Exogenous Appliance of Nano-Zeolite and Nano-Silicon Elevate Solidago canadensis Invasive Plant Tolerance to Water Deficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Plant Material, Transplant, and Harvest Date
2.3. Soil Preparation
2.4. Chemical Fertilizers
2.5. Synthesis of Nano-Zeolite (n-ZE)-Loaded Nitrogen Particles
2.6. Synthesis of Silicon Nanoparticles
2.7. Synthesis of Nitrogen, Phosphorus, and Potassium Nanoparticles
- NPK as a control, T1;
- Nano-silicon (n-Si), T2;
- Nano-NPK (n-NPK), T3;
- Nano-zeolite-loaded nitrogen (n-ZE-loaded N),T4;
- Nano-zeolite-loaded nitrogen (n-ZE-loaded N) + nano-silicon (n-Si), T5.
2.8. Data Recorded
2.8.1. Morphological Traits
2.8.2. Relative Water Content (RWC)
2.8.3. Net Photosynthesis
2.8.4. Chemical Analysis
2.8.5. Endogenous Phytohormones
2.8.6. The Activity of Antioxidant Enzymes
2.8.7. Essential Oil Analysis
2.9. Statistical Analysis
3. Results
3.1. Nanoparticles Enhanced Solidagocanadensis Plant Growth and Its Yield Characteristics
3.2. Nanoparticles Enhanced Leaf Endogenous Nutrient Contents of Solidago canadensis Plants
3.3. Nanoparticles Enhanced Photosynthetic Pigments and Biochemical Contents of Solidago canadensis Plants
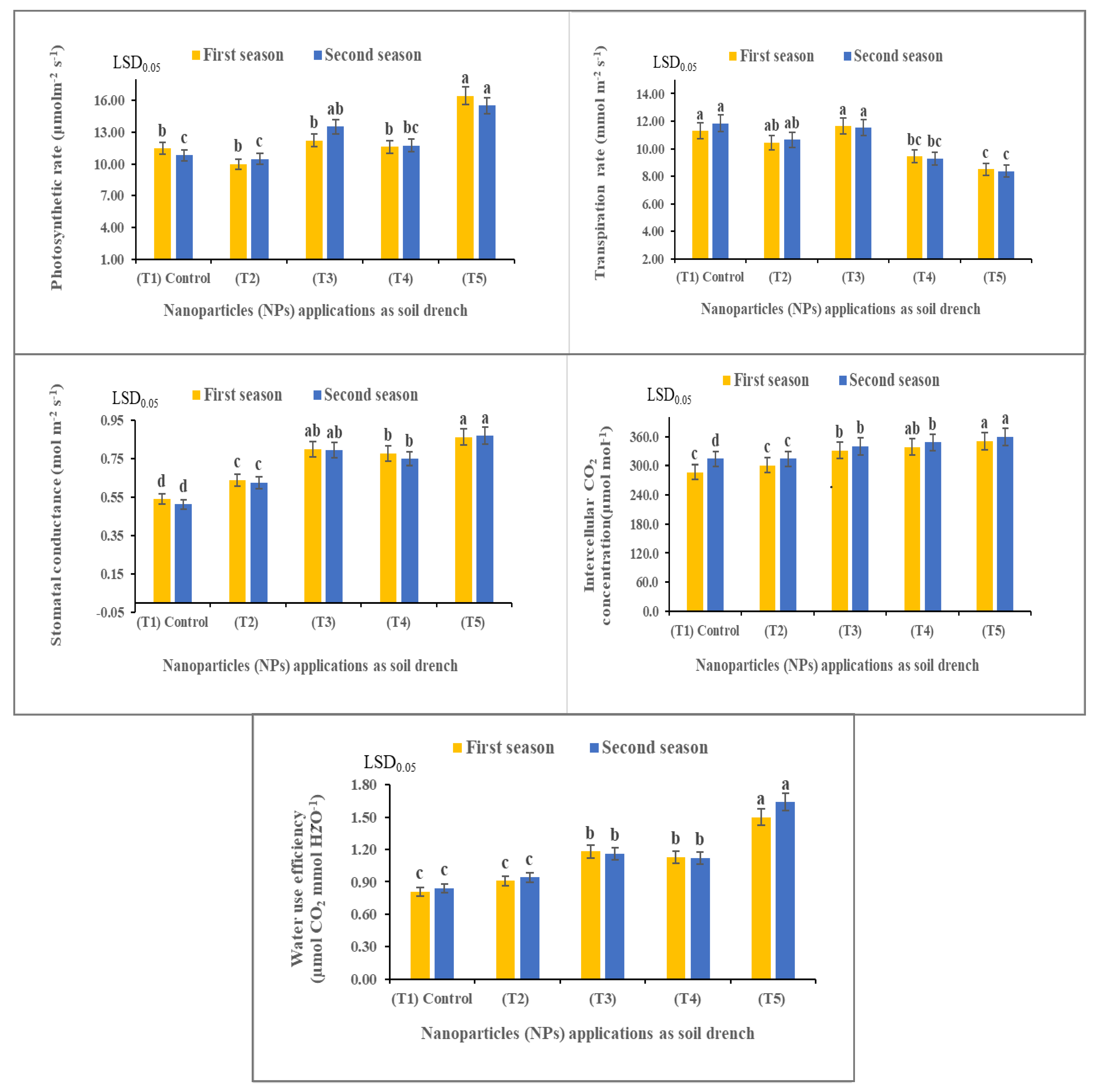
3.4. Nanoparticles Enhanced Photosynthetic and Transpiration Rate, Stomatal Conductance, Intercellular CO2 Concentration, and Water Use Efficiency of Solidago canadensis Plants
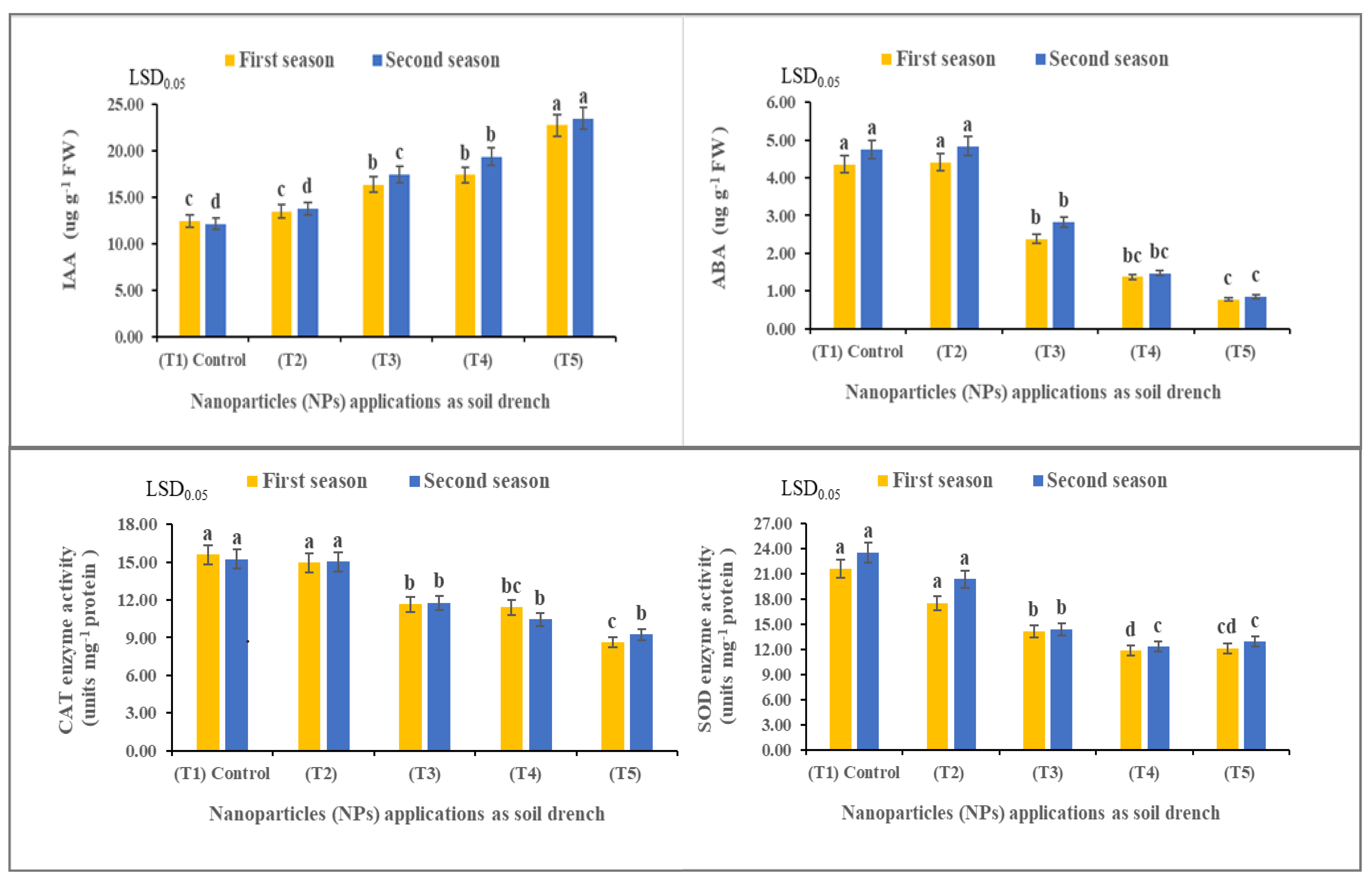
3.5. Nanoparticles Enhanced Leaf Hormones and Enzyme Activity of Solidago canadensis Plants
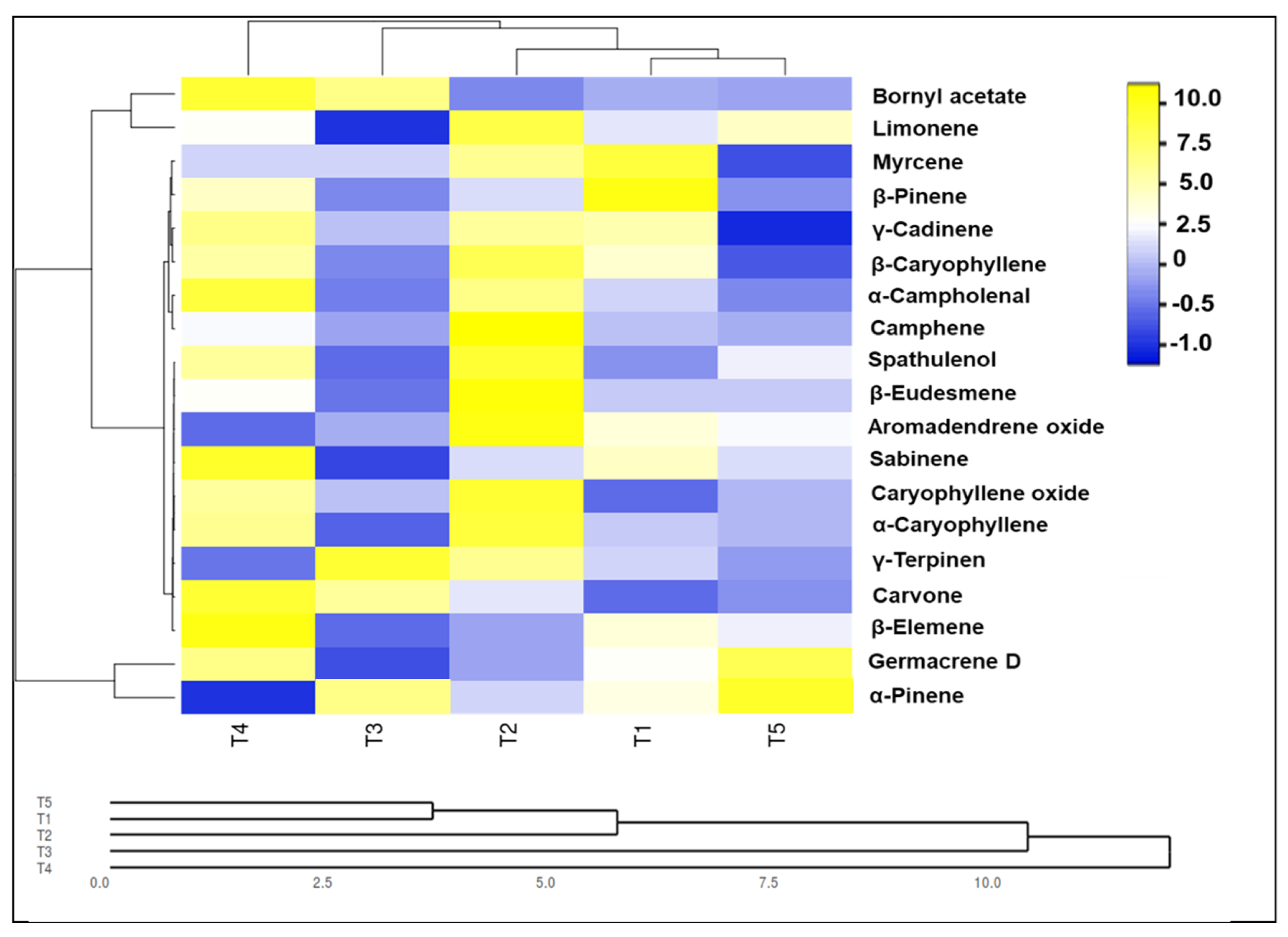
3.6. Nanoparticles Enhanced Productivity Essential Oil and Components of Solidago canadensis Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Badri, A.M.; Batool, M.A.A.; Mohamed, I.; Wang, Z.; Khatab, A.; Sherif, A.; Ahmad, H.; Khan, M.N.; Hassan, H.M.; Elrewainy, I.M.; et al. Antioxidative and Metabolic Contribution to Salinity Stress Responses in Two Rapeseed Cultivars during the Early Seedling Stage. Antioxidants 2021, 10, 1227. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.; Countryman, A.M. The Importance of Agriculture in the Economy: Impacts from COVID-19. Am. J. Agric. Econ. 2021, 103, 1595–1611. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Saud, S.; Chen, Y.; Fahad, S.; Hussain, S.; Na, L.; Xin, L.; Alhussien, S. Silicate application increases the photosynthesis and its associated metabolic activities in Kentucky bluegrass under drought stress and post-drought recovery. Environ. Sci. Pollut. Res. 2016, 23, 17647–17655. [Google Scholar] [CrossRef] [PubMed]
- Abdul Qados, A.M.S. Effect of salt stress on plant growth and metabolism of bean plant Vicia faba. J. Saudi Soc. Agric. Sci. 2011, 10, 7–15. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; Samy, M.M.; Sany, H.; Eid, R.R.; Rashad, H.M.; Abdeldaym, E.A. Nanopotassium, Nanosilicon, and Biochar Applications Improve Potato Salt Tolerance by Modulating Photosynthesis, Water Status, and Biochemical Constituents. Sustainability 2022, 14, 723. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; Esmail, S.E.A.; El-Attar, A.B.; Othman, E.Z.; El-Bahbohy, R.M. Prospective Practice for Compound Stress Tolerance in Thyme Plants Using Nanoparticles and Biochar for Photosynthesis and Biochemical Ingredient Stability. Agronomy 2022, 12, 1069. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Chen, J.; Finnegan, P.M.; Younis, A.; Nafees, M.; Zorrig, W.; Hamed, K.B. Application of Trehalose and Salicylic Acid Mitigates Drought Stress in Sweet Basil and Improves Plant Growth. Plants 2021, 10, 1078. [Google Scholar] [CrossRef]
- Pingping, W.; Chubin, W.; Biyan, Z. Drought Stress Induces Flowering and Enhances Carbohydrate Accumulation in Averrhoa Carambola. Hortic. Plant J. 2017, 3, 60–66. [Google Scholar] [CrossRef]
- Mansoor, S.; Kour, N.; Manhas, S.; Zahid, S.; Wani, O.A.; Sharma, V.; Wijaya, L.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; et al. Biochar as a tool for effective management of drought and heavy metal toxicity. Chemosphere 2021, 271, 129458. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; Ayad, A.A.; Abdel-Aziz, H.S.M.; Williams, L.L.; El-Shazoly, R.M.; Abdel-Wahab, A.; Abdeldaym, E.A. Foliar Application of Different Iron Sources Improves Morpho-Physiological Traits and Nutritional Quality of Broad Bean Grown in Sandy Soil. Plants 2022, 11, 2599. [Google Scholar] [CrossRef] [PubMed]
- Hafez, M.; Popov, A.I.; Rashad, M. Integrated use of bio-organic fertilizers for enhancing soil fertility–plant nutrition, germination status and initial growth of corn (Zea mays L). Environ. Technol. Innov. 2021, 21, 101329. [Google Scholar] [CrossRef]
- Marchiol, L.; Mattiello, A.; Pošćić, F.; Fellet, G.; Zavalloni, C.; Carlino, E.; Musetti, R. Changes in Physiological and Agronomical Parameters of Barley (Hordeum vulgare) Exposed to Cerium and Titanium Dioxide Nanoparticles. Int. J. Environ. Res. Public Health 2016, 13, 332. [Google Scholar] [CrossRef] [PubMed]
- Abou-Sreea, A.I.B.; Kamal, M.; El Sowfy, D.M.; Rady, M.M.; Mohamed, G.F.; Al-Dhumri, S.A.; AL-Harbi, M.S.; Abdou, N.M. Small-Sized Nanophosphorus Has a Positive Impact on the Performance of Fenugreek Plants under Soil-Water Deficit Stress: A Case Study under Field Conditions. Biology 2022, 11, 115. [Google Scholar] [CrossRef]
- Qureshi, A.; Singh, D.; Dwivedi, S. Nano-fertilizers: A Novel Way for Enhancing Nutrient Use Efficiency and Crop Productivity. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3325–3335. [Google Scholar] [CrossRef]
- Astaneh, N.; Bazrafshan, F.; Zare, M.; Amiri, B.; Bahrani, A. Nano-fertilizer prevents environmental pollution and improves physiological traits of wheat grown under drought stress conditions. Sci. Agropecu. 2021, 12, 41–47. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef]
- Xin, X.; Nepal, J.; Wright, A.L.; Yang, X.; He, Z. Carbon nanoparticles improve corn (Zea mays L.) growth and soil quality: Comparison of foliar spray and soil drench application. J. Clean. Prod. 2022, 363, 132630. [Google Scholar] [CrossRef]
- Asadpour, S.; Madani, H.; Mohammadi, G.N.; Heravan, I.M.; Abad, H.H.S. Improving Maize Yield with Advancing Planting Time and Nano-Silicon Foliar Spray Alone or Combined with Zinc. Silicon 2020, 14, 201–209. [Google Scholar] [CrossRef]
- Badem, A.; Söylemez, S. Effects of Nitric Oxide and Silicon Application on Growth and Productivity of Pepper under Salinity Stress. J. King Saud Univ. Sci. 2022, 34, 102189. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Feizi, M.; Kumari, A.; Khan, M.; Mandzhieva, S.; Sushkova, S.; El-Ramady, H.; Verma, K.K.; Singh, A.; et al. Effects of Silicon and Silicon-Based Nanoparticles on Rhizosphere Microbiome, Plant Stress and Growth. Biology 2021, 10, 791. [Google Scholar] [CrossRef]
- Ismail, L.M.; Soliman, M.I.; Abd El-Aziz, M.H.; Abdel-Aziz, H.M.M. Impact of Silica Ions and Nano Silica on Growth and Productivity of Pea Plants under Salinity Stress. Plants 2022, 11, 494. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.W.M.; Abdeldaym, E.A.; Abdelaziz, S.M.; El-Sawy, M.B.I.; Mottaleb, S.A. Synergetic Effects of Zinc, Boron, Silicon, and Zeolite Nanoparticles on Confer Tolerance in Potato Plants Subjected to Salinity. Agronomy 2019, 10, 19. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; Swaefy, H.M. Comparison between commercial and nano NPK in presence of nano zeolite on sage plant yield and its components under water stress. Agriculture 2020, 66, 24–39. [Google Scholar] [CrossRef]
- Hassan, A.Z.A.; Mahmoud, A.W.M. Strategy for Boosting Rock Phosphate Efficiency and Conversion into Nano Zeolite. Am. J. Nanomater. 2016, 4, 27–38. [Google Scholar] [CrossRef]
- Hassan, A.Z.A.; Mahmoud, A.W.M.; Turky, G. Rice husk derived nano zeolite (A.M.2) as fertilizer, hydrophilic and novel organophilic material. Am. J. Nanomater. 2017, 5, 11–23. [Google Scholar] [CrossRef]
- Yuvaraj, M.; Subramanian, K.S. Development of slow release Zn fertilizer using nano-zeolite as carrier. J. Plant Nutr. 2018, 41, 311–320. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; El-Attar, A.B.; Mahmoud, A.A. Economic evaluation of nano and organic fertilizers as an alternative source to chemical fertilizers on Carum carvi L. plant yield and components. Agriculture 2017, 63, 33–49. [Google Scholar] [CrossRef]
- Besharati, J.; Shirmardi, M.; Meftahizadeh, H.; Ardakani, M.D.; Ghorbanpour, M. Changes in growth and quality performance of Roselle (Hibiscus sabdariffa L.) in response to soil amendments with hydrogel and compost under drought stress. South Afr. J. Bot. 2022, 145, 334–347. [Google Scholar] [CrossRef]
- McGaw, L.J.; Omokhua-Uyi, A.G.; Finnie, J.F.; Van Staden, J. Invasive alien plants and weeds in South Africa: A review of their applications in traditional medicine and potential pharmaceutical properties. J. Ethnopharmacol. 2022, 283, 114564. [Google Scholar] [CrossRef]
- Dong, L.J.; Yu, H.W.; He, W.M. What determines positive, neutral, and negative impacts of Solidago canadensis invasion on native plant species richness? Sci. Rep. 2015, 5, 16804. [Google Scholar] [CrossRef] [PubMed]
- Weber, E. Current and Potential Ranges of Three Exotic Goldenrods (Solidago) in Europe. Conservat. Biol. 2001, 15, 122–128. [Google Scholar] [CrossRef]
- Bao, X.-Y.; Wang, Z.-X.; He, Z.-S.; He, W.-M. Enhanced precipitation offsets climate warming inhibition on Solidago canadensis growth and sustains its high tolerance. Global Ecol. Conservat. 2022, 34, e02023. [Google Scholar] [CrossRef]
- Mahdi, Z.A.H.; Hussein, J.K. Effect of humic acid, gibberellin and tryptophan on the growth and flowering characteristics of goldenrods plant Solidago canadensis. IOP Conf. Ser. Earth Environ. Sci. 2021, 910, 012128. [Google Scholar] [CrossRef]
- Fursenco, C.; Calalb, T.; Uncu, L.; Dinu, M.; Ancuceanu, R. Solidago virgaurea L.: A Review of Its Ethnomedicinal Uses, Phytochemistry, and Pharmacological Activities. Biomolecules 2020, 10, 1619. [Google Scholar] [CrossRef]
- Nkuimi-Wandjou, J.G.; Quassinti, L.; Gudžinskas, Z.; Nagy, D.U.; Cianfaglione, K.; Bramucci, M.; Maggi, F. Chemical composition and antiproliferative effect of essential oils of four Solidago species (S. canadensis, S. gigantea, S. virgaurea and S.× niederederi). Chem. Biodivers. 2020, 17, e2000685. [Google Scholar] [CrossRef]
- Radušienė, J.; Karpavičienė, B.; Marksa, M.; Ivanauskas, L.; Raudonė, L. Distribution Patterns of Essential Oil Terpenes in Native and Invasive Solidago Species and Their Comparative Assessment. Plants 2022, 11, 1159. [Google Scholar] [CrossRef]
- Wang, Z.X.; He, Z.S.; He, W.M. Nighttime climate warming enhances inhibitory effects of atmospheric nitrogen deposition on the success of invasive Solidago canadensis. Clim. Change 2021, 167, 20. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils, U.S.D.A. Agriculture Handbook No. 60; US Department of Agriculture: Washington, DC, USA, 1954; p. 158.
- Jackson, M. Soil Chemical Analysis; Pentice Hall of India Pvt. Ltd.: New Delhi, India, 1973; Volume 498, pp. 151–154. [Google Scholar]
- Keller, J.; Bliesner, R.D. Sprinkle and Trickle Irrigation; The Blackburn Press: New York, NY, USA, 2000; 652p. [Google Scholar]
- Hassan, A.Z.A.; Mahmoud, A.W.M. Hydrothermal synthesis of nano crystals (AM) zeolite using variable temperature programs. J. Nanomater. Mol. Nanotechnol. 2015, 4. [Google Scholar] [CrossRef]
- Li, J.; Wee, C.; Sohn, B. Effect of ammonium- and potassium-loaded zeolite on kale, growth, and soil property. Am. J. Plant Sci. 2013, 4, 1976–1982. [Google Scholar] [CrossRef]
- Helrich, K. Official Methods of Analysis, 15th ed.; Association of Official Agricultural Chemist: Arlington, VA, USA, 1990; Volume 1, p. 673. [Google Scholar]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2014, 34, 455–472. [Google Scholar] [CrossRef]
- Asiya, S.I.; Pal, K.; Kralj, S.; El-Sayyad, G.S.; de Souza, F.G.; Narayanan, T. Sustainable preparation of gold nanoparticles via green chemistry approach for biogenic applications. Mater. Today Chem. 2020, 17, 100327. [Google Scholar] [CrossRef]
- Lugojan, C.; Ciulca, S. Evaluation of relative water content in winter wheat. J. Hortic. Sci. Biotechnol. 2011, 15, 173–177. [Google Scholar]
- Moran, R. Formulae for determination of chlorophyllous pigments extracted with N,N-dimethylformamide. Plant Physiol. 1982, 69, 1376–1381. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144. [Google Scholar]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Fales, T.M.; Jaouni, J.F.; Babashak, I. Simple device for preparing ethereal diazomethane without resorting to codistillation. Ann. Chem. 1973, 45, 2302–2303. [Google Scholar] [CrossRef]
- Furniss, B.S. Vogel’s Textbook of Practical Organic Chemistry; Pearson Education India: Delhi, India, 1989; pp. 1174–1179. [Google Scholar]
- Polle, A.; Otter, T.; Mehne-Jakobs, B. Effect of magnesium deficiency on antioxidative systems in needles of Norway spruce [Piceaabies (L.) Karst.] grown with different ratios of nitrate and ammonium as nitrogen sources. New Phytol. 1994, 128, 621–628. [Google Scholar] [CrossRef]
- Nishikimi, M.; Appaji Rao, N.; Yagi, K. The Occurrence of Superoxide Anion in the Reaction of Reduced Phenazine Methosulfate and Molecular Oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. InMethods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; pp. 121–126. [Google Scholar] [CrossRef]
- Shelepova, O.; Vinogradova, Y.; Zaitchik, B.; Ruzhitsky, A.; Grygorieva, O.; Brindza, J. Constituents of the essential oil in Solidago canadensis L. from Eurasia. Potravin. Slovak J. Food Sci. 2018, 12, 20–25. [Google Scholar] [CrossRef]
- Amani Machiani, M.; Javanmard, A.; Morshedloo, M.R.; Maggi, F. Evaluation of Competition, Essential Oil Quality and Quantity of Peppermint Intercropped with Soybean. Ind. Crops Prod. 2018, 111, 743–754. [Google Scholar] [CrossRef]
- Stenhagen, E.; Abrahamsson, S.; McLafferty, F.W. Registry of Mass Spectral Data; John Wiley & Sons, Ltd.: New York, NY, USA, 1974. [Google Scholar]
- Sadtler, R. The Sadtler Standard Gas Chromatography Retention Index Library; Sadtler Research Laboratories: Philadelphia, PA, USA, 1986; Volume 4. [Google Scholar]
- Duncan, D.B. Multiple range and multiple ’F’ tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Kusvuran, S. Microalgae (Chlorella vulgaris Beijerinck) alleviates drought stress of broccoli plants by improving nutrient uptake, secondary metabolites, and antioxidative defense system. Hortic. Plant J. 2021, 7, 221–231. [Google Scholar] [CrossRef]
- El-Sayed, I.M.; Salim, R.G.; El-Haggar, E.F.; El-Ziat, R.A.; Soliman, D.M. Molecular Characterization and Positive Impact of Brassinosteroids and Chitosan on Solidago canadensis cv. Tara Characteristics. Horticulturae 2020, 6, 100. [Google Scholar] [CrossRef]
- Du, L.; Liu, H.; Guan, W.; Li, J.; Li, J. Drought affects the coordination of belowground and aboveground resource-related traits in Solidago canadensis in China. Ecol.Evolut. 2019, 9, 9948–9960. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Huang, P.; Hussain, S.; Shen, F.; Wang, H.; Du, D. Mild evidence for local adaptation of Solidago canadensis under different salinity, drought, and abscisic acid conditions. Pol. J. Environ. Stud. 2022, 31, 2987–2995. [Google Scholar] [CrossRef] [PubMed]
- Ostadi, A.; Javanmard, A.; Amani Machiani, M.; Kakaei, K. Optimizing antioxidant activity and phytochemical properties of peppermint (Mentha piperita L.) by integrative application of biofertilizer and stress-modulating nanoparticles under drought stress conditions. Plants 2023, 12, 151. [Google Scholar] [CrossRef]
- El-Attar, A.B.; Othman, E.Z.; El-Bahbohy, R.M.; Mahmoud, A.W.M. Efficiency of different potassium sources, and soil bio-fertilizers for growth, productivity, and biochemical constituents of Narcissus (Narcissus tazetta L.). J. Plant Nutr. 2022. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; Hassan, A.Z.A.; Mottaleb, S.A.; Rowezak, M.M.; Salama, A.M. The role of nano-silicon and other soil conditioners in improving physiology and yield of the drought-stressed barley crop. Agriculture 2021, 67, 124–143. [Google Scholar] [CrossRef]
- Golubkina, N.; Logvinenko, L.; Konovalov, D.; Garsiya, E.; Fedotov, M.; Alpatov, A.; Shevchuk, O.; Skrypnik, L.; Sekara, A.; Caruso, G. Foliar Application of Selenium under Nano Silicon on Artemisia annua: Effects on Yield, Antioxidant Status, Essential Oil, Artemisinin Content and Mineral Composition. Horticulture 2022, 8, 597. [Google Scholar] [CrossRef]
- Greger, M.; Landberg, T.; Vaculík, M. Silicon Influences Soil Availability and Accumulation of Mineral Nutrients in Various Plant Species. Plants 2018, 7, 41. [Google Scholar] [CrossRef]
- Bybordi, A. Influence of zeolite, selenium and silicon upon some agronomic and physiologic characteristics of canola grown under salinity. Commun. Soil Sci. Plant Anal. 2016, 47, 832–850. [Google Scholar] [CrossRef]
- Cataldo, E.; Fucile, M.; Mattii, G.B. Leaf Eco-Physiological Profile and Berries Technological Traits on Potted Vitis vinifera L. cv Pinot Noir Subordinated to Zeolite Treatments under Drought Stress. Plants 2022, 11, 1735. [Google Scholar] [CrossRef]
- Sil, P.; Das, P.; Biswas, A.K. Impact of Exogenous Silicate Amendments on Nitrogen Metabolism in Wheat Seedlings Subjected to Arsenate Stress. Silicon 2019, 12, 535–545. [Google Scholar] [CrossRef]
- Esmaili, S.; Tavallali, V.; Amiri, B. Nano-Silicon Complexes Enhance Growth, Yield, Water Relations and Mineral Composition in Tanacetum parthenium under Water Deficit Stress. Silicon 2021, 13, 2493–2508. [Google Scholar] [CrossRef]
- Namjoyan, S. Nano-silicon protects sugar beet plants against water deficit stress by improving the antioxidant systems and compatible solutes. Acta Physiol. Plant 2020, 42, 157. [Google Scholar] [CrossRef]
- Dehghanipoodeh, S.; Ghobadi, C.; Baninasab, B.; Gheysari, M.; Shiranibidabadi, S. Effect of Silicon on Growth and Development of Strawberry under Water Deficit Conditions. Hortic. Plant J. 2018, 4, 226–232. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Ren, H.; Guo, Z.; Li, N.; Zhao, C.; XIE, Z. Transcriptomic and physiological analyses identifying Lanzhou lily (Lilium davidii var. unicolor) drought adaptation strategies. Hortic. Plant J. 2022, in press. [Google Scholar] [CrossRef]
- González-García, Y.; Cárdenas-Álvarez, C.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Cabrera-de-la-Fuente, M.; Sandoval-Rangel, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Effect of Three Nanoparticles (Se, Si and Cu) on the Bioactive Compounds of Bell Pepper Fruits under Saline Stress. Plants 2021, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh, H.S.; Asadi, M.; Zahedi, S.M.; Hamzehpour, N.; Rasouli, F.; Helvacı, M.; Alas, M. Silicon dioxide-nanoparticle nutrition mitigates salinity in gerbera by modulating ion accumulation and antioxidants. Folia Hortic. 2021, 33, 91–105. [Google Scholar] [CrossRef]
- Teixeira, G.C.M.; de Prado, R.M.; Rocha, A.M.S.; de Oliveira Filho, A.S.B.; da Sousa Junior, G.S.; Gratão, A.S.B. Action of silicon on the activity of antioxidant enzymes and on physiological mechanisms mitigates water deficit in sugarcane and energy cane plants. Sci. Rep. 2022, 12, 17487. [Google Scholar] [CrossRef]
- Montanari, T.; Busca, G. On the mechanism of adsorption and separation of CO2 on LTA zeolites: An IR investigation. Vib. Spectrosc. 2008, 46, 45–51. [Google Scholar] [CrossRef]
- Palanivell, P.; Ahmed, O.H.; Omar, L.; Abdul Majid, N.M. Nitrogen, Phosphorus, and Potassium Adsorption and Desorption Improvement and Soil Buffering Capacity Using Clinoptilolite Zeolite. Agronomy 2021, 11, 379. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate Peroxidase and Catalase Activities and Their Genetic Regulation in Plants Subjected to Drought and Salinity Stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef]
- Zandi, P.; Schnug, E. Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Ahanger, M.A.; Alam, P.; Alyemeni, M.N.; Nasser, M.; Wijaya, L.; Ali, S.; Ashraf, M. Silicon (Si) Supplementation Alleviates NaCl Toxicity in Mung Bean [Vigna radiata (L.) Wilczek] Through the Modifications of Physio-biochemical Attributes and Key Antioxidant Enzymes. J. Plant Growth Regul. 2019, 38, 70–82. [Google Scholar] [CrossRef]
- Wen, J.; Dong, H.; Zeng, G. Application of zeolite in removing salinity/sodicity from wastewater: A review of mechanisms, challenges and opportunities. J. Clean. Prod. 2018, 197, 1435–1446. [Google Scholar] [CrossRef]
- Balakhnina, T.I.; Bulak, P.; Matichenkov, V.V.; Kosobryukhov, A.A.; Włodarczyk, T.M. The influence of Si-rich mineral zeolite on the growth processes and adaptive potential of barley plants under cadmium stress. Plant Growth Regul. 2015, 75, 557–565. [Google Scholar] [CrossRef]
- Valadabadi, S.A.; Yousefi, M. Effect of Zeolite and Biofertilizers on the Essential Oil Yield and Some Physiological Characteristics of Satureja hortensis L. under Water Deficit Stress. J. Med. Plants By-Prod. 2022, 5, 1327. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Romero, M.J.; Llanderal, A.; Cermeño, P.; Lao, M.T.; Segura, M.L. Effects of drought stress on biomass, essential oil content, nutritional parameters, and costs of production in six Lamiaceae species. Water 2019, 11, 573. [Google Scholar] [CrossRef]
- Caser, M.; Chitarra, W.; D’Angiolillo, F.; Perrone, I.; Demasi, S.; Lovisolo, C.; Pistelli, L.; Pistelli, L.; Scariot, V. Drought stress adaptation modulates plant secondary metabolite production in Salvia dolomitica Codd. Ind. Crops Prod. 2019, 129, 85–96. [Google Scholar] [CrossRef]
- Amani Machiani, M.; Javanmard, A.; Morshedloo, M.R.; Aghaee, A.; Maggi, F. Funneliformismosseae inoculation under water deficit stress improves the yield and phytochemical characteristics of thyme in intercropping with soybean. Sci. Rep. 2021, 11, 15279. [Google Scholar] [CrossRef] [PubMed]
- Afshari, M.; Pazoki, A.; Sadeghipour, O. Foliar-applied silicon and its nanoparticles stimulate physio-chemical changes to improve growth, yield and active constituents of coriander (Coriandrum sativum L.) essential oil under different irrigation regimes. Silicon 2021, 13, 4177–4188. [Google Scholar] [CrossRef]


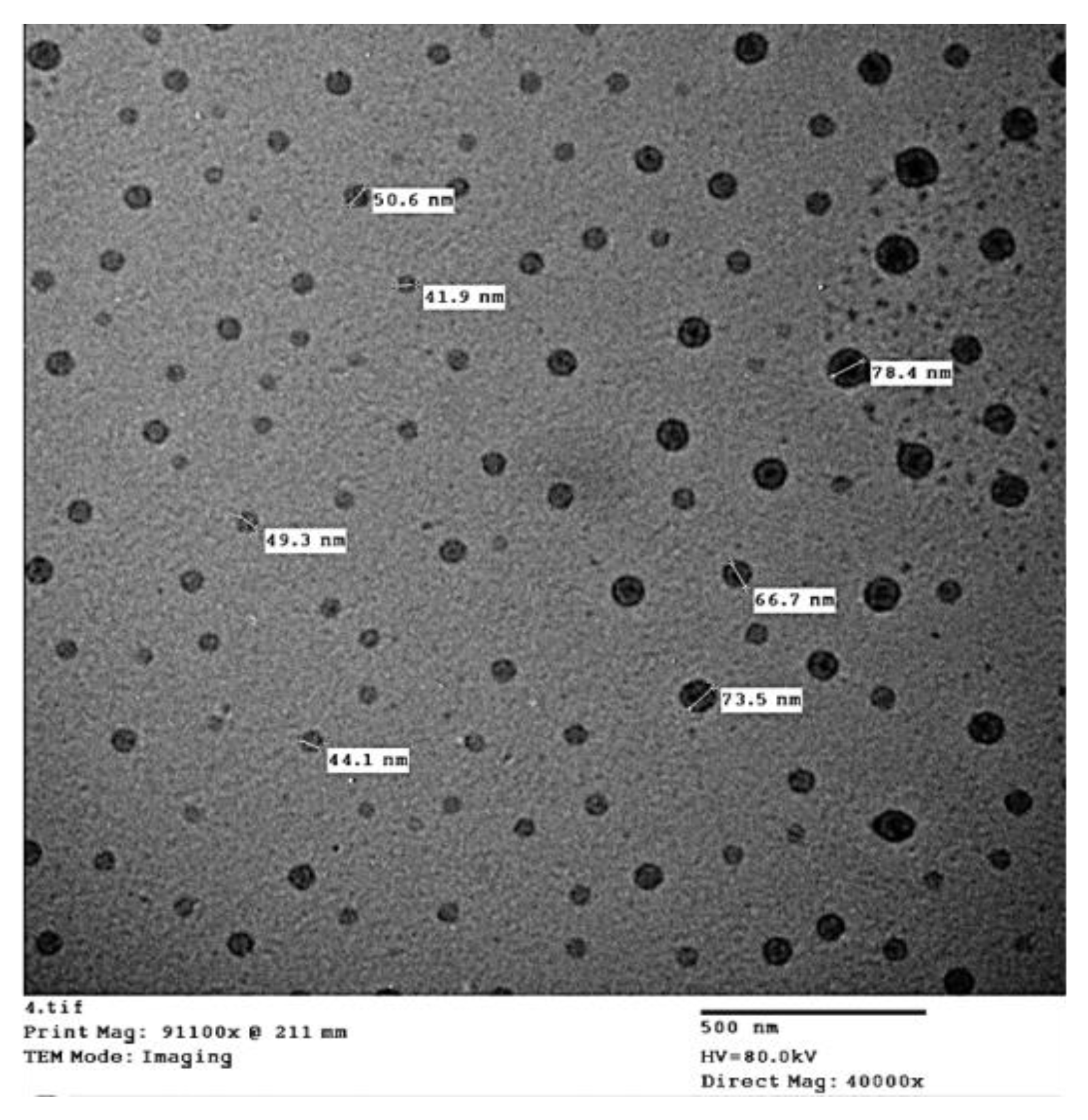
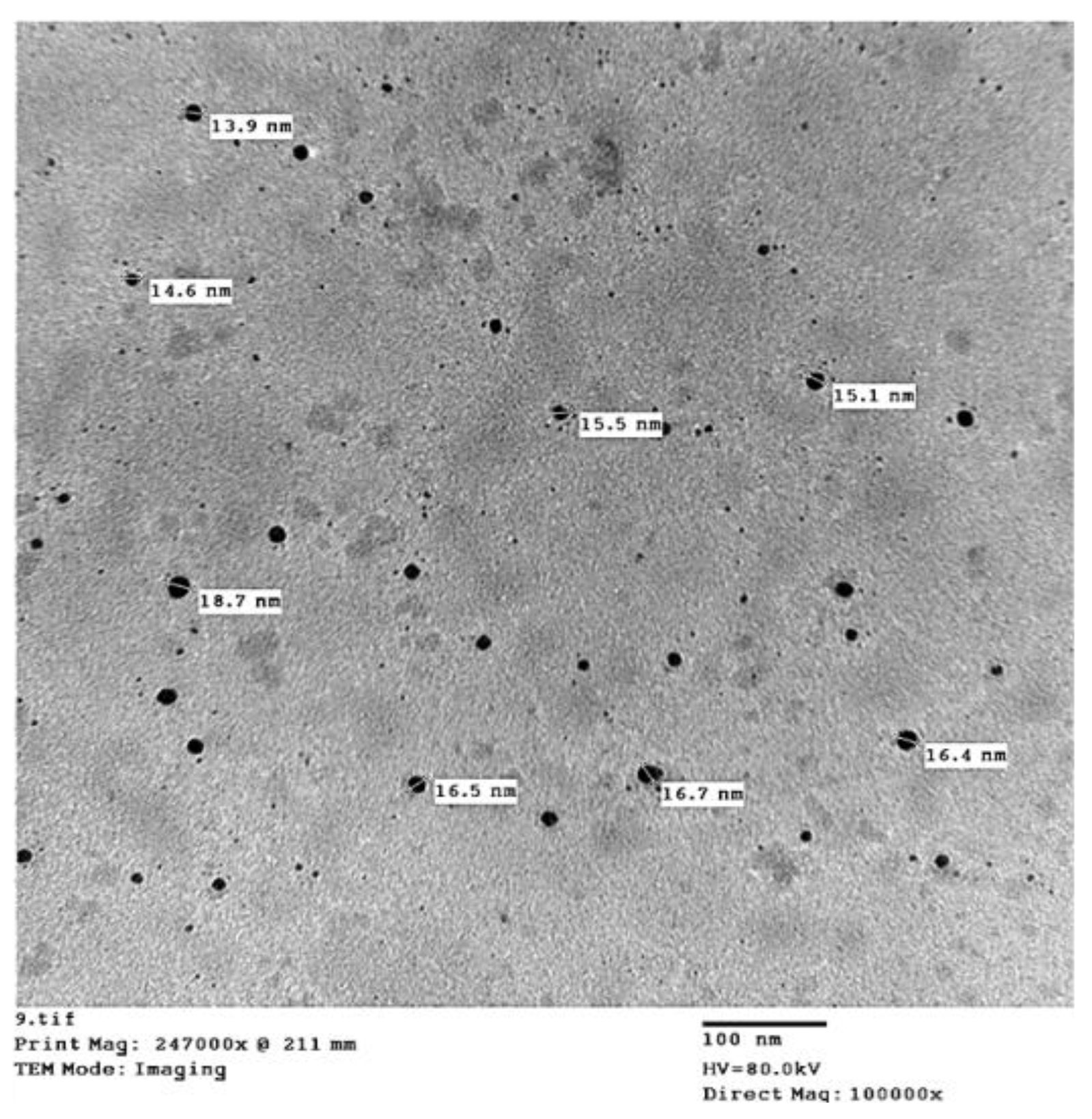
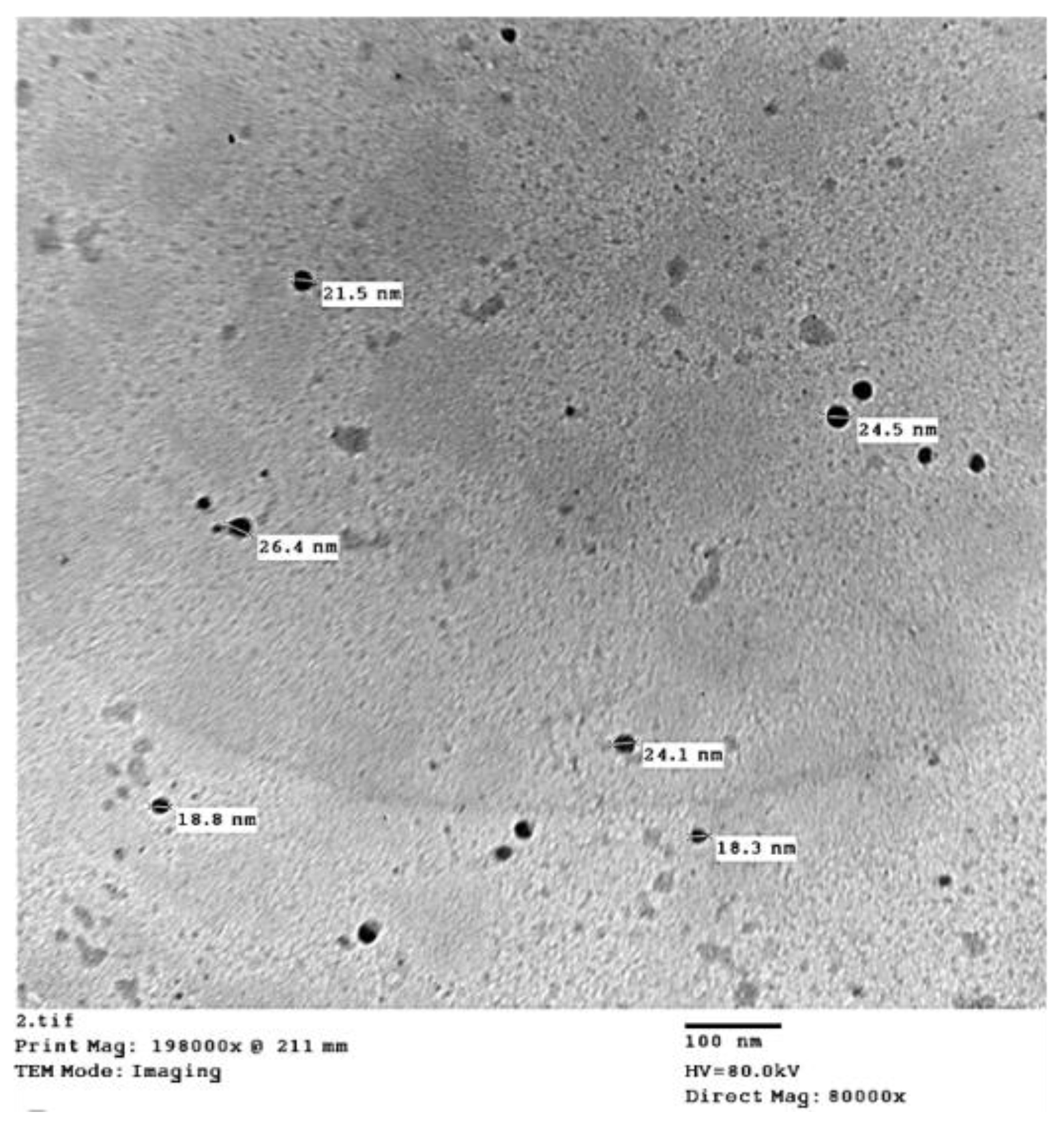
| Parameters | Soil Depth (cm) | |
|---|---|---|
| 0–30 | 30–60 | |
| Sand% | 90.10 | 90.00 |
| Silt% | 6.90 | 6.50 |
| Clay% | 3.00 | 3.50 |
| Textural class | Sand | Sand |
| Saturation water content (cm3 cm−3) | 0.385 | 0.396 |
| Field capacity (cm3cm−3) | 0.213 | 0.218 |
| Permanent wilting point (cm3cm−3) | 0.057 | 0.057 |
| Available water (cm3.cm−3) | 0.156 | 0.161 |
| Bulk density (mg m−3) | 1.64 | 1.65 |
| Saturated hydraulic conductivity (m day−1) | 2.40 | 2.34 |
| Organic matter (%) | 0.31 | 0.25 |
| Calcium carbonates (%) | 4.80 | 3.71 |
| pH (1: 1, soil: water suspension) | 7.70 | 7.81 |
| EC (1:1, soil: water extract) (dS m−1) | 4.02 | 4.13 |
| Soluble ions (meq 100 g−1 soil) | ||
| Ca2+ | 13.85 | 13.41 |
| Mg2+ | 12.15 | 10.59 |
| Na+ | 8.10 | 10.25 |
| K+ | 6.00 | 6.05 |
| CO3− | - | - |
| HCO3− | 11.92 | 9.75 |
| Cl− | 14.00 | 10.50 |
| SO4− | 15.08 | 21.30 |
| Available nutrients (mg g−1 soil) | ||
| N | 16.21 | 13.12 |
| P | 7.78 | 6.21 |
| K | 46.50 | 45.89 |
| Fe | 9.20 | 12.00 |
| Mn | 1.63 | 1.50 |
| Cu | 2.10 | 1.15 |
| B | 0.23 | 0.21 |
| Zn | 2.00 | 1.61 |
| Property | Value |
|---|---|
| Moisture content (%) | 25 |
| pH (1:5) | 7.5 |
| EC (1:5 extract) (dS m−1) | 3.1 |
| Organic-C (%) | 33.11 |
| Organic matter (%) | 70 |
| Total-N (%) | 1.82 |
| Total-K (%) | 1.25 |
| C/N ratio | 19:1 |
| Total-P (%) | 1.29 |
| Fe (mg L−1) | 1019 |
| Mn (mg L−1) | 111 |
| Cu (mg L−1) | 180 |
| Zn (mg L−1) | 280 |
| The total content of bacteria (CFU.g−1) | 2.5 × 107 |
| Phosphate dissolving bacteria (CFU.g−1) | 2.5 × 106 |
| Weed seeds | 0 |
| Chemical composition (%) | SiO2 | TiO2 | Al2O3 | Fe2O3 | FeO | MnO | MgO | CaO | Na2O | K2O | SrO | P2O3 | N |
| 45.50 | 2.81 | 13.30 | 5.40 | 8.31 | 0.51 | 6.30 | 9.52 | 2.83 | 0.87 | 0.22 | 0.67 | 2.70 | |
| Trace elements (mg L−1) | Ba | Co | Cr | Se | Cu | Zn | Zr | Nb | Ni | Rb | Y | ||
| 10 | 1.2 | 35 | 0.8 | 19 | 64 | 257 | 13 | 55 | 15 | 22 |
| Treatment | Plant Height (cm) | Inflorescence Length (cm) | Number of Inflorescences /Plant | Total Leaf Area (cm)2 | Leaf Length (cm) | Herb Fresh Weight (g/Plant) | Herb Dry Weight (g/Plant) | Yield Fresh Weight (ton/fed) | ||||||||
| 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | |
| (T1) control | 47.67 b ±1.316 | 49.36 c ±0.591 | 21.63 b ±2.009 | 21.60 b ±1.814 | 2.0 c ±0.000 | 2.0 e ±0.577 | 147.99 c ±1.436 | 162.42 c ±2.324 | 6.32 b ±0.447 | 6.91 cd ±0.344 | 167.75 c ±6.144 | 178.66 c ±5.989 | 38.78 cd ±1.058 | 39.72 d ±1.058 | 4.19 d ±0.006 | 4.47 d ±0.012 |
| (T2) | 42.22 c ±1.804 | 44.75 c ±2.074 | 19.75 b ±1.698 | 19.73 b ±0.858 | 2.0 c ±0.577 | 3.0 d ±0.000 | 136.17 d ±3.850 | 144.05 d ±2.138 | 5.09 c ±0.382 | 6.31 d ±0.413 | 150.95 c ±5.239 | 161.93 c ±5.292 | 33.35 d ±2.639 | 36.29 d ±0.132 | 3.77 e ±0.009 | 4.07 e ±0.012 |
| (T3) | 66.48 a ±2.001 | 64.33 b ±2.752 | 23.54 a ±1.624 | 26.33 a ±1.496 | 4.0 ab ±0.577 | 5.0 b ±0.000 | 165.90 b ±2.725 | 189.05 b ±2.380 | 8.10 a ±0.427 | 8.65 ab ±0.476 | 214.65 b ±2.451 | 233.13 b ±4.751 | 54.55 b ±2.117 | 58.85 b ±1.493 | 5.37 b ±0.012 | 5.84 b ±0.021 |
| (T4) | 65.25 a ±1.702 | 69.32 ab ±1.978 | 21.90 a ±1.939 | 27.23 a ±2.158 | 3.0 bc ±0.000 | 4.0 c ±0.000 | 155.32 c ±2.018 | 164.61 c ±2.289 | 7.83 a ±0.727 | 7.86 bc ±0.491 | 199.41 b ±4.678 | 222.47 b ±5.292 | 43.22 c ±2.166 | 51.47 c ±2.367 | 4.99 c ±0.000 | 5.60 c ±0.047 |
| (T5) | 68.37 a ±0.115 | 72.25 a ±0.509 | 27.83 a ±0.301 | 26.80 a ±0.327 | 5.0 a ±0.000 | 6.0 a ±0.000 | 186.05 a ±1.456 | 211.92 a ±2.110 | 8.66 a ±0.269 | 9.09 a ±0.226 | 234.67 a ±6.181 | 255.83 a ±6.289 | 64.40 a ±1.744 | 70.88 a ±3.966 | 5.88 a ±0.007 | 6.53 a ±0.133 |
| Treatment | Macronutrients (%) | Micronutrients (mg L−1) | ||||||||||||||||
| N | P | K | Ca | Mg | Cu | Zn | Fe | Mn | ||||||||||
| 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | |
| (T1) control | 2.94 bc ±0.156 | 3.12 c ±0.049 | 0.29 c ±0.015 | 0.32 c ±0.006 | 3.24 c ±0.124 | 3.15 d ±0.021 | 0.83 bc ±0.020 | 0.85 bc` ±0.006 | 0.17 cd ±0.012 | 0.19 c ±0.009 | 7.64 b ±0.316 | 7.79 b ±0.373 | 38.64 c ±1.695 | 42.05 c ±0.913 | 63.11 c ±1.650 | 65.73 c ±1.695 | 50.34 b ±1.411 | 51.01 d 0.973 |
| (T2) | 2.85 c ±0.154 | 2.90 cd ±0.105 | 0.31 bc ±0.015 | 0.32 bc ±0.009 | 4.41 a ±0.115 | 4.45 a ±0.020 | 0.78 c ±0.018 | 0.80 c ±0.028 | 0.15 d ±0.015 | 0.18 c ±0.009 | 7.43 b ±0.467 | 8.12 b ±0.088 | 40.81 bc ±2.075 | 43.20bc ±0.497 | 65.96 c ±1.140 | 67.68 c ±1.064 | 53.45 b ±1.097 | 54.19 c 0.990 |
| (T3) | 3.20 b ±0.047 | 3.38 b ±0.085 | 0.43 a ±0.006 | 0.43 a ±0.009 | 3.34 bc ±0.059 | 3.27 c ±0.024 | 0.85 b ±0.015 | 0.88 ab ±0.015 | 0.22 b ±0.006 | 0.24 b ±0.012 | 7.98 b ±0.437 | 8.01 b ±0.076 | 45.73 b ±1.346 | 47.20 b ±1.043 | 73.16 b ±1.580 | 75.37 b ±0.665 | 55.14ab ±0.852 | 57.57 b ±0.456 |
| (T4) | 2.87 c ±0.087 | 2.86 d ±0.059 | 0.33 b ±0.009 | 0.35 b ±0.009 | 3.27 c ±0.022 | 3.25 cd ±0.047 | 0.83 bc ±0.015 | 0.86 bc ±0.009 | 0.21 bc ±0.012 | 0.20 bc ±0.012 | 7.84 b ±0.148 | 7.87 b ±0.276 | 41.59 bc ±1.018 | 44.00bc ±1.165 | 72.85 b ±1.487 | 73.56 b ±1.320 | 54.15 b ±1.527 | 55.69 bc ±0.919 |
| (T5) | 4.28 a ±0.059 | 4.17 a ±0.050 | 0.45 a ±0.006 | 0.44 a ±0.006 | 3.55 b ±0.062 | 3.43 b ±0.055 | 0.94 a ±0.012 | 0.92 a ±0.009 | 0.27 a ±0.015 | 0.28 a ±0.012 | 9.20 a ±0.313 | 9.40 a ±0.321 | 52.47 a ±1.590 | 53.16 a ±2.232 | 80.09 a ±1.517 | 81.37 a ±1.224 | 59.63 a ±2.032 | 62.28 a ±1.069 |
| Treatment | Total Chlorophyll (mg/g) | Total Phenolic Content (mg/g) | Total Flavonoids Content (mg/g) | Total Carbohydrate % | ||||
| 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | |
| (T1) control | 15.42 d ±0.618 | 16.31 c ±0.414 | 29.52 c ±1.311 | 32.98 c ±1.251 | 0.39 d ±0.012 | 0.42 d ±0.017 | 29.96 bc ±1.554 | 27.98 bc ±1.805 |
| (T2) | 16.22 cd ±0.954 | 15.50 c ±1.100 | 32.64 c ±1.149 | 33.82 c ±0.874 | 0.46 c ±0.015 | 0.49 c ±0.015 | 28.12 c ±1.236 | 26.85 c ±0.813 |
| (T3) | 19.46 b ±0.610 | 21.80 b ±1.313 | 38.11 ab ±1.007 | 37.13 ab ±0.767 | 0.51 ab ±0.009 | 0.54 ab ±0.012 | 33.41 ab ±1.263 | 32.80 a ±1.537 |
| (T4) | 18.34 bc ±0.492 | 19.90 b ±0.969 | 35.93 b ±1.034 | 34.77 bc ±1.118 | 0.48 bc ±0.015 | 0.52 bc ±0.018 | 30.43 abc ±0.514 | 31.99 ab ±1.556 |
| (T5) | 22.90 a ±0.884 | 26.17 a ±0.921 | 39.37 a ±0.676 | 38.57 a ±0.366 | 0.53 a ±0.012 | 0.55 a ±0.012 | 34.17 a ±0.947 | 35.52 a ±1.274 |
| Treatment | Oil% of herb | Oil % of root | Oil yield/plant (ml) | Oil yield/fed. (L) | ||||
| 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | |
| (T1) control | 0.45 c ±0.018 | 0.46 d ±0.012 | 0.187 bc ±0.018 | 0.180 cd ±0.012 | 0.75 d ±0.058 | 0.82 d ±0.045 | 18.78 d ±1.407 | 20.57 d ±1.131 |
| (T2) | 0.34 d ±0.012 | 0.38 e ±0.012 | 0.140 c ±0.012 | 0.160 d ±0.012 | 0.51 e ±0.030 | 0.61 e ±0.020 | 12.84 e ±0.790 | 15.36 e ±0.452 |
| (T3) | 0.54 b ±0.012 | 0.58 b ±0.012 | 0.227 ab ±0.018 | 0.240 ab ±0.012 | 1.16 b ±0.038 | 1.35 b ±0.009 | 28.99 b ±0.943 | 33.77 b ±0.182 |
| (T4) | 0.50 b ±0.012 | 0.51 c ±0.018 | 0.207 b ±0.018 | 0.213 bc ±0.018 | 1.00 c ±0.047 | 1.14 c ±0.034 | 24.95 c ±1.139 | 28.53 c ±0.932 |
| (T5) | 0.62 a ±0.012 | 0.65 a ±0.018 | 0.260 a ±0.012 | 0.280 a ±0.012 | 1.45 a ±0.036 | 1.67 a ±0.038 | 36.37 a ±0.926 | 41.75 a ±0.983 |
| Components | (T1) Control | (T2) | (T3) | (T4) | (T5) |
| 1-Monoterpene | |||||
| α-Pinene | 30.89 | 28.51 | 31.11 | 22.14 | 31.87 |
| Camphene | 1.23 | 2.34 | 1.13 | 1.45 | 1.15 |
| Sabinene | 0.44 | 0.36 | 0.22 | 0.57 | 0.36 |
| β-Pinene | 2.75 | 2.31 | 2.16 | 2.47 | 2.18 |
| Myrcene | 3.25 | 3.05 | 2.65 | 2.65 | 2.29 |
| Limonene | 15.97 | 19.54 | 12.48 | 16.53 | 17.35 |
| γ-Terpinen | 0.32 | 0.48 | 0.58 | 0.22 | 0.26 |
| α-Campholenal | 1.34 | 1.66 | 1.14 | 1.82 | 1.17 |
| Carvone | 0.11 | 0.17 | 0.23 | 0.28 | 0.13 |
| Bornyl acetate | 9.48 | 8.81 | 12.87 | 14.33 | 9.42 |
| 2-Sesquiterpene | |||||
| β-Elemene | 0.71 | 0.63 | 0.59 | 0.84 | 0.68 |
| β-Caryophyllene | 2.38 | 2.86 | 1.65 | 2.53 | 1.45 |
| α-Caryophyllene | 0.46 | 0.74 | 0.32 | 0.65 | 0.43 |
| Germacrene D | 23.52 | 21.78 | 20.12 | 25.31 | 26.24 |
| γ-Cadinene | 2.15 | 2.19 | 1.76 | 2.25 | 1.33 |
| β-Eudesmene | 0.37 | 0.62 | 0.29 | 0.42 | 0.37 |
| Aromadendrene oxide | 0.38 | 0.49 | 0.31 | 0.27 | 0.36 |
| Caryophyllene oxide | 0.31 | 0.77 | 0.42 | 0.64 | 0.41 |
| Spathulenol | 0.23 | 0.58 | 0.19 | 0.47 | 0.34 |
| Total identified (%) | 96.77 | 96.41 | 96.22 | 96.15 | 97.79 |
| Grouped compounds (%) | |||||
| Monoterpenes | 65.78 | 66.23 | 70.57 | 62.77 | 66.18 |
| Sesquiterpenes | 30.99 | 30.18 | 25.65 | 33.38 | 31.61 |
| Others | 3.23 | 3.59 | 3.78 | 3.85 | 2.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Othman, E.Z.; El-Attar, A.B.; El-Bahbohy, R.M.; Abd El-Khalek, S.N.; Morgan, S.H.; Mahmoud, A.W.M. Exogenous Appliance of Nano-Zeolite and Nano-Silicon Elevate Solidago canadensis Invasive Plant Tolerance to Water Deficiency. Horticulturae 2023, 9, 172. https://doi.org/10.3390/horticulturae9020172
Othman EZ, El-Attar AB, El-Bahbohy RM, Abd El-Khalek SN, Morgan SH, Mahmoud AWM. Exogenous Appliance of Nano-Zeolite and Nano-Silicon Elevate Solidago canadensis Invasive Plant Tolerance to Water Deficiency. Horticulturae. 2023; 9(2):172. https://doi.org/10.3390/horticulturae9020172
Chicago/Turabian StyleOthman, Eman Z., Asmaa B. El-Attar, Reham M. El-Bahbohy, Sarah N. Abd El-Khalek, Sherif H. Morgan, and Abdel Wahab M. Mahmoud. 2023. "Exogenous Appliance of Nano-Zeolite and Nano-Silicon Elevate Solidago canadensis Invasive Plant Tolerance to Water Deficiency" Horticulturae 9, no. 2: 172. https://doi.org/10.3390/horticulturae9020172
APA StyleOthman, E. Z., El-Attar, A. B., El-Bahbohy, R. M., Abd El-Khalek, S. N., Morgan, S. H., & Mahmoud, A. W. M. (2023). Exogenous Appliance of Nano-Zeolite and Nano-Silicon Elevate Solidago canadensis Invasive Plant Tolerance to Water Deficiency. Horticulturae, 9(2), 172. https://doi.org/10.3390/horticulturae9020172







