Hydroalcoholic Extracts of Campomanesia lineatifolia R. & P. Seeds Inhibit the Germination of Rumex crispus and Amaranthus hybridus
Abstract
1. Introduction
2. Materials and Methods
2.1. Localization
2.2. Hydroalcoholic Extract Preparation for Germination Tests
Determination of Phenolic Compounds in C. lineatifolia Seeds
2.3. Experimental Design and Treatments
2.4. Germination Variables
2.5. Seed Viability (%)
2.6. Statistical Analysis
3. Results
3.1. Hydroalcoholic Extract of C. lineatifolia
3.2. Germination and Viability in Rumex crispus
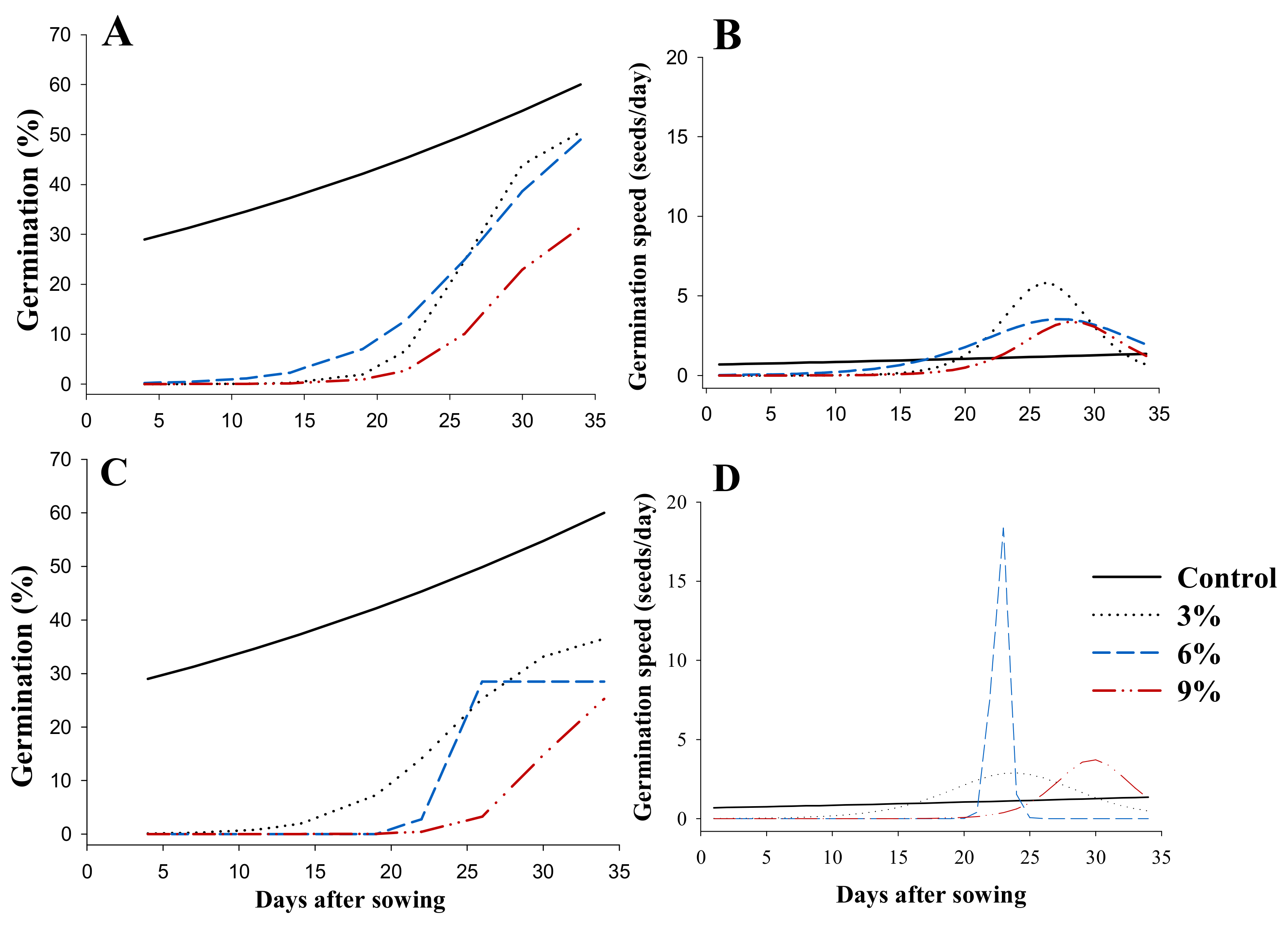
3.2.1. Behavior of Germination as a Function of Time
3.2.2. Germination Indices
3.2.3. Seed Viability
3.3. Amaranthus hybridus
3.3.1. Germination Behavior as a Function of Time
3.3.2. Germination Indices
3.3.3. Seed Viability
4. Discussion
4.1. Hydroalcoholic Extract of Campomanesia lineatifolia
4.2. Germination and Viability in R. crispus Seeds
4.3. Germination and Viability in A. hybridus Seeds
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrova, T.; Valcheva, G.; Velcheva, G. A case study of Allelopathic effect on weeds in wheat. Ecol. Balk. 2015, 7, 121–129. [Google Scholar]
- Dellaferrera, I.; Cortés, E.; Panigo, E.; De Prado, R.; Christoffoleti, P.; Perreta, M. First report of Amaranthus hybridus with multiple resistance to 2, 4-D, dicamba, and glyphosate. Agronomy 2018, 8, 140. [Google Scholar] [CrossRef]
- Van Volkenburg, H.; Guinel, F.C.; Vasseur, L. Impacts of smooth pigweed (Amaranthus hybridus) on cover crops in Southern Ontario. Agronomy 2020, 10, 529. [Google Scholar] [CrossRef]
- García, M.J.; Palma-Bautista, C.; Vazquez-Garcia, J.G.; Rojano-Delgado, A.M.; Osuna, M.D.; Torra, J.; De Prado, R. Multiple mutations in the EPSPS and ALS genes of Amaranthus hybridus underlie resistance to glyphosate and ALS inhibitors. Sci. Rep. 2020, 10, 17681. [Google Scholar] [CrossRef] [PubMed]
- Sardrood, P.; Goltapeh, M. Weeds, herbicides and plant disease management. In Sustainable Agriculture Reviews; Springer: Cham, Switzerland, 2018; Volume 31, pp. 41–178. [Google Scholar]
- Macías, A.; Mejías, J.; Molinillo, M. Recent advances in allelopathy for weed control: From knowledge to applications. Pest Manag. Sci. 2019, 75, 2413–2436. [Google Scholar] [CrossRef]
- Arafat, Y.; Ali, N. Allelopathic evaluation of selected plants extracts against broad and narrow leaves weeds and their associated crops. Acad. J. Agric. Res. 2015, 3, 226–234. [Google Scholar] [CrossRef]
- Nawaz, A.; Farooq, M.; Cheema, S.; Cheema, Z. Role of allelopathy in weed management. In Recent Advances in Weed Management; Chauhan, B., Mahajan, G., Eds.; Springer: New York, NY, USA, 2014; pp. 39–61. [Google Scholar]
- Kaab, S.; Rebey, I.; Hanafi, M.; Hammi, K.; Smaoui, A.; Fauconnier, L.; De Clerck, C.; Jijakli, M.; Ksouri, R. Screening of Tunisian plant extracts for herbicidal activity and formulation of a bioherbicide based on Cynara cardunculus. S. Afr. J. Bot. 2020, 128, 67–76. [Google Scholar] [CrossRef]
- Guglielmini, A.; Ghersa, C.; Satorre, E. Co-evolution of domesticated crops and associated weeds. Ecol. Austral. 2020, 17, 167–178. Available online: https://bibliotecadigital.exactas.uba.ar/download/ecologiaaustral/ecologiaaustral_v017_n01_p167.pdf (accessed on 29 June 2022).
- Vencill, W.K.; Nichols, R.L.; Webster, T.M.; Soteres, J.K.; Mallory-Smith, C.; Burgos, N.R.; Johnson, W.G.; Mc Clelland, M.R. Herbicide resistant: Toward and understanding of resistance development and the impact of herbicide-resistance crops. Weed Sci. 2012, 60, 2–30. [Google Scholar] [CrossRef]
- Menalled, F. Consideraciones ecológicas para el desarrollo de programas de manejo integrado de malezas. Agroecología 2010, 5, 73–78. Available online: https://revistas.um.es/agroecologia/article/view/160581 (accessed on 29 June 2022).
- Böcker, T.; Möhring, N.; Finger, R. Herbicide free agriculture? A bio-economic modelling application to Swiss wheat production. Agric. Syst. 2019, 173, 378–392. [Google Scholar] [CrossRef]
- Muñoz, C.; Chavez, W.; Pabón, L.; Rendón, R.; Patricia-Chaparro Otálvaro-Álvarez, M. Extracción de compuestos fenólicos con actividad antioxidante a partir de Champa (Campomanesia lineatifolia). Rev. CENIC Cienc. Químicas 2015, 46, 38–46. [Google Scholar]
- Álvarez-Herrera, J.; Galvis, J.; Balaguera-López, H. Determinación de cambios físicos y químicos durante la maduración de frutos de champa (Campomanesia lineatifolia R. & P.). Agron. Colomb. 2009, 27, 253–259. [Google Scholar]
- Bonilla, A.; Duque, C.; Garzón, C.; Takaishi, Y.; Yamaguchi, K.; Hara, N.; Fujimoto, Y. Champanones, yellow pigments from the seeds of champa (Campomanesia lineatifolia). Phytochemistry 2005, 66, 1736–1740. [Google Scholar] [CrossRef]
- Osorio, C.; Alarcón, M.; Moreno, C.; Bonilla, A.; Barrios, J.; Garzón, C.; Duque, C. Characterization of odor-active volatiles in Champa (Campomanesia lineatifolia R. & P.). J. Agric. Food Chem. 2006, 54, 509–516. [Google Scholar] [CrossRef]
- Cabeza-Cepeda, C.; Balaguera-López, H.E.; Useche de Vega, D.S. Alelopatía del extracto de Campomanesia lineatifolia sobre Taraxacum officinale. Cienc. Tecnol. Agropecu. 2021, 22, e2010. [Google Scholar] [CrossRef]
- Martinez, C.; Fonseca, J.; Balaguera-López, H.E. The bioherbicidal activity of seed extract of Campomanesia lineatifolia on the weed Sonchus oleraceus L. Agron. Colomb. 2022, 40, 49–57. [Google Scholar] [CrossRef]
- Niño-Hernandez, J.; Moreno, F.; Ruiz-Berrío, D.; Balaguera-López, E.; Magnitskiy, S. Light, gibberellins and burial depth affect seed germination of Amaranthus hybridus L. Rev. UDCA Actual. Divulg. Científica 2020, 23. [Google Scholar]
- Paraiso, A.; Brandão, S.; Avelar, S.; Costa, D.; Gomes, P.; Nascimento, W. Adjustments in the tetrazolium test methodology for assessing the physiological quality of chickpea seeds. J. Seed Sci. 2019, 41, 007–012. [Google Scholar] [CrossRef]
- Elias, G.; Copeland, O.; McDonald, B.; Baalbaki, Z. Seed Testing: Principles and Practices. 22–24; Michigan State University Press: East Lansing, MI, USA, 2012. [Google Scholar]
- Carranza, C.; Lanchero, O.; Miranda, D.; Chaves, B. Análisis del crecimiento de lechuga (Lactuca sativa L.) ‘Batavia’ cultivada en un suelo salino de la Sabana de Bogotá. Agron. Colomb. 2009, 27, 41–48. [Google Scholar]
- Madalosso, R.C.; Oliveira, G.C.; Martins, M.T.; Vieira, A.E.; Barbosa, J.; Caliari, M.V.; Castilho, R.O.; Tagliati, C.A. Campomanesia lineatifolia Ruiz & Pav. as a gastroprotective. J. Ethnopharmacol. 2012, 139, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Santos, E.; Camargo, M.; Araújo, J.; Couto, M.; Vinholes, J.; Olle, D.; Vizzotto, M.; Nora, L. Biological activity and chemical composition of fruits, seeds, and leaves of guabirobeira (Campomanesia xanthocarpa O. Berg–Myrtaceae): A review. Food Biosci. 2021, 40, 100899. [Google Scholar] [CrossRef]
- Catunda, M.G.; Souza, C.L.M.; Morais, V.; Carvalho, G.J.A.; Freitas, S.P. Efeitos de extratos aquosos de tiririca sobre a germinação de alface, pimentão e jiló e sobre a divisão celular na radícula de alface. Ceres 2002, 49, 1–11. Available online: http://www.ceres.ufv.br/ojs/index.php/ceres/article/view/2785 (accessed on 29 June 2022).
- Carvalho, M.S.S.; Andrade-Vieira, L.F.; Santos FEd Correa, F.F.; das Graças Cardoso, M.; Vilela, L.R. Allelopathic potential and phytochemical screening of ethanolic extracts from five species of Amaranthus spp. in the plant model Lactuca sativa. Sci. Hortic. 2019, 245, 90–98. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA, 2014; 169p. [Google Scholar]
- Motmainna, M.; Juraimi, A.S.; Uddin, M.K.; Asib, N.B.; Islam, A.K.M.M.; Ahmad-Hamdani, M.S.; Hasan, M. Phytochemical Constituents and Allelopathic Potential of Parthenium hysterophorus L. in Comparison to Commercial Herbicides to Control Weeds. Plants 2021, 10, 1445. [Google Scholar] [CrossRef]
- Seabra Júnior, E.; Dal-Pozzo, D.M.; Feiden, A.; Ferreira Santos, R.; Kazue Tokura, L. Allelopathic effects of jack bean leaf aqueous extract on safflower cultures. Rev. Colomb. Cienc. Hortícolas 2017, 11, 435–440. [Google Scholar] [CrossRef]
- Gil, A.I.; Celis, Á.; Cuevas, J.C. Efecto inhibitorio de extractos de Swinglea glutinosa (Blanco) Merr. y Lantana camara L. en preemergencia y posemergencia. Rev. Colomb. Cienc. Hortícolas 2010, 4, 209–222. [Google Scholar] [CrossRef]
- Gindri, D.M.; Coelho, C.M.M.; Uarrota, V.G.; Rebelo, A.M. Herbicidal bioactivity of natural compounds from Lantana camara on the germination and seedling growth of Bidens pilosa. Pesqui. Agropecuária Trop. 2020, 50, e57746. [Google Scholar] [CrossRef]
- Imatomi, M.; Novaes, P.; Gualtieri, G.C.J. Interspecific variation in the allelopathic potential of the family Myrtaceae. Acta Bot. Bras. 2013, 27, 54–61. [Google Scholar] [CrossRef]
- Fan, P.; Hostettmann, K.; Lou, H. Allelochemicals of the invasive neophyte Polygonum cuspidatum Sieb. & Zucc.(Polygonaceae). Chemoecology 2010, 20, 223–227. [Google Scholar]
- Ahammed, G.; Li, Y.; Cheng, Y.; Liu, A.; Chen, S.; Li, X. Abscisic Acid and Gibberellins Act Antagonistically to Mediate Epigallocatechin-3-Gallate-Retarded Seed Germination and Early Seedling Growth in Tomato. J. Plant Growth Regul. 2020, 39, 1414–1424. [Google Scholar] [CrossRef]
- Pereira, C.; Carvalho, C.; Serra, N.; Souza, I.; Costa, J.; Santana, A. Allelopathic effects of Cecropia pachystachya Trecul on germination and seedling growth of Lactuca sativa. Allelopath. J. 2017, 42, 265–280. [Google Scholar] [CrossRef]
- Ximenez, G.R.; Bianchin, M.; Carmona, J.M.P.; de Oliveira, S.M.; Ferrarese-Filho, O.; Pastorini, L.H. Reduction of weed growth under the influence of extracts and metabolites isolated from Miconia spp. Molecules 2022, 27, 5356. [Google Scholar] [CrossRef]
- Chauhan, A.; Kumari, N.; Saxena, D.C.; Singh, S. Effect of germination on fatty acid profile, amino acid profile and minerals of amaranth (Amaranthus spp.) grain. J. Food Meas. Charact. 2022, 16, 1777–1786. [Google Scholar] [CrossRef]
- El-Shora, H.M.; Alharbi, M.M.; Darwish, D.B.; Gad, D. Allelopathic potential of aqueous leaf extract of Rumex dentatus L. on metabolites and enzyme activities of common purslane leaves. J. Plant Interact. 2022, 17, 267–276. [Google Scholar] [CrossRef]
- Barco-Antoñanzas, M.; Gil-Monreal, M.; Eceiza, M.V.; Royuela, M.; Zabalza, A. Primary metabolism in an Amaranthus palmeri population with multiple resistance to glyphosate and pyrithiobac herbicides. Plant Sci. 2022, 318, 111212. [Google Scholar] [CrossRef]


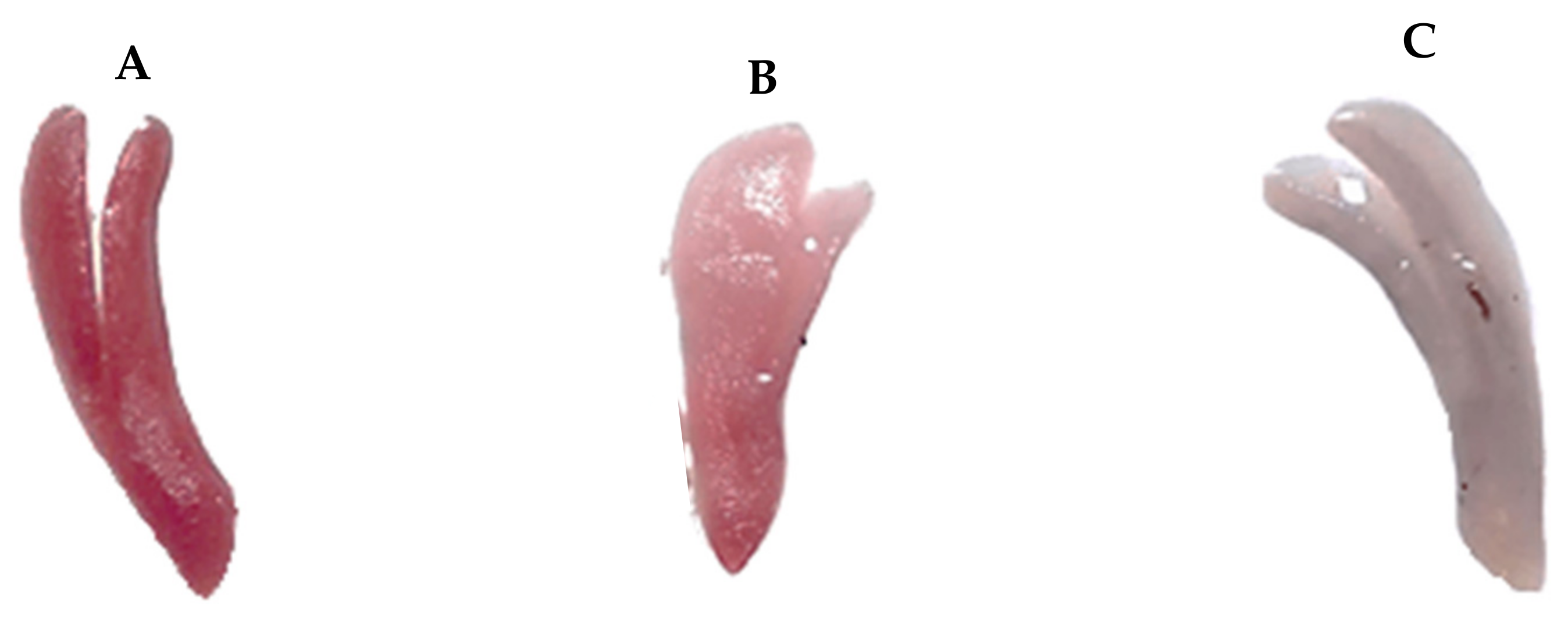
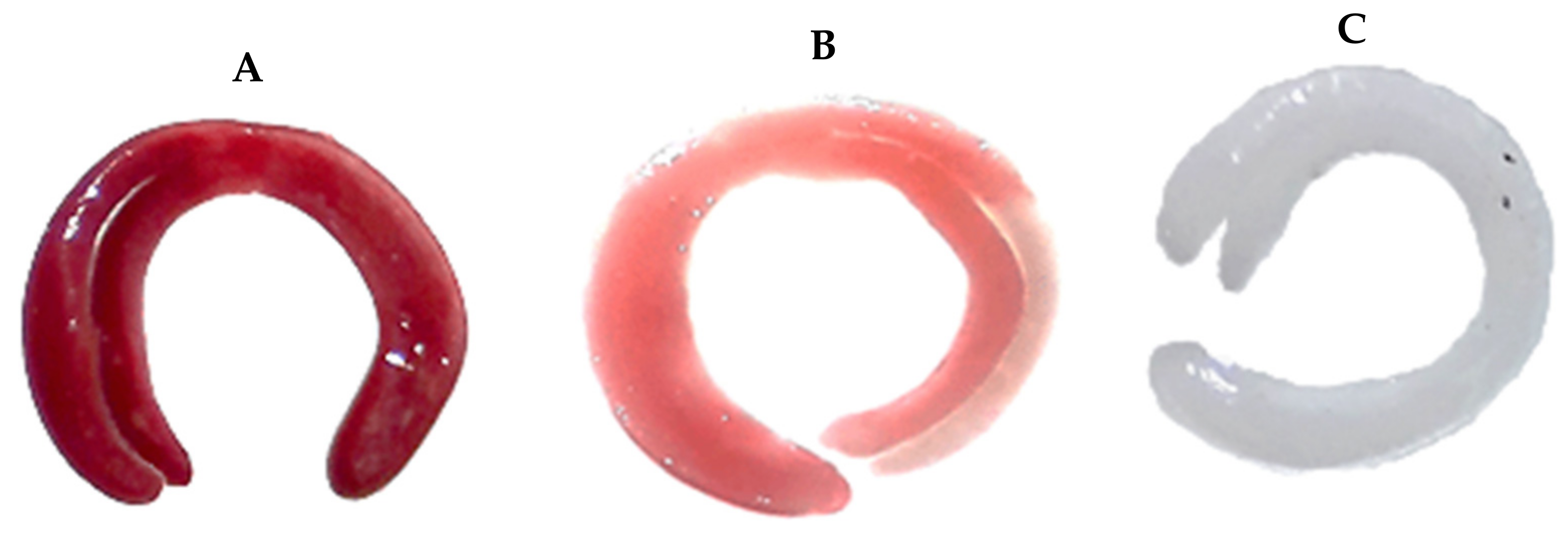
| Variable | Equations |
|---|---|
| Germination percentage GP (%) | |
| Mean germination speed (germinated seeds/day) | |
| Mean germination time (days) |
| Classification | Compound | Rt, min | [M + H]+ | Concentration (mg kg−1) |
|---|---|---|---|---|
| Methylxanthines | Caffeine | 4.12 | 195.09 | 4.30 |
| Theobromine | 3.57 | 181.07 | 15.60 | |
| Flavanol | Catechins | 3.96 | 291.09 | 5.47 |
| Epicatechin (EC) | 4.17 | 291.08 | 17.23 | |
| Epigallocatechin (EGC) | 3.80 | 307.08 | 53.50 | |
| Epicatechin gallate (ECG) | 4.58 | 443.10 | 83.30 | |
| Epigallocatechin gallate (EGCG) | 4.21 | 459.09 | 165.40 | |
| Kaempferol | 6.08 | 287.05 | 3.20 | |
| Quercetin | 5.59 | 303.05 | 40.00 | |
| Flavanone | Naringenin | 5.87 | 273.07 | 0.50 |
| Pinocembrin | 6.75 | 257.08 | 4.30 | |
| Flavone | Apigenin | 6.01 | 271.06 | 3.10 |
| Flavonol glycosides | Kaempferol-3-glucoside | 4.84 | 449.11 | 15.50 |
| Quercetin-3-glucoside | 4.66 | 465.10 | 138.40 | |
| Hydroxycinnamic acids | p-coumaric acid | 4.70 | 165.05 | 12.50 |
| Trans-cinnamic acid | 5.79 | 149.06 | 49.10 | |
| Hydroxybenzoic acids | p-hydroxybenzoic acid | 4.19 | 139.04 | 100.70 |
| Vanilic acid | 4.23 | 169.05 | 45.60 | |
| Anthocyanins | Cyanidin | 4.86 | 287.05 | 18.90 |
| Pentacyclic triterpenoid | Ursolic acid | 9.79 | 457.37 | 76.40 |
| Experiment and Treatm Ents | Equations | RMSE |
|---|---|---|
| [Extract] (% w/v) on R. crispus with one application | ||
| Control | Y = 60.93/1 + e−0.38×(days−9.20) | 4.20 *** |
| 3 | Y = 40.77/1 + e−0.20×(days−16.29) | 2.45 *** |
| 6 | Y = 50.91/1 + e−0.56×(days−8.39) | 3.07 *** |
| 9 | Y = 25.13/1 + e−0.66×(days−17.87) | 2.82 ** |
| [Extract] (% w/v) on R. crispus with repeated application | ||
| Control | Y = 60.93/1 + e−0.38×(days−9.20) | 4.20 *** |
| 3 | Y = 35.23/1 + e−0.23×(days−26.57) | 0.70 *** |
| 6 | Y = 23.48/1 + e−0.23×(days−26.57) | 1.33 *** |
| 9 | Y = 19.68/1 + e−0.28×(days−29.40) | 0.55 *** |
| Extract Application | Concentration (w/v) | Germination | Viability (%) | ||||
|---|---|---|---|---|---|---|---|
| GP (%) | MGS (Germinated Seeds/Days) | MGT (Days) | Viable | Doubtful | Nonviable | ||
| One application | Control | 65.0 ± 5.5 a | 6.4 ± 0.7 a | 10.5 ± 1.1 a | 3.5 ± 0.5 b | 11.2 ± 4.0 ab | 20.3 ± 4.3 b |
| 3% | 41.3 ± 3.1 b | 2.7 ± 0.3 b | 9.7 ± 1.6 a | 10.2 ± 1.4 a | 18.5 ± 3.1 a | 30.0 ± 2.8 ab | |
| 6% | 54.3 ± 2.9 ab | 5.3 ± 0.5 a | 8.2 ± 0.7 a | 9.5 ± 1.9 a | 8.0 ± 2.3 b | 27.5 ± 2.9 ab | |
| 9% | 40.0 ± 8.5 b | 1.7 ± 0.3 b | 11.8 ± 3.0 a | 10.8 ± 3.5 a | 17.5 ± 3.8 a | 37.5 ± 5.7 a | |
| Significance | * | * | ns | * | * | * | |
| Repeated application | Control | 65.0 ± 5.4 a | 6.4 ± 0.7 a | 10.5 ± 1.1 a | 3.5 ± 0.5 b | 11.2 ± 4.09 b | 20.3 ± 4.3 b |
| 3% | 30.0 ± 2.5 b | 1.2 ± 0.2 b | 9.9 ± 0.6 a | 30.8 ± 2.2 a | 14.0 ± 2.3 ab | 25.3 ± 4.1 ab | |
| 6% | 22.3 ± 4.6 b | 0.9 ± 0.2 b | 6.9 ± 1.4 a | 21.3 ± 5.7 a | 20.8 ± 2.4 ab | 35.8 ± 3.2 a | |
| 9% | 15.5 ± 4.6 b | 0.6 ± 0.1 b | 5.6 ± 1.8 a | 17.0 ± 3.2 ab | 31.5 ± 2.8 a | 36.0 ± 3.6 a | |
| Significance | * | * | ns | * | * | * | |
| Experiment and Treatments | Equations | RMSE |
|---|---|---|
| [Extract] (% w/v) on A. hybridus with one application | ||
| 0 | Y = 301.65/1 + e−0.03×(days−83.23) | 2.31 * |
| 3 | Y = 51.98/1 + e−0.45×(days−26.23) | 1.00 *** |
| 6 | Y = 58.61/1 + e−0.24×(days−27.27) | 2.92 ** |
| 9 | Y = 34.88/1 + e−0.39×(days−28.32) | 1.28 *** |
| [Extract] (% w/v) on A. hybridus with repeated application | ||
| 0 | Y = 301.65/1 + e−0.03×(days−83.23) | 2.31 * |
| 3 | Y = 38.14/1 + e−0.30×(days−23.77) | 1.16 *** |
| 6 | Y = 28.50/1 + e−3.13×(days−22.71) | 4.54 ** |
| 9 | Y = 27.92/1 + e−0.53×(days−29.79) | 0.05 *** |
| Extract Application | Concentration (w/v) | Germination | Viability (%) | ||||
|---|---|---|---|---|---|---|---|
| GP (%) | MGS (Germinated Seeds/Days) | MGT (Days) | Viable | Doubtful | Nonviable | ||
| One application | Control | 60.5 ± 6.5 a | 9.0 ± 2.8 a | 11.2 ± 3.3 b | 36.0 ± 5.8 a | 1.3 ± 0.5 b | 1.8 ± 0.7 c |
| 3% | 50.0 ± 1.8 a | 1.8 ± 0.4 b | 18.8 ± 0.7 a | 10.5 ± 1.9 b | 24.8 ± 2.2 a | 12.0 ± 3.7 bc | |
| 6% | 47.5 ± 3.4 a | 2.1 ± 0.4 b | 16.7 ± 0.6 ab | 7.3 ± 2.2 b | 19.0 ± 3.7 a | 23.8 ± 3.4 ab | |
| 9% | 31.0 ± 1.0 b | 1.1 ± 0.1 b | 12.1 ± 0.6 ab | 15.5 ± 1.6 b | 25.8 ± 0.8 a | 28.8 ± 3.1 a | |
| Significance | * | * | * | * | * | * | |
| Several application | Control | 60.5 ± 6.5 a | 9.0 ± 2.853 a | 11.2 ± 3.4 a | 36.0 ± 5.8 a | 1.3 ± 0.5 b | 1.8 ± 0.7 c |
| 3% | 35.0 ± 3.1 b | 1.5 ± 0.2 ab | 12.4 ± 1.5 a | 5.5 ± 0.7 b | 25.5 ± 0.7 a | 34.0 ± 2.9 b | |
| 6% | 27.0 ± 0.9 b | 0.9 ± 0.6 bc | 10.4 ± 0.3 a | 6.5 ± 0.7 b | 20.5 ± 2.4 a | 46.0 ± 2.1 a | |
| 9% | 25.2 ± 1.7 b | 0.8 ± 0.1 c | 10.6 ± 0.8 a | 5.8 ± 0.9 b | 22.5 ± 1.9 a | 46.5 ± 2.8 a | |
| Significance | * | * | ns | * | * | * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maestre Rodríguez, L.; Palacios Ortega, E.; Moreno Medina, B.L.; Balaguera-López, H.E.; Hernandez, J.P. Hydroalcoholic Extracts of Campomanesia lineatifolia R. & P. Seeds Inhibit the Germination of Rumex crispus and Amaranthus hybridus. Horticulturae 2023, 9, 177. https://doi.org/10.3390/horticulturae9020177
Maestre Rodríguez L, Palacios Ortega E, Moreno Medina BL, Balaguera-López HE, Hernandez JP. Hydroalcoholic Extracts of Campomanesia lineatifolia R. & P. Seeds Inhibit the Germination of Rumex crispus and Amaranthus hybridus. Horticulturae. 2023; 9(2):177. https://doi.org/10.3390/horticulturae9020177
Chicago/Turabian StyleMaestre Rodríguez, Laura, Edgar Palacios Ortega, Brigitte Liliana Moreno Medina, Helber Enrique Balaguera-López, and Juan Pablo Hernandez. 2023. "Hydroalcoholic Extracts of Campomanesia lineatifolia R. & P. Seeds Inhibit the Germination of Rumex crispus and Amaranthus hybridus" Horticulturae 9, no. 2: 177. https://doi.org/10.3390/horticulturae9020177
APA StyleMaestre Rodríguez, L., Palacios Ortega, E., Moreno Medina, B. L., Balaguera-López, H. E., & Hernandez, J. P. (2023). Hydroalcoholic Extracts of Campomanesia lineatifolia R. & P. Seeds Inhibit the Germination of Rumex crispus and Amaranthus hybridus. Horticulturae, 9(2), 177. https://doi.org/10.3390/horticulturae9020177








