Abstract
The positive effect of silicon on plants is thought to be mediated by a modification of phenolic metabolism. The purpose of the study was to evaluate the effect of a silicon-based mechanocomposite (MC) on alterations of the phenolic profile of strawberry plants in the course of development under in vitro, ex vitro, and in vivo conditions. Aqueous ethanol extracts of aboveground parts of in vitro–derived plants (Fragaria × ananassa cv. ‘Solnechnaya polyanka’) were subjected to HPLC. Nineteen individual phenolic compounds (hydroxybenzoic and hydroxycinnamic acids, catechins, ellagic acid derivatives, and flavonol glycosides) were quantified. The results revealed phenolic profiles specific to each studied stage and significant transformations of the profiles by the MC. It induced strong upregulation of hydroxycinnamic acid during in vitro rooting and of catechins and hydroxybenzoic acids during ex vitro acclimation. At ex vitro and in vivo stages, the emergence of quercetin glycosides and ellagitannins was registered, and the MC elevated their levels during ex vitro acclimation and field growth. Principal component analysis confirmed the significant effect of the MC on the phenolic profile at all stages, and this effect was the strongest during ex vitro acclimation. The results are consistent with previous reports on the modification of phenolic profiles of plants by silicon-derived biostimulants.
1. Introduction
Strawberry (Fragaria × ananassa (Duchesne ex Weston) Duchesne ex Rozier) is highly regarded throughout the world as a crop of considerable interest for research and berry production. The world demand for this crop increases substantially every year owing to its superior flavor, fragrance, and health-restoring qualities. Among many tools of modern horticulture for plant propagation and production, the most promising ones are biostimulants derived from various organic or inorganic substances and microorganisms. The application of biostimulants improves the growth and nutrition of plants and alleviates the impact of environmental stressors [1,2,3]. Currently, much emphasis is placed on organic agriculture. Properly selected organic biostimulants may improve crop yields and the quality of production and can minimize harm to the environment [4,5]. Moreover, biostimulants are ecologically friendly chemicals and address the population’s need for environmentally friendly and toxic-chemical-free foods [6].
Among biostimulants, sources of silicon compounds have attracted much interest. One way in which silicon-based biostimulants may be applied to plants is in the form of silicon chelates based on plant raw materials produced by solid-phase mechanochemical methods without organic solvents [7,8,9]. As a result of this treatment, plant raw materials and reagents remain in a stable solid form, which completely prevents their oxidation and loss of biological activity. Silicon chelates obtained by mechanochemical activation easily dissolve in an aqueous solution and are more efficiently absorbed by plants in comparison with inorganic silicates. The practical significance of mechanocomposites (MCs) is high because they enable “green chemistry” as a component of organic farming for the cultivation of valuable strawberry cultivars.
In addition to improving product quality and quantity, silicon-based biostimulants increase the tolerance of plants to various environmental stressors [6,10,11,12]. In particular, a positive effect of silicon-based biostimulants can be explained by the activation of a plant’s defensive mechanisms through the production of secondary metabolites, such as phenolic compounds [10,13,14,15,16]. The latter are considered essential and active cellular metabolites. Their participation has been demonstrated in many redox processes, in the regulation of enzymatic reactions, in processes of photosynthesis, in growth, in development, in reproduction, in the construction of cell walls protecting plants from mechanical damage and pathogens, and in other processes [17,18]. The diversity of structures and of corresponding functions of phenolic compounds helps plants to respond to a wide range of environmental factors [19]. Molecular structure determines specific roles of individual phenolic compounds in plant development and defense [18].
In strawberry leaves, phenolic compounds from several classes have been identified: hydroxybenzoic and hydroxycinnamic acids, ellagic acid derivatives (including ellagitannins), catechins, and flavonols [20,21]. Previously, we investigated the phenolic profile of strawberry leaves and its changes throughout plant development in tissue culture, including under the influence of a silicon-based biostimulant; we revealed upregulation of some phenolic acids and ellagic acid derivatives [22,23].
Alterations of the phenolic profile have been associated with other considerable physiological transformations: enhanced antioxidant enzymatic activity, vegetative growth, dry matter production, and formation of photosynthetic pigments, as well as reduced hydrogen peroxide accumulation and modulation of concentrations of endogenous plant growth regulators [9,23,24,25]. Some authors have suggested that the silicon-stimulated production of flavonoids and organic acids may be a strategy used by plants to cope with exogenous stressors [26,27,28]. Therefore, further research is needed to investigate this type of MCs as a trigger of various adaptive changes in strawberry.
Only limited information is available on the transformation of the phenolic profile of F. × ananassa leaves under different conditions and its specific features in tissue culture. Phenolic compounds in leaves and roots of F. × ananassa and the effect of inorganic silicon on them at three developmental stages (vegetation, flowering, and fruiting) have been investigated under greenhouse conditions [29]. There is an influence of nitrogen nutrition on the phenolic profile of leaves of greenhouse plants [30]. There are many reports dealing with changes in concentrations of phenolic compounds in fruits of F. × ananassa [31,32]. Meanwhile, the characteristics of the phenolic profile of leaves of in vitro–grown Fragaria vesca plants have been described in additional detail [33,34].
In the current study, for the first time, the phenolic profile of aboveground parts of strawberry was investigated during adaptation to in vitro, ex vitro, and in vivo conditions, including under the action of an MC based on silicon chelates from plant raw materials. Each stage of the micropropagation technique is characterized by a set of specific negative environmental factors [35,36], and we focused on quantitative and qualitative changes in the phenolic profile correlating with the main developmental stages and with coping with adverse environmental factors. Thus, the study was aimed at evaluating an influence of the MC on the transformation of phenolic profiles in the aboveground part of strawberry in the course of plant development during the micropropagation and adaptation of the plants to in vivo conditions.
2. Materials and Methods
2.1. Plant Material and Growth Conditions
In vitro–derived strawberry plants (F. × ananassa cv. ‘Solnechnaya polyanka’) from the collection of the Laboratory of Biotechnology in the Central Siberian Botanical Garden (CSBG), SB RAS (Novosibirsk, Russia), were used for the assessment of phenolic profiles during micropropagation (under in vitro or ex vitro conditions) and in vivo (in a greenhouse or in the field). Single-fruiting cv. ‘Solnechnaya polyanka’ is characterized by stable high productivity and is recommended for cultivation under Western Siberia conditions.
2.1.1. Micropropagation
In vitro multiplication of plants was carried out by the activation of axillary meristems of microshoots on Gamborg-Eveleg’s (B5) [37] basal salt medium supplemented with 0.75 mg L−1 6-benzylaminopurine (plant cell culture tested, BioReagent, Sigma-Aldrich®) for one passage of 8 weeks in accordance with Ambros et al. [38]. In vitro rooting was performed on a hormone-free medium of the same composition. The duration of the rooting stage was 4 weeks. The in vitro cultures were maintained in culture jars (15 mL of medium per vessel) at 23 ± 2 °C under a 16 h photoperiod with 40 μmol m−2 s−1 light intensity provided by cool white fluorescent lamps (Philips, Pila, Poland). For ex vitro acclimation, the microclones were cultivated in plastic trays where individual tray cells (90 cm3) contained peat with 20% of perlite (pH 5.5–6.0) and a coconut substrate (at 1.0:0.5, v/v) during 4 weeks in accordance with Ambros et al. [23].
2.1.2. In Vivo Conditions
The experiment in a greenhouse was carried out from mid-April to mid-May 2022 under natural outdoor conditions of temperature and illumination (temperature of 30 ± 2 °C during the day and 23 ± 2 °C at night, relative humidity of 60 ± 10%, photoperiod of 16 h, and light intensity of 200–250 μmol m−2 s−1). Strawberry plants (one plant per pot) grown in plastic pots containing 250 cm3 of a soil substrate from a mixture of peat with perlite, humus, sand, and a coco substrate (1:1:0.25:0.25, v/v/v/v) were used as plant material for assaying the adaptability of strawberry plants to closed ground conditions.
For the field experiments, seedlings of strawberry with a closed root system [39] were planted on the experimental plot of the CSBG (geographical coordinates 54°49′9.87″ N and 83°6′6.95″ E) at the end of May 2022 and analyzed in August 2022. The mechanical properties of the soil in this area are as follows: medium loamy with a large amount of coarse dust fractions, structureless, with poor air and water permeability, rapid settlement and compaction after processing, and a propensity for swimming and crusting. Its pH is slightly acidic: 6.3–6.9. The soil layer from 0 to 20 cm contains 2–4% humus, and at a depth of 50–60 cm, no more than 0.8%. The total natural reserves of nutrients are low; therefore, humus was introduced into the holes during the planting. A two-line planting scheme was chosen that features the distance between plants in a row of 40 cm (between rows: 35 cm) and rows of 50 cm long.
2.1.3. Description of the MC, Treatments, and Experimental Design
The MC was prepared from rice husks (Oryza sativa, cv. ‘Liman’, Krasnodar Krai, Russia) and green tea (Camellia sinensis L.) (Krasnodar Krai, Russia) by mechanical activation in a water-cooled flow activator (roller mill RM-20, manufactured at the Institute of Solid State Chemistry and Mechanochemistry, SB RAS, Novosibirsk, Russia). The MC was found to contain 1.4 ± 0.2% of catechins (mean ± SE), 16.7 ± 0.9% of ash, and 15.2 ± 0.7% of silica. In the MC, the mass ratio of rice husks to green tea was 10:1 [23].
The experiments were set up without (−MC) and with the mechanocomposite (+MC) treatment:
- In vitro, MC was added to the B5 medium at a concentration of 5.0 mg L−1 before autoclaving (121 °C, 1.2 atm for 20 min) (+MC). The B5 medium without the MC served as a control (−MC). Plant microshoots were collected for analysis at the end of each cultivation cycle (in vitro multiplication and in vitro rooting after 4 and 8 weeks, respectively);
- At the ex vitro acclimation stage, the plants were moistened with the MC slurry at a concentration of 0.3 g L−1 dissolved in a ¼-strength Murashige and Skoog’s (MS) [40] salt solution (27 mL per cell) once a period (4 weeks) (+MC). A control group of plants was moistened with a ¼-strength MS salt solution (−MC). Shoots were sampled for analysis after 4 weeks of ex vitro acclimation of the microclones;
- In the greenhouse, each plant was watered under roots with an MC slurry at a concentration of 0.3 g L−1 in tap water (90 mL per pot) at 50% of full moisture capacity twice a period at regular intervals (2 weeks). Control specimens were moistened with tap water (−MC);
- In the field, root treatment of plants with 0.3 g L−1 MC dissolved in tap water (2.7 L per plant) was carried out at 2 weeks after planting (+MC). Control plants were watered with tap water (−MC). The number of treatments during the growing season was three at regular intervals (3 weeks).
In between, when the soils dried out, the plants were moistened with water under in vivo conditions. The plant’s aboveground parts were selected for analysis 1 week after the last treatment with the MC.
2.2. High Performance Liquid Chromatography (HPLC) Analysis of Phenolic Compounds
For this analysis, the aboveground part from three randomly selected plants (without roots) was air-dried completely (for 1 day). Dry weight concentrations in the samples of plant material were calculated by the gravimetric method. Then, the samples of plant material were ground up, weighed (0.2 g), and extracted three times with an ethanol–water mixture (70:30, v/v) in a water bath at 60–70 °C. HPLC analysis of the aqueous ethanol extracts was carried out using an Agilent 1200 system with a diode array detector and the ChemStation software for data processing (Agilent Technologies, Santa Clara, CA, USA). The chromatographic separation was performed at 25 °C on a Zorbax SB-C18 column (4.6 × 150 mm, 5 μm internal diameter). The mobile phase consisted of MeOH (solvent A) and 0.1% orthophosphoric acid in water (solvent B).
The separation of compounds in the extracts was performed through gradient 1. The run via this gradient was started with an A–B mixture at 22:78 (v/v), followed by a linear gradient to 70:30 (v/v) for the first 30 min, and then to 100:0 (v/v) from minute 30 to minute 32. A return of the mobile phase to 22:78 (v/v) was implemented from minute 32 to minute 36.
Flavonoid aglycones were identified after acid hydrolysis of the extracts to confirm glycoside composition. The aqueous ethanol extracts of aboveground parts of F. × ananassa were hydrolyzed with 2N HCl for 1 h in a boiling water bath. The hydrolyzed extracts were separated via gradient 2. The run via this gradient was started with a solvent A–solvent B mixture at 50:50 (v/v), followed by a linear transition to 52:48 (v/v) for the first 15 min, then to 100:0 (v/v) from minute 15 to minute 17, with a return to 50:50 (v/v) from minute 17 to minute 20. The flow rate in both gradients was set to 1 mL min−1. The sample injection volume was 10 μL. Tracking of chromatograms was conducted by means of absorbance at 220, 255, 270, 290, 325, 340, 350, and 370 nm.
Phenolic compounds were quantified by the external standard method. Reference standards of gallic, p-hydroxybenzoic, syringic, neochlorogenic, p-coumaric, caffeic, and ellagic acids, (+)-catechin, quercetin, kaempferol, kaempferol rutinoside (Sigma-Aldrich, St. Louis, MO, USA), epigallocatechin gallate (Teavigo, Gevelsberg, Germany), vanillic acid (Serva, Heidelberg, Germany), and ferulic acid (Koch-Light Lab Ltd., Haverhill, Suffolk, UK) were used for building calibration curves in the concentration range of 10–100 μg mL−1. Ellagic acid derivatives and quercetin glycosides were quantified as ellagic acid and rutin, respectively.
2.3. Statistical Analysis
For micropropagation, 30 shoots or plants were used, and the experiments were conducted thrice. The greenhouse experiments were carried out in duplicate at 20 plants per treatment group. The field experiment was carried out two times independently at 40 plants per treatment group.
The presented data are means of three independent experiments with three technical replicates each. Concentrations of the compounds were expressed in milligrams per gram of absolutely dry mass. The data were analyzed in the STATISTICA 10.0 software (Statsoft Inc., Tulsa, OK, USA), are reported as mean ± standard error from three biological and three technical replicates, and were compared by Duncan’s multiple-range test. Differences between the means were considered statistically significant at p < 0.05.
To evaluate variations among the samples of strawberry, principal components analysis (PCA) was performed.
3. Results
3.1. The Phenolic Profile of the Aboveground Parts of F. × ananassa
In the aqueous ethanol extracts from the aboveground parts of F. × ananassa, more than 20 peaks of individual phenolic compounds were detected. The quantified compounds included hydroxybenzoic acids (compounds 1, 4, 8, and 9), hydroxycinnamic acids (2, 7, 10, and 13), catechins (3 and 5), flavonol glycosides (14, 15, and 19), ellagic acid (18), and its derivatives (6, 11–12, and 16–17) (Supplementary Materials Table S1).
Not all these compounds were present at all the stages. Under the in vitro conditions, ellagic acid derivatives 1 and 2 (6 and 11) and quercetin glycosides (14 and 15) were absent. Ellagic acid derivative 1, however, was also absent during ex vitro acclimation, and at this stage, two hydroxycinnamic acids (p-coumaric and ferulic) disappeared. Except for ferulic acid, all the other compounds were present under the greenhouse conditions, and all the compounds were detectable in the field (Table S2; Figure 1).
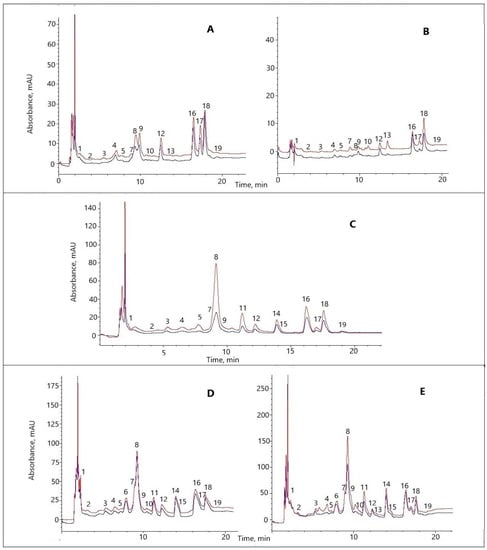
Figure 1.
Representative sections of HPLC chromatograms of the extracts of the aboveground part of strawberry cv. ‘Solnechnaya polyanka’ plants. (A) The in vitro multiplication stage, (B) in vitro rooting stage, (C) ex vitro acclimation stage, (D) greenhouse conditions, (E) field conditions. Black curve: −MC treatment; red curve: +MC treatment. MC: mechanocomposite. Peaks: 1, gallic acids; 2, neochlorogenic acid; 3, (+)-catechin; 4, p-hydroxybenzoic acid; 5, epigallocatechin gallate; 6, ellagic acid derivative 1; 7, caffeic acid; 8, vanillic acid; 9, syringic acid; 10, p-coumaric acid; 11, ellagic acid derivative 2; 12, ellagic acid derivative 3; 13, ferulic acid; 14, quercetin glycoside 1; 15, quercetin glycoside 2; 16, ellagic acid derivative 4; 17, ellagic acid derivative 5; 18, ellagic acid; and 19, kaempferol rutinoside.
The total phenolic content without the MC decreased from in vitro multiplication to in vitro rooting; then, it slightly increased until ex vitro acclimation and, after that, drastically rose during greenhouse growth and significantly declined in the field (Figure 2). The decrease at the in vitro rooting stage was mediated by a reduction or insignificant variation of levels of most of the phenolic compounds. Concurrent shifts up and down of concentrations of the majority of the phenolic compounds (approximately half of them) resulted in a small increase in the total phenolic content during ex vitro acclimation. A sharp rise of the total phenolic content during greenhouse growth occurred due to an increase in concentrations of the vast majority of the phenolic compounds (except for three), including the compounds that emerged at this stage. A more modest upregulation of the total phenolic content in field-derived plants took place because of an increase in concentrations of a smaller number of compounds: 13.
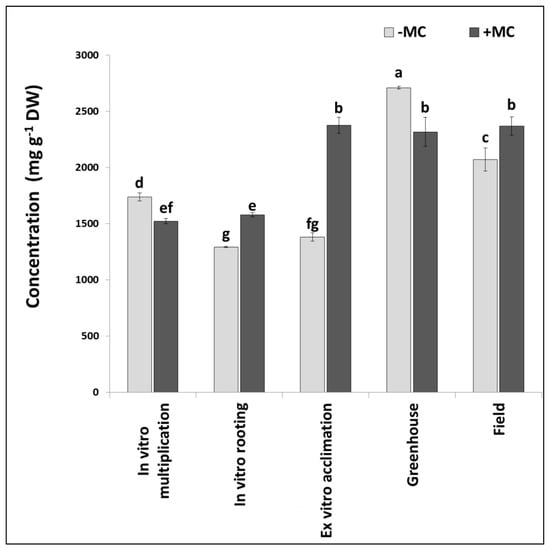
Figure 2.
The total phenolic content in the aboveground part of strawberry cv. ‘Solnechnaya polyanka’ plants at different propagation stages without (−MC) or with mechanocomposite (+MC) treatment. The same lowercase letters for each compound indicate the absence of significant differences in concentration between groups −MC and +MC according to Duncan’s multiple-range test (p < 0.05). Abbreviations: MC, mechanocomposite; −MC, mechanocomposite free; +MC, mechanocomposite treatment; DW, dry weight.
3.2. The Effect of the MC on the Phenolic Profile
The MC modified this course. Plants showed a slight increase (approximately equal between the groups −MC and +MC) in the total phenolic content under in vitro conditions, but during ex vitro acclimation, the total phenolic content of +MC plants (in contrast to MC-untreated plants) substantially went up, and thereafter, it did not show significant changes under different conditions (Figure 2). At all the stages, the compounds responded differently to the MC. Downregulation, upregulation, and statistically insignificant effects were registered. As compared with untreated plants, the total phenolic content decreased in the +MC group during in vitro multiplication and greenhouse growth (Figure 3). Concentrations of neochlorogenic acid, (+)-catechin, caffeic acid, p-coumaric acid, ellagic acid, and its derivatives 3 and 4 diminished under the influence of the MC during both stages.
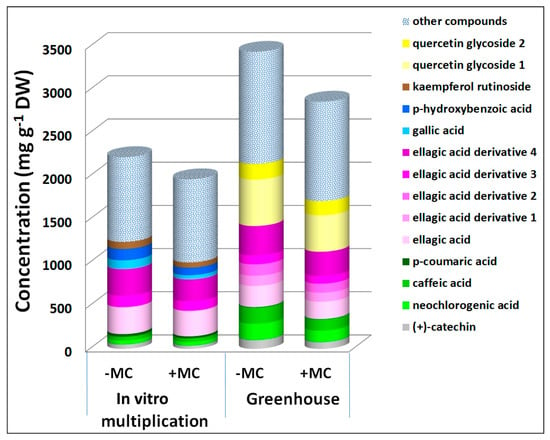
Figure 3.
Concentrations of phenolic compounds in the aboveground part of strawberry cv. ‘Solnechnaya polyanka’ plants contributed to the downregulation of the total phenolic content under the influence of the MC during in vitro multiplication and greenhouse growth. Abbreviations: MC, mechanocomposite; −MC, mechanocomposite free; +MC, mechanocomposite treatment; DW, dry weight.
At the in vitro multiplication stage, a decrease was also manifested by gallic acid by 2.1-fold, p-hydroxybenzoic acid by 1.5-fold, p-coumaric acid by 1.3-fold, ellagic acid derivative 3 by 1.2-fold, and kaempferol rutinoside by 1.3-fold (p < 0.05), whereas during greenhouse growth, concentrations of caffeic acid dropped by 1.4-fold, quercetin glycosides 1 and 2 by 1.3- to 1.1-fold, ellagic acid by 1.2-fold, and ellagic acid derivatives 1 and 2 by 1.1- to 1.3-fold (p < 0.05) (Figure 3). At the other three stages, the MC induced an increase in the total phenolic content as compared with untreated plants. Some phenolic compounds’ levels diminished during the multiplication stage and contributed to the positive effects of the MC on the total phenolic content during in vitro rooting (gallic acid by 1.4-fold, caffeic acid by 2.3-fold, and p-coumaric acid by 1.3-fold, p < 0.05) and ex vitro acclimation (gallic acid by 2.4-fold, (+)-catechin by 1.9-fold, caffeic acid by 1.3-fold, ellagic acid by 1.3-fold, and its derivatives 3 and 4 by 1.8- and 1.4-fold, respectively, p < 0.05; Figure 4).
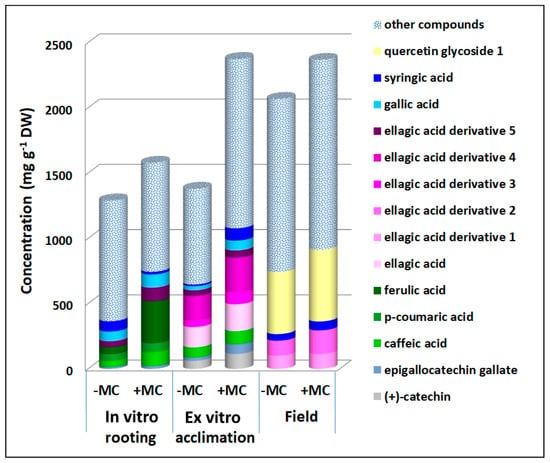
Figure 4.
Concentrations of phenolic compounds in the aboveground part of strawberry cv. ‘Solnechnaya polyanka’ plants contributed to the increase in the total phenolic content under the influence of the MC during in vitro rooting, ex vitro acclimation, and field growth. Abbreviations: MC, mechanocomposite; −MC, mechanocomposite free; +MC, mechanocomposite treatment; DW, dry weight.
The compounds whose levels declined during greenhouse growth contributed to the positive effect of the MC on the total phenolic content during field growth (syringic acid, quercetin glycoside 1, and ellagic acid derivatives 1 and 2 by 1.3-, 1.1-, 1.1-, and 1.6-fold, respectively, p < 0.05; Figure 4).
The levels of other phenolic compounds mostly increased during the growth of the total phenolic content. Epigallocatechin gallate, ellagic acid derivative 5, and ferulic acid showed a substantial increase by 1.5-, 2.3-, and 5.9-fold, respectively (p < 0.05), under the influence of the MC during in vitro rooting (Figure 4). The first two compounds also contributed to the rise of the total phenolic content by 2.8- and 1.3-fold, respectively (p < 0.05), during ex vitro acclimation (Figure 4). On the other hand, vanillic acid, ellagic acid derivative 2, and quercetin glycoside 1 (Figure 5) made a contribution to the increase in the total phenolic content during both ex vitro acclimation (their levels rose by 2.6-, 1.8-, and 1.3-fold, respectively, p < 0.05) and field growth (by 1.4-, 1.6-, and 1.1-fold, respectively, p < 0.05). At the ex vitro acclimation stage, the MC caused an upregulation of the largest number of compounds and the greatest increase in the total phenolic content.
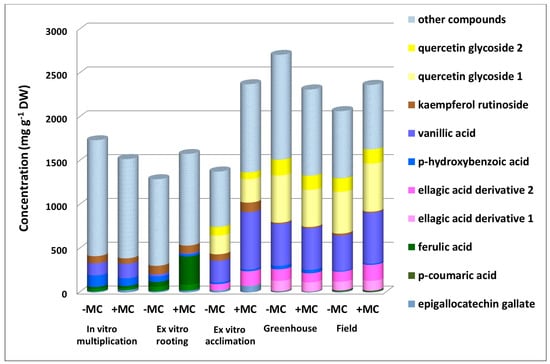
Figure 5.
Phenolic compounds with the greatest concentration shift during development under in vitro, ex vitro, and in vivo conditions under the influence of the MC treatment. MC, mechanocomposite.
Consequently, the impact of the MC manifested itself mostly at the ex vitro acclimation stage, when the total phenolic content of MC-treated plants was 1.5 times higher than that of the same plants at the previous stage and 1.7 times higher than that of untreated plants at the same stage (p < 0.05) (Figure 2).
A comparison of phenolic profiles at different stages revealed compounds most responsive to environmental conditions: their concentrations varied ≥3-fold. In both groups of plants, there were epigallocatechin gallate, gallic, neochlorogenic, p-hydroxybenzoic, syringic, vanillic acids, ellagic acid derivative 5, and kaempferol rutinoside (Supplementary Materials Figures S1–S5). The MC made concentration shifts more substantial. For instance, during the transition to the ex vitro stage, the concentration of vanillic acid increased 9.9-fold without the MC treatment, whereas in treated plants, its concentration increased 53.9-fold (p < 0.05). The concentration of epigallocatechin gallate diminished considerably only under the MC influence in in vivo conditions. The transition to the greenhouse from the ex vitro stage caused its reduction by 10.3-fold, and the transfer to the field led to a 13.4-fold decrease (Figure 5). In addition, phenolic compounds that emerged only under ex vitro (quercetin glycosides and ellagic acid derivative 2) or in vivo conditions (ellagic acid derivative 1; Figure 3 and Figure 5) or disappeared at these stages (p-coumaric and ferulic acids; Figure 5) revealed a transformation of the phenolic profile of the plants during these big environmental changes.
A comparison of the levels of the main classes of phenolic compounds indicated a significant difference between the classes in their response to the MC treatment (Figure 6). Concentrations of hydroxybenzoic acids diminished by 1.3- and 1.5-fold (p < 0.05) during in vitro stages and went up during ex vitro acclimation and field growth by 2.7- and 1.4-fold, respectively (p < 0.05). Upregulation of catechins by 2.2-fold (p < 0.05) during ex vitro acclimation coincided with the increase in hydroxybenzoic acids’ content.
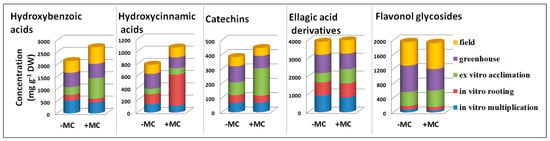
Figure 6.
The distribution of phenolic compounds in the aboveground part of strawberry cv. ‘Solnechnaya polyanka’ plants at different propagation stages under the influence of the MC treatment. MC, mechanocomposite.
Changes in the levels of ellagic acid derivatives matched those of hydroxybenzoic acids in general. Meanwhile, alterations of contents of hydroxycinnamic acids differed drastically from them. Hydroxycinnamic acids underwent considerable upregulation by 3.5-fold (p < 0.05) at the in vitro rooting stage and a decrease by 1.5-fold (p < 0.05) during greenhouse growth with the MC treatment. Under the influence of the MC, concentrations of flavonoid glycosides were significantly higher during ex vitro acclimation (1.2-fold, p < 0.05) and field growth (1.1-fold, p < 0.05) than without the MC, and the opposite was registered during greenhouse growth (a decrease by 1.2-fold, p < 0.05) (Figure 6).
3.3. PCA
This analysis confirmed most of these responsive compounds as variables that contributed to the observed variation (Figure 7). Vanillic acid showed a strong positive correlation (0.87) with the first principal component (PC1). Meanwhile, p-coumaric acid (−0.79), gallic acid (−0.85), p-hydroxybenzoic acid (−0.66), and kaempferol rutinoside (−0.77) highly negatively correlated with this component. Neochlorogenic acid (−0.88), syringic acid (−0.63), and ellagic acid derivative 5 (−0.67) strongly negatively correlated with the second principal component (PC2), whereas epigallocatechin gallate (0.87) showed a strong positive correlation with the third principal component (PC3). Quercetin glycosides (0.98) and ellagic acid derivative 2 (0.91), which were absent at in vitro stages, manifested the highest positive correlation with PC1. The total phenolic content strongly positively correlated with this component too (0.80). Ellagic acid derivative 1 (0.85), which was detected only during greenhouse growth and in the field, also strongly positively correlated with PC1 (Figure 7C,D).
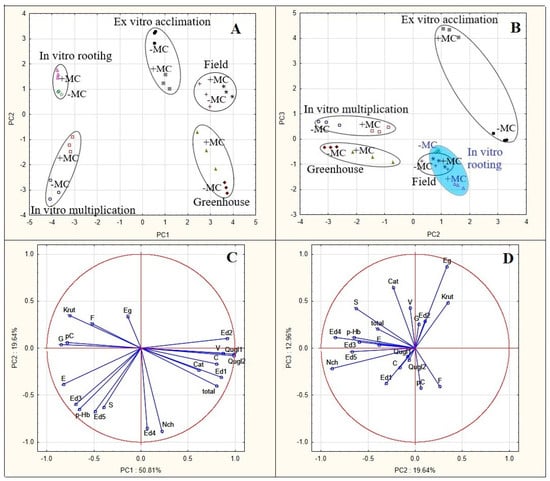
Figure 7.
PCA score plots of PC1 versus PC2 (A) and PC2 versus PC3 (B) from PCA of a 20-variable matrix for the five different propagation stages without (−MC) and with mechanocomposite (+MC) treatment of strawberry cv. ‘Solnechnaya polyanka’ plants. Projections of the variables on the factor plane of PCA of the 20 parameters of the phenolic profile of the plants: (C) loading plots for the compounds’ variables contributing to PC1 and PC2; (D) loading plots for the compounds’ variables contributing to PC2 and PC3. Abbreviations: MC, mechanocomposite; −MC, mechanocomposite free; +MC, mechanocomposite treatment; G, gallic acid; Nch, neochlorogenic acid; Cat, (+)-catechin; p-Hb, p-hydroxybenzoic acid; Eg, epigallocatechin gallate; Ed 1–5, ellagic acid derivatives 1–5; C, caffeic acid; V, vanillic acid; S, syringic acid; pC, p-coumaric acid; F, ferulic acid; Qugl 1 and 2, quercetin glycosides 1 and 2; E, ellagic acid; Krut, kaempferol rutinoside; and Total, total phenolic content.
Some compounds—whose levels did not vary widely under the influence of environmental changes or of the MC—also strongly correlated with principal components. Caffeic acid showed a strong positive correlation (0.80) with PC1, whereas ellagic acid and ellagic acid derivative 3 strongly correlated with it negatively (−0.82 and −0.69, respectively). Ellagic acid derivative 4 showed a strong negative correlation with PC2 (−0.85) (Figure 7C).
PCA revealed relations among all the stages of micropropagation and in vivo adaptation with and without MC treatment (Figure 7A). Two principal components explaining 71% of the overall variance (51% and 20% for PC1 and PC2, respectively) divided the analyzed stages of development into a separate cluster, and data points involving the MC treatment were found to be separated from data points without the treatment.
The location of in vitro stages in the scatterplot of PC2 against PC1 characterized their phenolic profiles as correlating negatively with PC1. They featured high levels of gallic, p-hydroxybenzoic, p-coumaric, and ellagic acids and of kaempferol rutinoside (Figure 7A). Meanwhile, in vitro multiplication and rooting stages occupy opposite positions relative to the PC2 axis. Phenolic profiles at the multiplication stage were found to be located in an area of an elevated influence of neochlorogenic acid and ellagic acid derivative 4. The MC weakened this correlation. On the other hand, the location of phenolic profiles during in vitro rooting indicated a moderate positive correlation with PC2, and the MC strengthened this correlation. Stages ex vitro and in vivo proved to be located in an area of a positive correlation with PC1 under the influence of quercetin glycosides, ellagic acid derivatives 1 and 2, caffeic acid, and vanillic acids and of the total phenolic content. Meanwhile, greenhouse plants, just as plants during in vitro multiplication, occupy an area with a negative correlation with PC2, and the MC weakened this correlation under the influence neochlorogenic acid. Ex vitro phenolic profiles ended up in an area with a positive correlation with PC2, and the MC attenuated this relation. The phenolic profiles of the greenhouse plants and plants in the field are characterized by the strongest positive correlation with PC1. For the former, the MC weakened this correlation for the transition from the area of quercetin glycosides to the area dominated by ellagic acid derivatives 1 and 2 and caffeic and vanillic acid. For the plants in the field, on the contrary, the MC strengthened this positive correlation. Thus, in this regard, the field-grown plants treated with the MC are under the strongest influence of quercetin glycosides.
The score plot of PC2 versus PC3 illustrates considerable differentiation between ex vitro plants treated and untreated with the MC in terms of the correlation with PC3 (Figure 7B). Plants treated with the MC showed the highest positive correlation with the third principal component in an area of the highest level of epigallocatechin gallate; however, the phenolic profile of untreated plants under the same conditions barely correlated with PC3. Other strong correlations with PC3 were not detectable. Therefore, PCA revealed phenolic profiles specific to each stage of micropropagation and in vivo adaptation as well as significant effects of the MC at all these stages.
4. Discussion
Our phenolic profiling of all five stages of propagation indicates transformations of the phenolic profile in the course of plant development and under the influence of environmental changes. Moreover, the results point to specific features of in vitro stages. The absence of quercetin glycosides and ellagic acid derivatives 1 and 2 is the main characteristic of these stages (Figures S3 and S5). Phenolic acids and ellagic acid derivatives 4 and 5 primarily constitute a pool of biologically active compounds presumably helping to overcome the effects of negative environmental factors. The original profiles of phenolic compounds and the prevalence and accumulation of phenolic acids, including ellagic acid, in tissue culture have been described previously [19,41,42], including our work [43]. Aside from having antioxidant properties, phenolic acids participate in cellular respiration and photosynthesis, lignin metabolism processes, water movements, and hormonal balance [44].
In our work, the effects of the MC were specific to each in vitro stage. The decrease in the total phenolic content caused by the MC during in vitro multiplication as compared with the −MC group most likely can be explained by the optimization of gas and water exchange in plantlets and a consequent reduction in the stress load. At the in vitro rooting stage, when the total phenolic content of untreated plants decreased, MC-treated plants showed a significant (for gallic, caffeic, and p-coumaric acids) or manyfold (for ferulic acid) increase in the levels of certain phenolic acids, although the total phenolic content was approximately equal to that at the previous stage (Figures S1 and S4). This increase means more intensive development of MC-treated plants and involvement of the majority of these phenolic acids in lignin synthesis. Esterified and etherified cinnamic acids (primarily ferulic, caffeic, and p-coumaric acids) are structural components of the cell wall, including lignin [18,45].
Our analysis of subsequent stages indicates that concentrations of p-coumaric and ferulic acids significantly declined under ex vitro and in vivo conditions compared with in vitro conditions, down to an undetectable level during ex vitro acclimation (Figure S1). Then, ferulic acid was found only in field plants in trace quantities, and p-coumaric acid was barely detectable at both in vivo stages. This variation confirms that these components are sources of lignin and shows the propensity of ferulic and p-coumaric acids to convert to a bound form usually. Some authors have also reported on the absence of ferulic acid in Fragaria leaves even in the cell-wall-bound fraction; they explained this phenomenon by extremely tight bonds via ether linkages in lignin and by consequent difficulties with extraction [46,47]. It is noteworthy that p-coumaric acid, along with some other compounds (ellagic acid, gallic acid, and kaempferol hexoside), has been also identified in the cell-wall-bound fraction [47]. This finding leads us to believe that the significant variation and transient absence of p-coumaric acid may be caused by cell wall binding. Cell-wall-associated phenolics have been shown to protect epidermal cells from UV radiation [48] and to limit water loss by maintaining the integrity and hydrophobicity of the cell wall [49].
Our results suggest that the MC induced upregulation of precursors of lignin especially at the rooting stage. Roots are reported to accumulate large amounts of lignin in xylem vessels and in the endodermal cells surrounding vascular tissues for Casparian strip formation [50]. In our previous study, we demonstrated an increase in the mass, length, and number of roots per microshoot under the influence of the same MC [25].
A reduction in p-coumaric acid concentration in F. vesca leaves was also observed during greenhouse growth [29]. Those authors associated this decrease with higher utilization of this intermediate for the synthesis of flavonols and flavanols. Our results confirm this supposition because an undetectable level of p-coumaric acid at the ex vitro stage coincided with the emergence of quercetin glycosides.
The overproduction of vanillic acid under the action of the MC during ex vitro acclimation is also worth noting (Figure S4). The levels of catechins (Figure S2) and syringic acid (Figure S4) considerably increased too, and the concentrations of the majority of the phenolic compounds also rose. Vanillic acid, just as syringic acid, was shown to participate in lignification [51]. However, in this stage, antioxidant properties of phenolic compounds can exert an action too. This expansion of the total antioxidant pool probably occurs in response to an increase in the stress load from the environment. Individual phenolic compounds contribute to total antioxidant activity in accordance with their molecular structure [52,53]. The upregulation of catechins is very important because these flavonoids are considered highly effective antioxidants [54]. Some authors demonstrated a similar ability of silicon to reinforce the antioxidant pool of phenolic compounds [55].
On the other hand, vanillic acid, along with some other phenolic acids, catechins, and tannins, has been found to take part in plants’ antiherbivore and antimicrobial mechanisms [56,57,58]. Meanwhile, silicon is reported to upregulate toxic compounds and antinutrients. For example, in miniature roses, silicon causes the accumulation of fungitoxic phenolic compounds (chlorogenic acids and flavonoids), which correlates with an approximately 50% reduction in the incidence of infection by the biotrophic pathogen Podosphaera pannosa [59].
Although quercetin derivatives are the most abundant class of low-molecular-weight phenolic compounds in strawberry leaves [60], results of the present study suggest that quercetin derivatives are specific only to ex vitro and in vivo plants (Figure S5). By contrast, kaempferol rutinoside was detected at all the tested stages. This finding once again indicates the unique properties of quercetin glycosides as antioxidants and defensive compounds whose content usually grows in response to harsh stressors [61,62]. Similar ratios between quercetin and kaempferol glycosides have been registered in in vitro–grown leaves of F. vesca; the level of rutin is negligible, whereas the concentration of kaempferol glucoside is substantial, albeit significantly lower as compared with field-grown plants [33].
The opposite effect in strawberry leaves has been reported too: after treatment with a high concentration of nitrogen, flavonol content diminishes, while the kaempferol/quercetin ratio rises [30].
Ellagic acid derivative 1 (Figure S3) proved to be another phenolic compound characteristic of in vivo stages. Ellagic acid derivative 2 was not detectable at in vitro stages either and was first detected in the plants during ex vitro acclimation. The increase in the concentrations of ellagic acid derivatives, along with phenolic acids’ levels, under various stresses has been documented previously [20,47]. On the other hand, our results point to the functional heterogeneity of ellagic acid derivatives similar to that of the above-mentioned flavonol glycosides. According to the spectral maxima of these derivatives, they are agrimoniin-like ellagitannin [63]. Similar compounds are abundant in strawberry leaves [47]. The sensitivity of ellagitannins to external factors has been reported before. Their biological properties, including their valuable therapeutic activities, are very diverse [64]. In this context, it is noteworthy that our findings expand the knowledge about the usefulness of strawberry leaves for biotechnology. Obviously, the spectrum of phenolic compounds in tissue culture here was limited, and the alterations induced by exogenous active compounds (MC) consisted of only quantitative shifts of certain compounds, but a qualitative transformation of the phenolic profile occurred during environmental changes.
5. Conclusions
For the first time, we present results about the phenolic profile of aboveground parts of F. × ananassa plants during the propagation cycle under in vitro, ex vitro, and in vivo conditions after treatment with a silicon-derived biostimulant. The results revealed phenolic profiles specific to each stage and transformations of the profiles by the MC treatment. Quantitative changes induced by the MC, especially significant for phenolic acids, were documented at the in vitro rooting and ex vitro acclimation stages. A strong increase in the hydroxycinnamic acid content during in vitro rooting correlated with elevated lignin synthesis in MC-treated plants. During ex vitro acclimation, the MC triggered considerable upregulation of antioxidants (primarily catechins and hydroxybenzoic acids). In ex vitro and in vivo stages, the emergence of the most effective flavonoid antioxidants (quercetin glycosides) and ellagitannins was registered, and the MC boosted their levels during ex vitro acclimation and field growth. PCA uncovered a significant influence of the MC on phenolic profiles at all stages, and this effect was most pronounced during ex vitro acclimation. Our findings expand the knowledge about poorly studied phenolic compounds of strawberry plantlets during in vitro development and may help to improve the yield of biologically active compounds. On the other hand, clarifying the roles of individual phenolic compounds opens up prospects for the modulation of the adaptation of strawberry plants to fruit production. An MC based on silicon chelates from plant raw materials apparently is useful for these processes and technologies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9020157/s1, Figure S1: Concentrations of hydroxycinnamic acids in the aboveground part of strawberry cv. ‘Solnechnaya polyanka’ plants at different propagation stages without (−MC) or with mechanocomposite (+MC) treatment; Figure S2: Concentrations of catechins in the aboveground part of strawberry cv. ‘Solnechnaya polyanka’ plants at different propagation stages without (−MC) or with mechanocomposite (+MC) treatment; Figure S3: Concentrations of ellagic acid derivatives in the aboveground part of strawberry cv. ‘Solnechnaya polyanka’ plants at different propagation stages without (−MC) or with mechanocomposite (+MC) treatment; Figure S4: Concentrations of hydroxybenzoic acids in the aboveground part of strawberry cv. ‘Solnechnaya polyanka’ plants at different propagation stages without (−MC) or with mechanocomposite (+MC) treatment; Figure S5: Concentrations of flavonol glycosides in the aboveground part of strawberry cv. ‘Solnechnaya polyanka’ plants at different propagation stages without (−MC) or with mechanocomposite (+MC) treatment; Table S1: Phenolic compounds in the aboveground part of strawberry cv. ‘Solnechnaya polyanka’ plants; Table S2: Total phenolic concentrations of compounds (mg g−1 DW) in the aboveground part of strawberry cv. ‘Solnechnaya polyanka’ plants at different propagation stages without (−MC) and with mechanocomposite (+MC) treatment.
Author Contributions
Conceptualization, E.A., E.K., and O.K.; methodology, E.K. and O.K.; validation, E.K. and O.K.; formal analysis, E.K. and O.K.; investigation, E.K., O.K., and E.A.; resources, E.A., E.K., O.K., E.T., and T.N.; data curation, E.A., E.K., and O.K.; writing—original draft preparation, E.A., E.K., and O.K.; writing—review and editing, E.A., E.K., and O.K.; visualization, E.A. and O.K.; supervision, E.A.; project administration, E.A.; funding acquisition, E.A. and O.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation and the Government of the Novosibirsk Region, grant number 22-26-20061.
Data Availability Statement
Raw data of this study are available upon request from the corresponding author.
Acknowledgments
In vitro material from the collection of CSBG, SB RAS, was used: unique scientific unit (USU) 440534: «Collection of living plants indoors and outdoors». The maintenance of in vitro collection were supported by the Laboratory of Biotechnology of the CSBG, project number AAAA-A21-121011290025-2.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Siletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Nephali, L.; Piater, L.A.; Dubery, I.A.; Patterson, V.; Huyser, J.; Burgess, K.; Tugizimana, F. Biostimulants for plant growth and mitigation of abiotic stresses: A metabolomics perspective. Metabolites 2020, 10, 505. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Durán-Lara, E.F.; Valderrama, A.; Marican, A. Natural organic compounds for application in organic farming. Agriculture 2020, 10, 41. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Toward a sustainable agriculture through plant biostimulants: From experimental data to practical applications. Agronomy 2020, 10, 1461. [Google Scholar] [CrossRef]
- Lomovsky, O.I.; Lomovskiy, I.O.; Orlov, D.V. Mechanochemical solid acid/base reactions for obtaining biologically active preparations and extracting plant materials. Green Chem. Lett. Rev. 2017, 10, 171–185. [Google Scholar] [CrossRef]
- Trofimova, E.G.; Podgorbunskikh, E.M.; Skripkina, T.S.; Bychkov, A.L.; Lomovsky, O.I. Scaling of the mechanochemical process of production of silicon chelates. Bulg. Chem. Commun. 2018, 50, 45–48. [Google Scholar]
- Ambros, E.; Karpova, E.; Kotsupiy, O.; Zaytseva, Y.; Trofmova, E.; Novikova, T. Silicon chelates from plant waste promote in vitro shoot production and physiological changes in strawberry plantlets. Plant Cell Tissue Organ. Cult. 2021, 145, 209–221. [Google Scholar] [CrossRef]
- Savvas, D.; Ntatsi, G. Biostimulant activity of silicon in horticulture. Sci. Hortic. 2015, 196, 66–81. [Google Scholar] [CrossRef]
- Szulc, W.; Rutkowska, B.; Hoch, M.; Ptasinski, D.; Kazberuk, W. Plant available silicon in differentiated fertilizing conditions. Plant Soil Environ. 2019, 65, 233–237. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.-P.; Tian, D.-D.; Guo, D.-J.; Chen, Z.-L.; Zhong, C.-S.; Nikpay, A.; Singh, M.; Rajput, V.D.; Singh, R.K.; et al. Influence of silicon on biocontrol strategies to manage biotic stress for crop protection, performance, and improvement. Plants 2021, 10, 2163. [Google Scholar] [CrossRef]
- Guerriero, G.; Hausman, J.F.; Legay, S. Silicon and the plant extracellular matrix. Front. Plant Sci. 2016, 7, 463. [Google Scholar] [CrossRef]
- Carneiro-Carvalho, A.; Aires, A.; Anjos, R.; Martins, L.; Pinto, T.; Peixoto, F.; Gomes-Laranjo, J. The role of silicon fertilization in the synthesis of phenolic compounds on chestnut plants infected with P. cinnamomi and C. parasitica. J. Plant Dis. Prot. 2020, 127, 211–227. [Google Scholar] [CrossRef]
- Vega, I.; Rumpel, C.; Ruíz, A.; Mora, M.d.l.L.; Calderini, D.F.; Cartes, P. Silicon modulates the production and composition of phenols in barley under aluminum stress. Agronomy 2020, 10, 1138. [Google Scholar] [CrossRef]
- Araújo, W.B.S.; Teixeira, G.C.M.; de Mello Prado, R.; Rocha, A.M.S. Silicon mitigates nutritional stress of nitrogen, phosphorus, and calcium deficiency in two forages plants. Sci. Rep. 2022, 12, 6611. [Google Scholar] [CrossRef]
- Kulbat, K. The role of phenolic compounds in plant resistance. Biotechnol. Food Sci. 2016, 80, 97–108. [Google Scholar]
- Šamec, D.; Karalija, E.; Šola, I.; Vujcic Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Matkowski, A. Plant in vitro culture for the production of antioxidants—A review. Biotechnol. Adv. 2008, 26, 548–560. [Google Scholar] [CrossRef]
- Kårlund, A.; Salminen, J.-P.; Koskinen, P.; Ahern, J.R.; Karonen, M.; Tiilikkala, K.; Karjalainen, R.O. Polyphenols in strawberry (Fragaria × ananassa) leaves induced by plant activators. J. Agric. Food Chem. 2014, 62, 4592–4600. [Google Scholar] [CrossRef]
- Dongju, L.; Qing, M.; Yiwen, Z.; Zeyan, P. Phenolic compounds with antioxidant activity from strawberry leaves: A study on microwave-assisted extraction optimization. Prep. Biochem. Biotechnol. 2020, 50, 874–882. [Google Scholar] [CrossRef]
- Kotsupiy, O.; Karpova, E.; Ambros, E. Phenolic compounds in strawberry (Fragaria × ananassa Duch.) microshoots. BIO Web Conf. 2020, 24, 00041. [Google Scholar] [CrossRef]
- Ambros, E.; Karpova, E.; Kotsupiy, O.; Trofimova, E.; Zakabluk, G.; Chernonosov, A.; Koval, V.; Novikova, T. A mechanocomposite based on biogenic silica and green tea flavonoids modulates adaptability of strawberry microclones to in vitro and ex vitro conditions. J. Soil Sci. Plant Nutr. 2022, 1–16. [Google Scholar] [CrossRef]
- Ambros, E.V.; Toluzakova, S.Y.; Shrainer, L.S.; Trofimova, E.G.; Novikova, T.I. An innovative approach to ex vitro rooting and acclimatization of Fragaria × ananassa Duch. microshoots using a biogenic silica and green-tea-catechin-based mechanocomposite. In Vitro Cell. Dev. Biol. Plant 2018, 54, 436–443. [Google Scholar] [CrossRef]
- Ambros, E.V.; Kotsupy, O.V.; Karpova, E.A.; Trofimova, E.G.; Zaytseva, Y.G.; Novikova, T.I. In vitro adaptive responses of Fragaria ananassa Duch. plantlets induced by the mechanocomposite based on amorphous silica and flavonoids of green tea. Theor. Prikl. Ecol. 2019, 4, 116–122. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Zia-Ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Qayyum, M.F.; Irshad, M. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2015, 119, 186–197. [Google Scholar] [CrossRef]
- Rahman, A.; Wallis, C.M.; Uddin, W. Silicon-induced systemic defense responses in perennial ryegrass against infection by Magnaporthe oryzae. Phytopathology 2015, 105, 748–757. [Google Scholar] [CrossRef]
- Gengmao, Z.; Shihui, L.; Xing, S.; Yizhou, W.; Zipan, C. The role of silicon in physiology of the medicinal plant (Lonicera japonica L.) under salt stress. Sci. Rep. 2015, 5, 12696. [Google Scholar] [CrossRef]
- Hajiboland, R.; Moradtalab, N.; Eshaghi, Z.; Feizy, J. Effect of silicon supplementation on growth and metabolism of strawberry plants at three developmental stages. N. Z. J. Crop Hortic. Sci. 2018, 46, 144–161. [Google Scholar] [CrossRef]
- Narvekar, A.S.; Tharayil, N. Nitrogen fertilization influences the quantity, composition, and tissue association of foliar phenolics in strawberries. Front. Plant Sci. 2021, 12, 613839. [Google Scholar] [CrossRef]
- Mahmood, T.; Anwar, F.; Abbas, M.; Saari, N. Effect of maturity on phenolics (phenolic acids and flavonoids) profile of strawberry cultivars and mulberry species from Pakistan. Int. J. Mol. Sci. 2012, 13, 4591–4607. [Google Scholar] [CrossRef]
- Pešaković, M.; Milenković, S.; Đukić, D.; Mandić, L.; Karaklajić-Stajić, Ž.; Tomić, J.; Miletić, N. Phenolic composition and antioxidant capacity of integrated and conventionally grown strawberry (Fragaria × ananassa Duch.). Hort. Sci. 2016, 43, 17–24. [Google Scholar] [CrossRef]
- Yildirim, A.B.; Turker, A.U. Effects of regeneration enhancers on micropropagation of Fragaria vesca L. and phenolic content comparison of field-grown and in vitro-grown plant materials by liquid chromatography-electrospray tandem mass spectrometry (LC–ESI-MS/MS). Sci. Hortic. 2014, 169, 169–178. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Sousa, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Enhancement of nutritional and bioactive compounds by in vitro culture of wild Fragaria vesca L. vegetative parts. Food Chem. 2017, 235, 212–219. [Google Scholar] [CrossRef]
- Hazarika, B.N. Morpho-physiological disorders in in vitro culture of plants. Sci. Hort. 2006, 108, 105–120. [Google Scholar] [CrossRef]
- Pospisilova, J.; Synková, H.; Haisel, D.; Semorádová, Š. Acclimation of plantlets to ex vitro conditions: Effects of air humidity, irradiance, CO2 concentration and abscisic acid (a review). Acta Hort. 2007, 748, 29–38. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Eveleigh, D.E. Culture methods and detection of glucanases in suspension cultures of wheat and barley. Can. J. Biochem. 1968, 46, 417–421. [Google Scholar] [CrossRef]
- Ambros, E.V.; Zaytseva, Y.G.; Krasnikov, A.A.; Novikova, T.I. Optimization of microshoots regeneration systems of Fragaria × ananassa (Rosaceae) genotypes perspectived for Siberian region. Rastit. Mir Aziat. Ross. 2017, 4, 73–80. [Google Scholar] [CrossRef]
- GOST R 53135-2008; Planting Material of Fruit, Berry, Subtropical, Nuts, Citrus Cultures and Tea. Technical Conditions. Standartinform: Moscow, Russia, 2009; 41p.
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Kikowska, M.; Thiem, B.; Szopa, A.; Klimek-Szczykutowicz, M.; ·Rewers, M.; ·Sliwinska, E.; Ekiert, H. Comparative analysis of phenolic acids and flavonoids in shoot cultures of Eryngium alpinum L.: An endangered and protected species with medicinal value. Plant Cell Tiss. Organ Cult. 2019, 139, 167–175. [Google Scholar] [CrossRef]
- Gutierrez-Sanchez, A.; Monribot-Villanueva, J.L.; Cocotle-Ronzón, Y.; Martínez-Cruz, N.S.; Guerrero-Analco, J.A. Phenolic profile and antioxidant activity from wild and in vitro cultivated Rhynchostele rossii (Orchidaceae). Act. Bot. Mex. 2020, 127, e1665. [Google Scholar] [CrossRef]
- Karpova, E.A.; Nabieva, A.Y.; Fershalova, T.D. Leaf pigments and concentrations of phenolic compound in Begonia grandis plantlets obtained from the floral explants. Rend. Lincei. Sci. Fis. E Naturali. 2021, 32, 921–930. [Google Scholar] [CrossRef]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; de Paiva Foletto-Felipe, M.; Ferrarese-Filho, J.A.O. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Ralph, J.; Bunzel, M.; Marita, J.; Lu, F.; Kim, H.; Schatz, P.; Grabber, J.; Steinhart, H. Peroxidase-dependent cross-linking reactions of p-hydroxycinnamates in plant cell walls. Phytochem. Rev. 2004, 3, 79–96. [Google Scholar] [CrossRef]
- Hukkanen, A.T.; Kokko, H.I.; Buchala, A.J.; McDougall, G.J.; Stewart, D.; Karenlampi, S.O.; Karjalainen, R.O. Benzothiadiazole induces the accumulation of phenolics and improves resistance to powdery mildew in strawberries. J. Agric. Food Chem. 2007, 5, 1862–1870. [Google Scholar] [CrossRef]
- González Moreno, A.; de Cózar, A.; Prieto, P.; Domínguez, E.; Heredia, A. Radiationless mechanism of UV deactivation by cuticle phenolics in plants. Nat. Commun. 2022, 13, 1786. [Google Scholar] [CrossRef]
- Hura, T.; Tyrka, M.; Hura, K.; Ostrowska, A.; Dziurka, K. QTLs for cell wall-bound phenolics in relation to the photosynthetic apparatus activity and leaf water status under drought stress at different growth stages of triticale. Mol. Genet. Genom. 2017, 292, 415–433. [Google Scholar] [CrossRef]
- Reyt, G.; Ramakrishna, P.; Salas-González, I.; Fujita, S.; Love, A.; Tiemessen, D.; Lapierre, C.; Morreel, K.; Calvo-Polanco, M.; Flis, P.; et al. Two chemically distinct root lignin barriers control solute and water balance. Nat. Commun. 2021, 12, 2320. [Google Scholar] [CrossRef]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Surech Kumar, C. Syringic acid (SA)—A review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure–antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Temocico, G.; Fierascu, I.; Ortan, A.; Babeanu, N.E. Fragaria genus: Chemical composition and biological activities. Molecules 2020, 25, 498. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The role of catechins in cellular responses to oxidative stress. Molecules. 2018, 23, 965. [Google Scholar] [CrossRef]
- Maksimovic, J.D.; Bogdanovic, J.; Maksimovic, V.; Nikolic, M. Silicon modulates the metabolism and utilization of phenolic compounds in cucumber (Cucumis sativus L.) grown at excess manganese. J. Plant Nutr. Soil Sci. 2007, 170, 739–744. [Google Scholar] [CrossRef]
- Summers, C.B.; Felton, G.W. Prooxidant effects of phenolic acids on the generalist herbivore Helicoverpa zea (Lepidoptera: Noctuidae): Potential mode of action for phenolic compounds in plant anti-herbivore chemistry. Insect. Biochem. Mol. Biol. 1994, 24, 943–953. [Google Scholar] [CrossRef]
- War, A.R.; Sharma, S.P.; Sharma, H.C. Differential induction of flavonoids in groundnut in response to Helicoverpa armigera and Aphis craccivora infestation. Int. J. Insect Sci. 2020, 8, 55–64. [Google Scholar] [CrossRef]
- Singh, P.; Yamshi, A.; Bajguz, A.; Hayat, S. The role of quercetin in plants. Plant Physiol. Biochem. 2021, 166, 10–19. [Google Scholar] [CrossRef]
- Shetty, R.; Jensen, B.; Shetty, N.P.; Hansen, M.; Hansen, C.W.; Starkey, K.R.; Jørgensen, H.J.L. Silicon induced resistance against powdery mildew of roses caused by Podosphaera pannosa. Plant Pathol. 2012, 61, 120–131. [Google Scholar] [CrossRef]
- Kårlund, A.; Hanhineva, K.; Lehtonen, M.; McDougall, G.J.; Stewart, D.; Karjalainen, R.O. Non-targeted metabolite profiling highlights the potential of strawberry leaves as a resource for specific bioactive compounds. J. Sci. Food Agric. 2017, 97, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Karpova, E.A.; Fershalova, T.D.; Petruk, A.A. Flavonoids in adaptation of Begonia grandis Dryander subsp. grandis introduced in West Siberia (Novosibirsk). J. Stress Physiol. Biochem. 2016, 12, 44–56. [Google Scholar]
- Fraser, D.P.; Sharma, A.; Fletcher, T.; Budge, S.; Moncrieff, C.; Dodd, A.N.; Franklin, K.A. UV-B antagonises shade avoidance and increases levels of the flavonoid quercetin in coriander (Coriandrum sativum). Sci. Rep. 2017, 7, 17758. [Google Scholar] [CrossRef]
- Duckstein, S.M.; Lotter, E.M.; Meyer, U.; Lindequist, U.; Stintzing, F.C. Phenolic constituents from Alchemilla vulgaris L. and Alchemilla mollis (Buser) Rothm. at different dates of harvest. Z. Naturforsch. C J. Biosci. 2012, 67, 529–540. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Skalicka-Woźniak, K.; Orhan, I.E.; Xiao, J.; Locatelli, M.; Piwowarski, J.P.; Granica, S.; Tomczyk, M. A comprehensive review of agrimoniin. Ann. N. Y. Acad. Sci. 2017, 1401, 166–180. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).