress. J. Plant Interact. 2013, 8, 350–359. [Google Scholar] [CrossRef]
- Perveen, S.; Iqbal, M.; Parveen, A.; Akram, M.S.; Shahbaz, M.; Akber, S.; Mehboob, A. Exogenous Triacontanol-Mediated Increase in Phenolics, Proline, Activity of Nitrate Reductase, and Shoot K+ Confers Salt Tolerance in Maize (Zea mays L.). Braz. J. Bot. 2017, 40, 1–11. [Google Scholar] [CrossRef]
- Khanam, D.; Mohammad, F. Plant Growth Regulators Ameliorate the Ill Effect of Salt Stress through Improved Growth, Photosynthesis, Antioxidant System, Yield and Quality Attributes in Mentha piperita L. Acta Physiol. Plant. 2018, 40, 188. [Google Scholar] [CrossRef]
- Shafiq, S.; Aisha, N.; Ashraf, M.; Arshad, A. Synergistic Effects of Drought and Ascorbic Acid on Growth, Mineral Nutrients and Oxidative Defense System in Canola (Brassica napus L.) Plants. Acta Physiol. Plant. 2014, 36, 1539–1553. [Google Scholar] [CrossRef]
- Genisel, M.; Erdal, S.; Kizilkaya, M. The Mitigating Effect of Cysteine on Growth Inhibition in Salt-Stressed Barley Seeds Is Related to Its Own Reducing Capacity Rather than Its Effects on Antioxidant System. Plant Growth Regul. 2015, 75, 187–197. [Google Scholar] [CrossRef]
- Nasibi, F.; Kalantari, K.; Zanganeh, R.; Mohammadi-Nejad, G.; Oloumi, H. Seed Priming with Cysteine Modulates the Growth and Metabolic Activity of Wheat Plants under Salinity and Osmotic Stresses at Early Stages of Growth. Indian J. Plant Physiol. 2016, 21, 279–286. [Google Scholar] [CrossRef]
- Fodorpataki, L.; Molnár, K.; Sebastian, P.; Tompa, B. Priming with Vitamin U Enhances Cold Tolerance of Lettuce (Lactuca sativa). Not. Bot. 2019, 47, 592–598. [Google Scholar]
- Zhu, Z.H.; Sami, A.; Xu, Q.Q.; Wu, L.L.; Zheng, W.Y.; Chen, Z.P.; Jin, X.Z.; Zhang, H.; Li, Y.; Yu, Y.; et al. Effects of Seed Priming Treatments on the Germination and Development of Two Rapeseed (Brassica napus L.) Varieties under the Co-Influence of Low Temperature and Drought. PLoS ONE 2021, 16, e0257236. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Abiotic Stress, the Field Environment and Stress Combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and Biotic Stress Combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Jędrszczyk, E.; Skowera, B.; Kopcińska, J.; Ambroszczyk, A. The Influence of Weather Conditions During Vegetation Period on Yielding of Twelve Determinate Tomato Cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 203–209. [Google Scholar] [CrossRef]
- Skowera, B. Changes of Hydrothermal Conditions in the Polish Area (1971–2010). Fragm. Agron. 2014, 31, 74–87. [Google Scholar]
- FACTOR KWS-Rapiţă-Produse-KWS SAAT SE & Co. KGaA. Available online: https://www.kws.com/md/ro/produse/rapita/factor-kws/ (accessed on 5 December 2022).
- Molnár, K.; Biró-Janka, B.; Nyárádi, I.-I.; Fodorpataki, L.; Varga, B.-E.; Bálint, J.; Duda, M.-M. Effects of Priming with Ascorbic Acid, L-Cystein and Triacontanol on Germination of Rapeseed (Brassica napus L.). Acta Biol. Marisiensis 2020, 3, 48–55. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants: BBCH Monograph; Julius Kühn-Institut (JKI): Quedlinburg, Germany, 2018. [Google Scholar] [CrossRef]
- Nagy, N.; Pepó, P. Comparative Study of Different Soybean Genotypes in Irrigation Technology. Acta Agrar. Debr. 2019, 1, 91–95. [Google Scholar] [CrossRef]
- Ortiz-Espín, A.; Sánchez-Guerrero, A.; Sevilla, F.; Jiménez, A. The Role of Ascorbate in Plant Growth and Development. In Ascorbic Acid in Plant Growth, Development and Stress Tolerance; Hossain, M.A., Munné-Bosch, S., Burritt, D.J., Diaz-Vivancos, P., Fujita, M., Lorence, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 25–45. ISBN 978-3-319-74057-7. [Google Scholar]
- Draganic, I.; lekić, S. Seed Priming with Antioxidants Improves Sunflower Seed Germination and Seedling Growth under Unfavorable Germination Conditions. Turk. J. Agric. For. 2012, 36, 421–428. [Google Scholar] [CrossRef]
- Afzal, I.; Basra, S.; Farooq, M.; Nawaz, A. Alleviation of Salinity Stress in Spring Wheat by Hormonal Priming with ABA, Salicylic Acid and Ascorbic Acid. Int. J. Agric. Biol. 2006, 8, 8530. [Google Scholar]
- Sadak, M.S.; Abd El-Hameid, A.R.; Zaki, F.S.A.; Dawood, M.G.; El-Awadi, M.E. Physiological and Biochemical Responses of Soybean (Glycine max L.) to Cysteine Application under Sea Salt Stress. Bull. Natl. Res. Cent. 2020, 44, 1. [Google Scholar] [CrossRef]
- Ma, B.-L.; Zheng, Z.; Whalen, J.K.; Caldwell, C.; Vanasse, A.; Pageau, D.; Scott, P.; Earl, H.; Smith, D.L. Uptake and Nutrient Balance of Nitrogen, Sulfur, and Boron for Optimal Canola Production in Eastern Canada. J. Plant Nutr. Soil Sci. 2019, 182, 252–264. [Google Scholar] [CrossRef]
- Lucas, F.T.; Coutinho, E.L.M.; Paes, J.M.V.; Barbosa, J.C. Yield and Quality of Canola Grains Due to Nitrogen and Sulfur Fertilization. Semin. Ciências Agrárias 2013, 34, 3205–3218. [Google Scholar] [CrossRef]
- Varga, L.; Ložek, O.; Ducsay, L.; Kováčik, P.; Lošák, T.; Hlušek, J. Effect of Topdressing with Nitrogen and Boron on the Yield and Quality of Rapeseed. Acta Univ. Agric. Et Silvic. Mendel. Brun. 2010, 58, 391–398. [Google Scholar] [CrossRef]
- Ahmadi SA, K.; Ebadi, A.; Daneshian, J.; Siadat, S.A.; Jahanbakhsh, S. Effect of Drought Stress and Foliar Application of Growth Regulators on Photosynthetic Pigments and Seed Yield of Rapeseed (Brassica napus L. Cv. Hyola 401). Iran. J. Crop Sci. 2016, 18, 196–217. [Google Scholar]
- Sikorska, A.; Gugała, M.; Zarzecka, K. The Impact of Foliar Feeding on the Yield Components of Three Winter Rape Morphotypes (Brassica napus L.). Open Agric. 2020, 5, 107–116. [Google Scholar] [CrossRef]
- Malhi, S.S.; Raza, M.; Schoenau, J.J.; Mermut, A.R.; Kutcher, R.; Johnston, A.M.; Gill, K.S. Feasibility of Boron Fertilization for Yield, Seed Quality and B Uptake of Canola in Northeastern Saskatchewan. Can. J. Soil. Sci. 2003, 83, 99–108. [Google Scholar] [CrossRef]
- Weymann, W.; Böttcher, U.; Sieling, K.; Kage, H. Effects of Weather Conditions during Different Growth Phases on Yield Formation of Winter Oilseed Rape. Field Crops Res. 2015, 173, 41–48. [Google Scholar] [CrossRef]
- Gugała, M.; Sikorska, A.; Findura, P.; Kapela, K.; Malaga-Toboła, U.; Zarzecka, K.; Domański, Ł. Effect of Selected Plant Preparations Containing Biologically Active Compounds on Winter Rape (Brassica napus L.) Yielding. Appl. Ecol. Environ. Res. 2019, 17, 2779–2789. [Google Scholar] [CrossRef]
- Luo, T.; Sheng, Z.; Zhang, C.; Li, Q.; Liu, X.; Qu, Z.; Xu, Z. Seed Characteristics Affect Low-Temperature Stress Tolerance Performance of Rapeseed (Brassica napus L.) during Seed Germination and Seedling Emergence Stages. Agronomy 2022, 12, 1969. [Google Scholar] [CrossRef]
- Bhandari, S.; Bhandari, A.; Shrestha, J. Effect of Different Doses of Triacontanol on Growth and Yield of Kohlrabi (Brassica oleracea L. var. gongylodes). Heliyon 2021, 7, e08242. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Roychoudhury, A. Penconazole, Paclobutrazol, and Triacontanol in Overcoming Environmental Stress in Plants. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 510–534. ISBN 978-1-119-55215-4. [Google Scholar]
- Perveen, S.; Shahbaz, M.; Ashraf, M. Triacontanol-Induced Changes in Growth, Yield, Leaf Water Relations, Oxidative Defense System, Minerals, and Some Key Osmoprotectants in Triticum aestivum under Saline Conditions. Turk. J. Bot. 2014, 38, 896–913. [Google Scholar] [CrossRef]
- Razaji, A.; Farzanian, M.; Sayfzadeh, S. The Effects of Seed Priming by Ascorbic Acid on Some Morphological and Biochemical Aspects of Rapeseed (Brassica napus L.) under Drought Stress Condition. J. Biodivers. Environ. Sci. 2014, 4, 432–442. [Google Scholar]
- El-Sabagh, A.; Abdelaal, K.A.A.; Barutcular, C. Impact of Antioxidants Supplementation on Growth, Yield and Quality Traits of Canola (Brassica napus L.) under Irrigation Intervals in North Nile Delta of Egypt. J. Exp. Biol. Agric. Sci. 2017, 5, 163–172. [Google Scholar] [CrossRef]
- Raza, M.A.S.; Shahid, A.M.; Saleem, M.F.; Khan, I.H.; Ahmad, S.; Ali, M.; Iqbal, R. Effects and Management Strategies to Mitigate Drought Stress in Oilseed Rape (Brassica napus L.): A Review. Zemdirb. Agric. 2017, 104, 85–94. [Google Scholar] [CrossRef]
- El Kelish, A.; El-Mogy, M.; Niedbała, G.; Piekutowska, M.; Omar, M.A.; Hamada, M.; Shahin, M.; Mukherjee, S.; Abou El-Yazied, A.; Shebl, M.; et al. Roles of Exogenous α-Lipoic Acid and Cysteine in Mitigation of Drought Stress and Restoration of Grain Quality in Wheat. Plants 2021, 10, 2318. [Google Scholar] [CrossRef]
- Alam, M.M.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Alleviation of Osmotic Stress in Brassica napus, B. campestris, and B. juncea by Ascorbic Acid Application. Biol. Plant. 2014, 58, 697–708. [Google Scholar] [CrossRef]
- Brown, J.K.M.; Beeby, R.; Penfield, S. Yield Instability of Winter Oilseed Rape Modulated by Early Winter Temperature. Sci. Rep. 2019, 9, 6953. [Google Scholar] [CrossRef] [PubMed]
- Eőri, T.; Wenszky, Á. Versenyképes Repcetermesztés: A Jövedelmezőség Kulcstényezői a Szántóföldi Gyakorlatban; Magyar Agrárkamara: Budapest, Hungary, 2012; ISBN 978-963-286-667-3. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).