1. Introduction
Plant-parasitic nematode-caused disease creates tremendous losses in agricultural production [
1]. Root-knot nematodes (RKNs;
Meloidogyne spp.) are widely distributed in soil and can infect most higher plants [
2]. RKN larvae invade plant bodies through the root tips and induce root cells to differentiate into hypertrophic multinucleated giant cells, through which larvae obtain water and nutrients necessary to develop into adults [
3,
4,
5]. The formation of these giant cells seriously affects the structure and function of roots, as well as the metabolism and shoot growth of host crops [
6].
Physical, chemical, and biological methods have been used in attempts to control RKNs [
7,
8]. Chemical pesticides are common: for example, halogenated hydrocarbons such as methyl bromide were once used to kill nematodes by soil fumigation [
9]. However, since the residues of these compounds in agricultural products and the environment pose serious threats to human health, they have recently been banned [
10]. Alternatives of methyl bromide that are currently and effectively being used for nematode control include aluminum phosphide, methane iodide, carbon oxysulfide (COS), chlorpyrine-proprietary solvent, 1, 3-D-methylsodium, carbamate, and methyl isothiocyanate [
11,
12]. However, these methods lead residues of pesticides in agricultural products and accumulation in the soil, threatening food and environmental security [
13]. In addition, long-term use of pesticides may induce the resistance of nematodes to these chemicals [
14].
To control rhizomatous nematodes effectively and safely, biological methods are now being tested. For instance, the application of urea can inhibit or kill nematodes, with the inhibitory effect correlated with the activity of urease in soil [
15].
Aspergillus niger,
Xanthophorus,
Xanthophyore,
Bacillus subtilis, and
Pseudomonas fluorescein can also effectively control RKNs in rice plants [
16]. El-Atta et al. (2014) found that mites (
Sancassania (Caloglyphus)
berlesei (Michael)) reduced nematode abundance in soil by feeding on nematode eggs [
17]. Addition of chitin from marine shell powder can also effectively reduce nematode larvae and eggs in tomato roots and RKNs in the soil [
18]. A parasitic bacterium
Bacillus licheniformis pt361 can control RKNs in cucumber [
19]. Furthermore, the addition of
Trichoderma spp. TRI2 killed 72% of RKNs in cucumber planting soil [
20]. Nevertheless, many biological methods do not completely control nematode infestations [
21], the production process of some microbial agents can be complicated, the activity of microbial strains can be difficult to maintain, and the actual application effect in field is largely affected by soil properties [
22]. As such, these methods are currently insufficient for large-scale deployment.
Some physical methods have also been used to control RKNs, such as soil airing at high temperature [
23], addition of organic conditioners [
24], and soil fumigation by ozone (O
3) smog [
25]. Heating soil using steam or flame can effectively reduce RKN concentrations [
26,
27]. In another instance, red light supplementation at night improved tomato plants’ resistance to nematodes (
Meloidogyne javanica and
Meloidogyne incognita) [
28,
29]. Microwave irradiation can also reduce the occurrence rate of root knot in melons [
30]. Physical control methods are environmental-friendly but can be complicated and costly.
Research on RKN biology suggests that RKNs are sensitive to long-term high or low temperature [
31]. For instance, the half-lethal and lethal low temperatures of the second instar larvae of RKN (
Meloidogyne hapla) were −2.22 °C and −6 °C, respectively [
32]. Addition of dry ice to soil caused fast cooling and local high CO
2 concentration, low temperature, and hypoxia, which significantly repress nematode populations [
33,
34]. High temperatures can also substantially inhibit RKNs [
35]. Along with the temperature elevation, RKN egg hatching was strongly inhibited, and most second instar larvae died [
36]. Nematodes and their eggs were destroyed when soil temperature was kept at 40 °C for 10 days [
37]. If there is no host root system to invade, nematodes extend their lives as dormant eggs, which are more resistant to extreme high or low temperature [
38]. Once plant roots grow in the soil under suitable temperature, RKNs activate from dormant state and rapidly infect the host roots [
39]. Based on these habits, we designed a new method for controlling RKNs in soil. We planted water spinach in RKN-infested soil to activate the RKNs, after which point the soil was naturally heated by sunlight and then suddenly cooled via the addition of dry ice. We tested how effective this method was at eliminating RKNs.
2. Materials and Methods
2.1. Materials
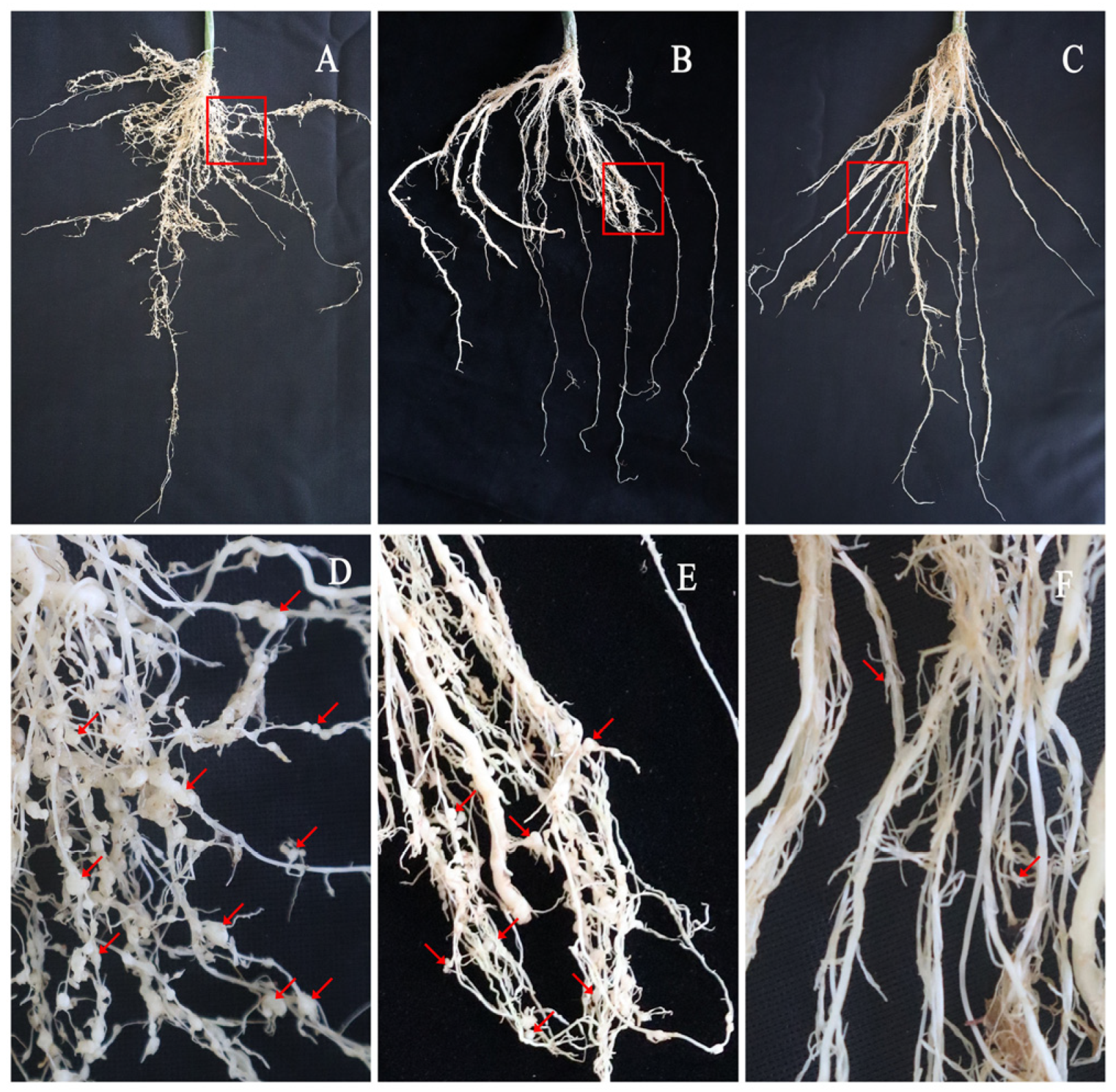
Soil was taken from a greenhouse in Hebei Yidong Agriculture and Animal Husbandry Technology Co., Ltd., Longyao, Hebei Province. In the previous year, cucumber plants grown in the soil had numerous root knots (
Figure S1). The soil samples were collected in May 2020. The experiment was conducted in a greenhouse in Shijiazhuang Academy of Agriculture and Forestry Sciences. The cucumber (
Cucumis sativus L.) and water spinach (
Ipomoea aquatica Forsk) cultivars used were Jinyou 301 and Gangzhong Dabaigu Tongxincai 310, respectively.
2.2. Temperature Switching Treatment
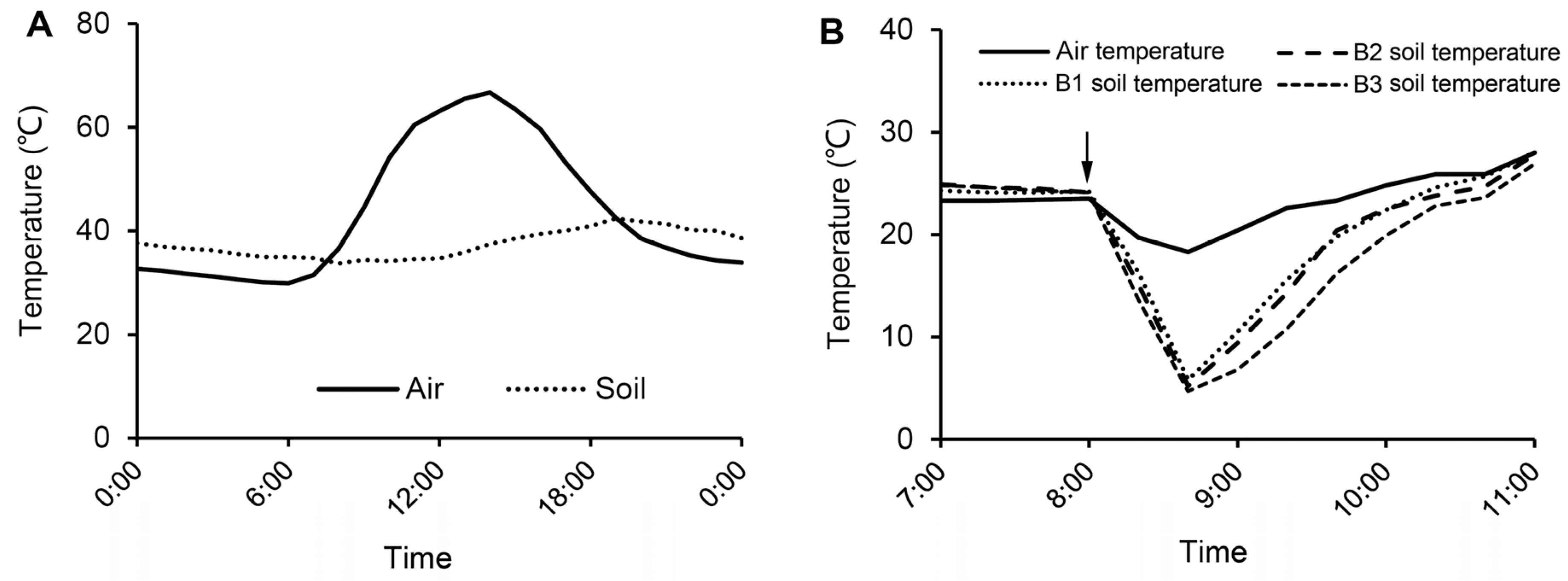
The experiment was conducted from 1 June to 25 September 2020. Soil samples were put into 20 × 25 cm plant pots, each containing 1.6 kg (dry weight) of soil. Five hundred plant pots were divided into group A (n = 250) and B (n = 250). In group A, no host plants were seeded. In group B, five water spinach seeds were sowed in each pot. The pots were irrigated once during the experimental process and the incidence of RKNs in water spinach roots was continuously monitored. When root knots appeared (50 days after sowing), 50 pots in each group were left as controls while the remainders were subject to the following treatments:
Over a three-day course of sunny days, 200 pots in groups A and B were heated by sunlight. Diurnal temperature dynamics of the air and soil was monitored using temperature sensors. After this heating treatment, 50 pots in each group were retained as controls while the rest were further treated.
On the 4th day, dry ice was added into high temperature treated soil. One hundred and fifty pots from group A or B were divided into three subgroups. Fifty, 80, or 110 g dry ice was added into each of 50 pots of the three subgroups, respectively (
Table 1). In each pot, 8–10 holes with diameter of 1.5 cm and depth of 10–15 cm were drilled. Dry ice was added into the holes and covered with soil. After 24 h, number of nematodes in root systems of water spinach roots in group B was measured. Soil in group A was used to measure soil physical and chemical properties and soil enzyme activities.
After the switched temperature treatment, the water spinach was removed from the experimental pots and cucumbers were planted in the soil in groups A and B. In each group, 50 plant pots were filled with the treated soil. In each pot, one cucumber seedling with at least three young leaves was transplanted. During the following 35 days, the growth and physiological parameters of the cucumbers were monitored.
2.3. Quantitative Evaluation of Nematodes in Roots
The Baermann funnel method was used to isolate root knot nematodes (
Meloidogyne incognita) [
40]. Water spinach or cucumber roots were washed with water and then cut into 0.5 cm segments. Ten gram root segments were ground in 20 mL deionized water. The homogenate was transferred into a funnel in which a piece of nematode filter paper was placed. A rubber tube was connected with the funnel and sealed with a clip. After 6 h, the clip was released and filtrate was collected. Twenty milliliters of deionized water was slowly added into the funnel to wash the homogenate; filtrate was collected after 6 h. The collected filtrate was centrifuged at 2000 rpm for 3 min. Sediment was re-suspended with 10 mL deionized water. Half a milliliter of suspension was transferred into a counting dish and observed with a microscope (E800i, Nikon, Japan), which equipped with an image acquisition system (DS-Fi2, Nikon, Japan), to count the number of nematode larvae (L2–L4). In each experiment, data were collected from at least three replicates.
2.4. Monitoring of Air and Soil Temperature
During the heating and cooling treatment, air temperature was monitored using a STARK TEC ST-WS05 sensor (Stark Tec, Handan, China), and soil temperature at a depth of 10–15 cm was monitored using a STARK TEC ST-TWS sensor (Stark Tec, Handan, China).
2.5. Detection of Soil Physical and Chemical Properties
Soil organic carbon, water-soluble carbon, total nitrogen, available nitrogen, available phosphorus, rapidly available potassium, pH value, electric conductivity (EC), and soil porosity were measured according to Bao [
41].
To measure organic carbon content, 0.1 g soil was transferred into a Kelvin tube, added 0.1 g silver sulfate powder, 5 mL 0.8 mol·L−1 potassium dichromate solution, and 5 mL concentrated sulfuric acid. The mixture was heated to boiling. After being boiled for 5 min, the mixture was cooled and diluted by adding 50 mL deionized water. Three drops of o-phenanthroline indicator was added into the solution, and ferrous sulfate titration was performed. Organic carbon content was calculated based on the consumption of ferrous sulfate.
To measure water-soluble carbon content, 10 g soil was mixed with 50 mL deionized water and stirred for 30 min. The suspension was then centrifuged at 5000 rpm for 20 min. The supernatant was filtered by using a microporous membrane (0.45 mm). The filtrate was detected by using a TOC analyzer (TOC-LCSH/CPH, Shimadzu, Japan).
To measure total nitrogen content, 0.5 g soil was transferred into a Kelvin tube, mixed with 5 mL concentrated sulfuric acid and heat-digested in a digester until the solution became light blue. The mixture was cooled and diluted by adding 30 mL deionized water. After adding 5 mL H3BO3 indicator solution and 20 mL 10 mol·L−1 NaOH solution, the mixture was distilled. Collected distillate (50 mL) was titrated by using 0.01 mol·L−1 HCl. Total nitrogen content was calculated based on the consumption of HCl.
To measure available nitrogen content, 2 g soil was mixed with 0.2 g FeSO4 powder and evenly spread into the outer chamber of a diffusion dish. 2 mL H3BO3 indicator solution was added into the inner chamber of the diffusion dish. After adding 10 mL 1.8 mol·L−1 NaOH solution into the outer chamber, the diffusion dish was quickly sealed with a glass cover and being incubated at 40 °C for 24 h. The solution in inner chamber was collected and titrated by using 0.01 mol·L−1 HCl. Available nitrogen content was calculated based on the consumption of HCl.
To measure available phosphorus content, 5 g soil was mixed with 100 mL 0.5 mol·L−1 NaHCO3 solution and being vibrated for 30 min. The suspension was filtered with a phosphorus-free filter paper. Ten milliliters of filtrate was mixed with 5 mL molybdenum antimony antichromogenic agent solution and 10 mL deionized water. After 30 min, the absorbance of the solution to 880 nm wavelength light was detected by using a spectrophotometer (722N, INESA, China). The absorbance was converted into concentration of reaction products based on the detected result of standard solution.
To measure rapidly available potassium content, 5 g soil was mixed with 25 mL 1 mol·L−1 NaOH solution. The mixture was vibrated for 5 min and then filtered with filter paper. Eight milliliters of filtrate was mixed with 1 mL formaldehyde-EDTA masking agent, 1 mL 3% sodium tetraphenylborate solution. After 15 min, the absorbance of the solution to 420 nm wavelength light was detected by using a spectrophotometer (722N, INESA, China). The absorbance was converted into concentration of reaction products based on the detected result of standard solution.
To measure soil pH value, 10 g soil was mixed with 25 mL 0.01 mol·L−1 CaCl2 solution and stirred for 2 min. Thirty minutes after, pH value of the suspension was detected using a pH meter (PHS-3C, INESA, Shanghai, China).
To measure soil EC value, 10 g soil was mixed with 50 mL CO2-free deionized water and sealed. The suspension was vibrated for 5 min and then filtered with filter paper. EC value of filtrate was detected by using an EC meter (DDSJ-319L, INESA, Shanghai, China).
Soil porosity was detected according to Zhang et al. [
42]. Soil was sampled by using a ring shear and transferred to an aluminum box. The soil was weighed and then dried in a 105 °C oven. Dry soil was weighed to determine water content. The soil porosity was calculated by using formula: soil total porosity = (1 − soil bulk density/soil density) × 100%. Soil bulk density = soil dry weight(g)/[ring shear volume(cm
3) × (1 + soil water content)].
2.6. Detection of Soil Enzymes’ Activity
Soil enzymes’ activity were assessed using specific kits, which were purchased from Keming Biotechnology Co. LTD., Suzhou, China.
To measure sucrase activity, 5 g soil was mixed with 0.25 mL methylbenzene, 5 mL 8% sucrose solution, and 5 mL phosphate buffer solution (pH5.5). After being incubated at 37 °C for 24 h, the mixture was centrifuged at 2000 rpm for 5 min. 1 mL supernatant was mixed with 3 mL 0.5% 3,5-dinitrosalicylic acid solution and being heated in boiling water bath for 5 min. The absorbance of the solution to 508 nm wavelength light was detected.
To measure dehydrogenase activity, 5 g soil was mixed with 5 mL reaction buffer, which consists of 0.5% TTC (2,3,5-triphenyltetrazolium chloride) and 0.5% glucose, and was incubated at 37 °C for 24 h. After adding 5 mL methanol, the mixture was centrifuged at 2000 rpm for 5 min. The absorbance of the supernatant to 485 nm wavelength light was detected.
To measure urease activity, 5 g soil was mixed with 1 mL methylbenzene, 10 mL 10% urea solution, and 20 mL citrate buffer (pH6.8), and incubated at 37 °C for 24 h. The mixture was centrifuged at 2000 rpm for 5 min. One milliliter of supernatant was mixed with 4 mL 1.35 mol·L−1 sodium phenolate and 3 mL 1% sodium hypochlorite. After 20 min, the absorbance of the solution to 578 nm wavelength light was detected.
To measure neutral phosphatase activity, 5 g soil was mixed with 0.25 mL methylbenzene, 20 mL 0.5% disodium phenyl phosphate solution, and being incubated at 37 °C for 2 h. The mixture was centrifuged at 2000 rpm for 5 min. Five milliliters supernatant was mixed with 0.5 mL 2% 4-aminoantipyrine solution, 0.5 mL 8% potassium ferricyanide solution. After 15 min, the absorbance of the solution to 510 nm wavelength light was detected.
To measure neutral protease activity, 5 g soil was mixed with 25 mL 50 mM Tris-HCl buffer (pH6.8), 25 mL 2% casein solution, and being incubated at 40 °C for 2 h. After adding 25 mL 15% trichloroacetic acid solution, the mixture was vibrated and then centrifuged at 2000 rpm for 5 min. 5 mL supernatant was mixed with 7.5 mL solution consisting of 5% NaOH-Na2CO3, 0.01% CuSO4, 0.02% potassium sodium tartrate, and 5 mL 33% Folin reagent. After 1 h, the absorbance of the solution to 700 nm wavelength light was detected.
Absorbance of above solution was detected by using a microplate reader (Cytation 1, Biotek, Germany) and converted into concentration of reaction products based on the detected result of standard solution.
2.7. Detection of Growth and Physiological Parameters of Cucumber Plants
Thirty-five days after transplanting, plant height, stem diameter, and leaf number of cucumber plants were measured. In each experiment, the parameters of at least 20 plants were measured; data from three replicates were calculated and analyzed.
A photosynthometer (1102G, Yaxin-Li Instruments Co. Beijing, China) was used to measure net photosynthetic rate, transpiration rate, intercellular CO2 content, and stomatal conductance of the cucumber plants. Cucumber chlorophyll content (SPAD value) was measured using SPAD-502 chlorophyll analyzer (Konica Minolta, Japan).
2.8. Data Processing and Statistical Analysis
Statistical analysis of the data was performed using Microsoft Office Excel 2021 and IBM SPSS Statistics 22.0 software (IBM, New York, NY, USA). All results were presented as the mean ± SD of at least three replicates. Data were statistically evaluated by analysis of variance (one-way or two-way ANOVA), and the mean values were compared by Duncan’s multiple comparisons. The Kolmogorov–Smirnov test was used to inspect the normality and homogeneity of variance of all the data. If the data did not satisfy the homogeneity of variance and the normality distribution, the logarithmic function transformation was performed before multiple comparison. Significant differences between different treatments were determined at p < 0.05 levels.
4. Discussion
RKNs are active in aqueous environments or in proper structural soil particles that have a thin water layer on the surface [
43]. They can also invade living cells of host plants [
44]. Soil temperature, humidity, and ventilation strongly affect the growth of RKNs [
45]. Secreted plant hormones, including auxin, ethylene, and mitogen, are essential for inducing the invasion of RKNs [
46].
High-temperature treatment and chemical elimination are methods commonly used to eliminate RKNs. Low temperature has also been used for elimination of nematodes [
33,
34,
47]. However, the temperature resistance of RKNs varies across species [
48]. The high temperature treatment method was designed according to the intolerance of RKNs to high temperatures, coupled with the high-temperature conversion of nitrogen-containing organic compounds in soil into volatile compounds such as ammonia, which may also kill RKNs [
49,
50,
51].
The activation of RKNs using host plants and the elimination of RKNs using switched temperature treatment were not used in previous reports [
13,
52]. Herein, water spinach was selected to activate RKNs based on its wide temperature tolerance range [
53] and excellent ability for activating RKNs [
54]. Our method also considers the biological characteristics of RKNs, specifically, their sensitivity to extreme temperature switching [
55]. Our results showed that active RKNs were strongly suppressed by sustained high temperature and further destroyed by sudden cooling. The combination of high temperature and low temperature treatment killed most of the RKNs in the soil. This method is simple, low cost, and effective for controlling soil nematodes in small batches of facility soil.
It has been reported that high-temperature treatments reduce alkali-hydrolyzed nitrogen and available phosphorus content in soil and an increase potassium content and soil EC values [
56,
57]. Consistent with these reports, we found that soil-available K content, EC, and porosity increased after temperature change treatment, while the contents of organic matter, total nitrogen, and alkali-hydrolyzed nitrogen were not altered. Soil enzyme activity is related to soil moisture, temperature, and ventilation [
58,
59]. We found that soil sucrase activity was enhanced after temperature switching, while the activity of other enzymes increased slightly as well. These data indicate that the switched temperature treatment had no adverse effects on soil physicochemical properties and soil enzyme activities, while benefiting soil nutrient content and porosity.
The roots of most crops grown in tropical or subtropical regions can be invaded by RKNs [
60]. In a preliminary work, we noticed some cucumber plants attacked by serious root-knot nematode disease (
Figure S1). Hence, the soils were collected and used for this research. In preliminary work, soil samples were directly heated and cooled. However, nematodes were not effectively eliminated. Moving forward, we tested the method of host plant induction and switched temperature treatment. In treated soil, growth and physiological parameters of cucumber plants were much better than those in control soils, together with substantially decreased numbers of root knots and RKNs in root systems. From these results, it can be inferred that there is a negative correlation between the number of root knot nematodes and the growth and development state of cucumber plants. That is, that the variation of growth and physiological parameter possibly results from variation of root knot occurrence. A healthy root system is essential for nutrient absorption and hormone metabolism, which in turn is crucial for plant growth and development. Cucumber plants were grown in the treated soil for 35 days, during which time RKN disease did not occur. As such, we suggest that this may be a long-term RKN control method.
A variety of physical, chemical, and biological methods have been developed for RKN control, including breeding resistant cultivars [
61], developing microbial pesticides [
62], searching for RKN-inhibitory fungi [
63], developing novel chemical agents [
64], and exposing soil to natural sunlight [
65]. However, these methods have drawbacks such as long development cycles, high costs, and substantial side effects on the soil, as well as unsatisfactory control effects. Here, switched temperature treatment in RKN-activated soil significantly reduced the number of RKNs in the soil. The dry ice had no adverse effects on the soil and can be used as a photosynthetic resource. As such, this method is low-cost and environmentally friendly. As RKN reproduction may take 25 to 30 days [
66], this method can be used to treat the soil before each round of vegetable planting to control RKN disease in facility crops.