Nitric Oxide Is Essential to Keep the Postharvest Quality of Fruits and Vegetables
Abstract
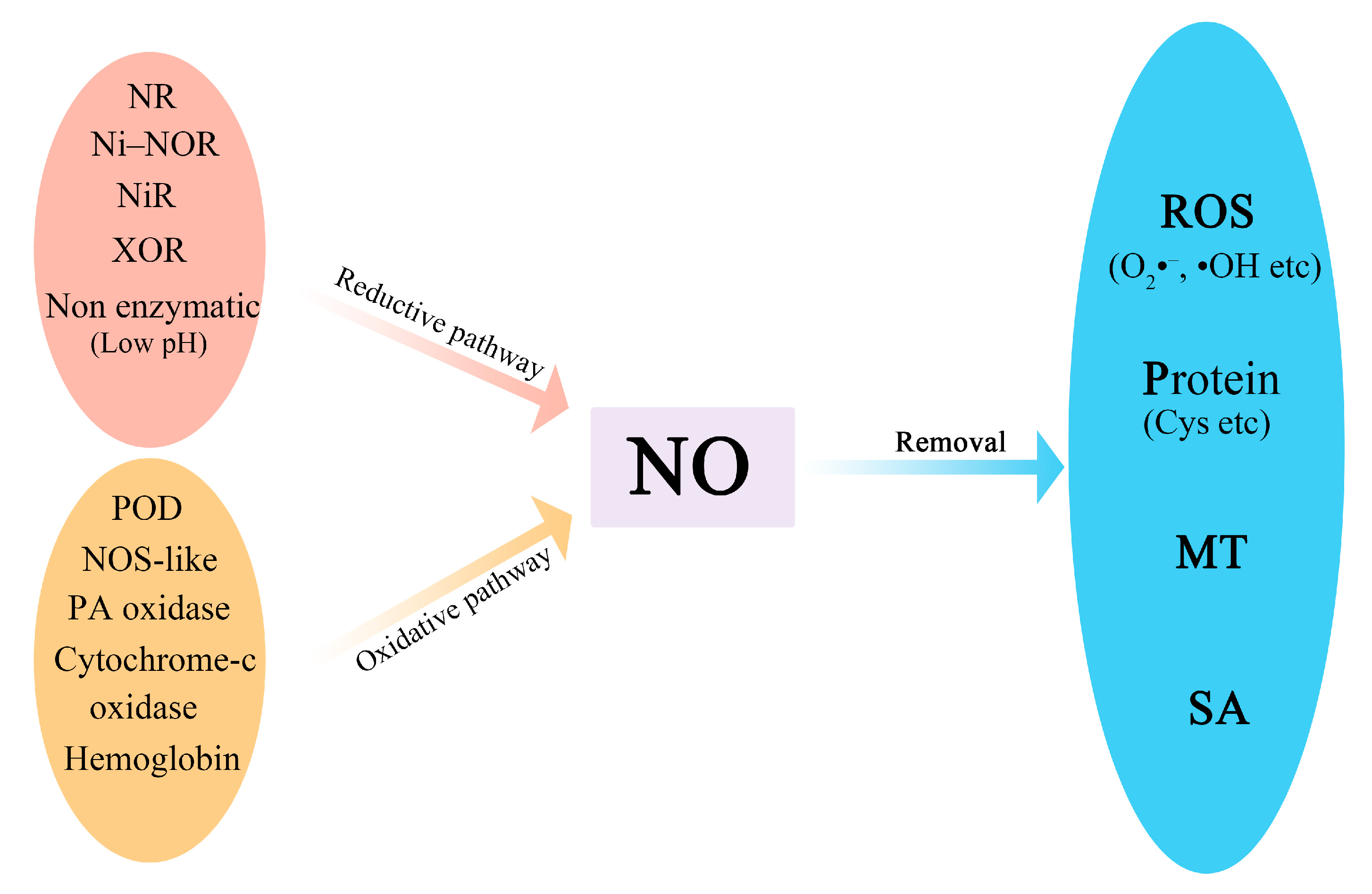
1. Introduction
2. Effects of NO on Fruit Ripening
3. NO Can Enhance the Defense of Fruits and Vegetables against Chilling Injury
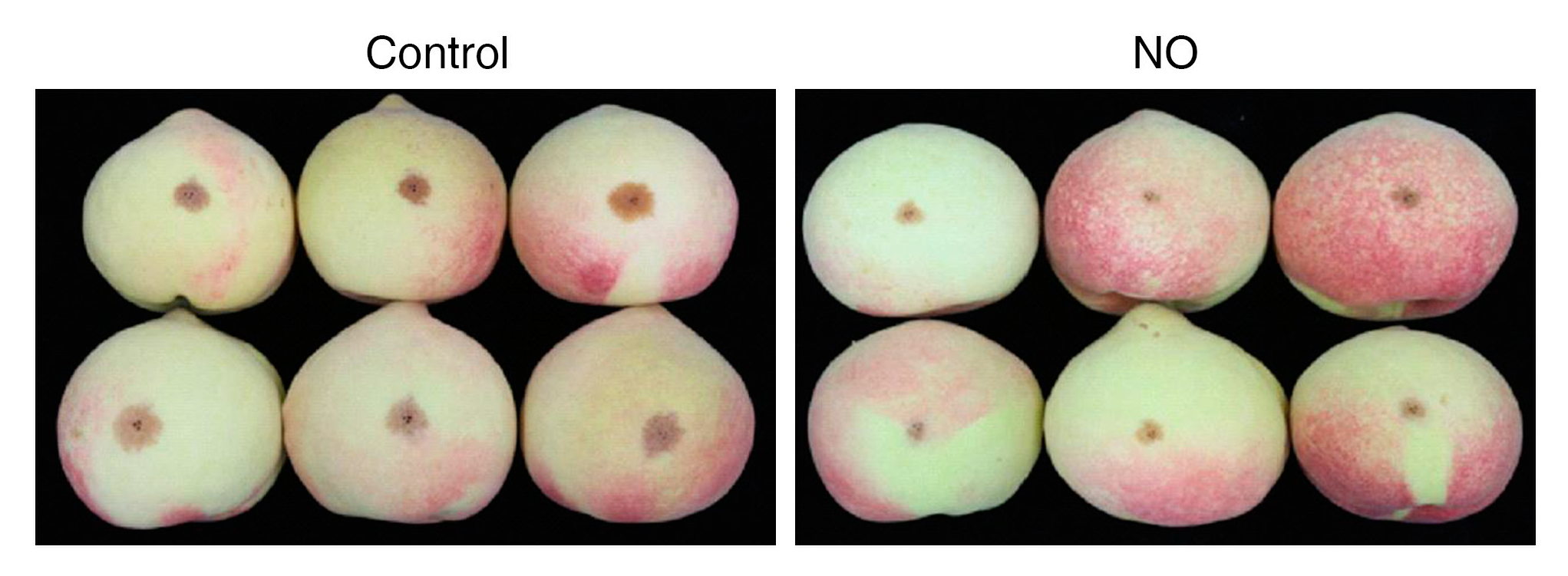
4. Effects of NO on Disease Resistance and Pest Control after Harvest
5. Effects of NO on Browning
6. The Application Methods of NO
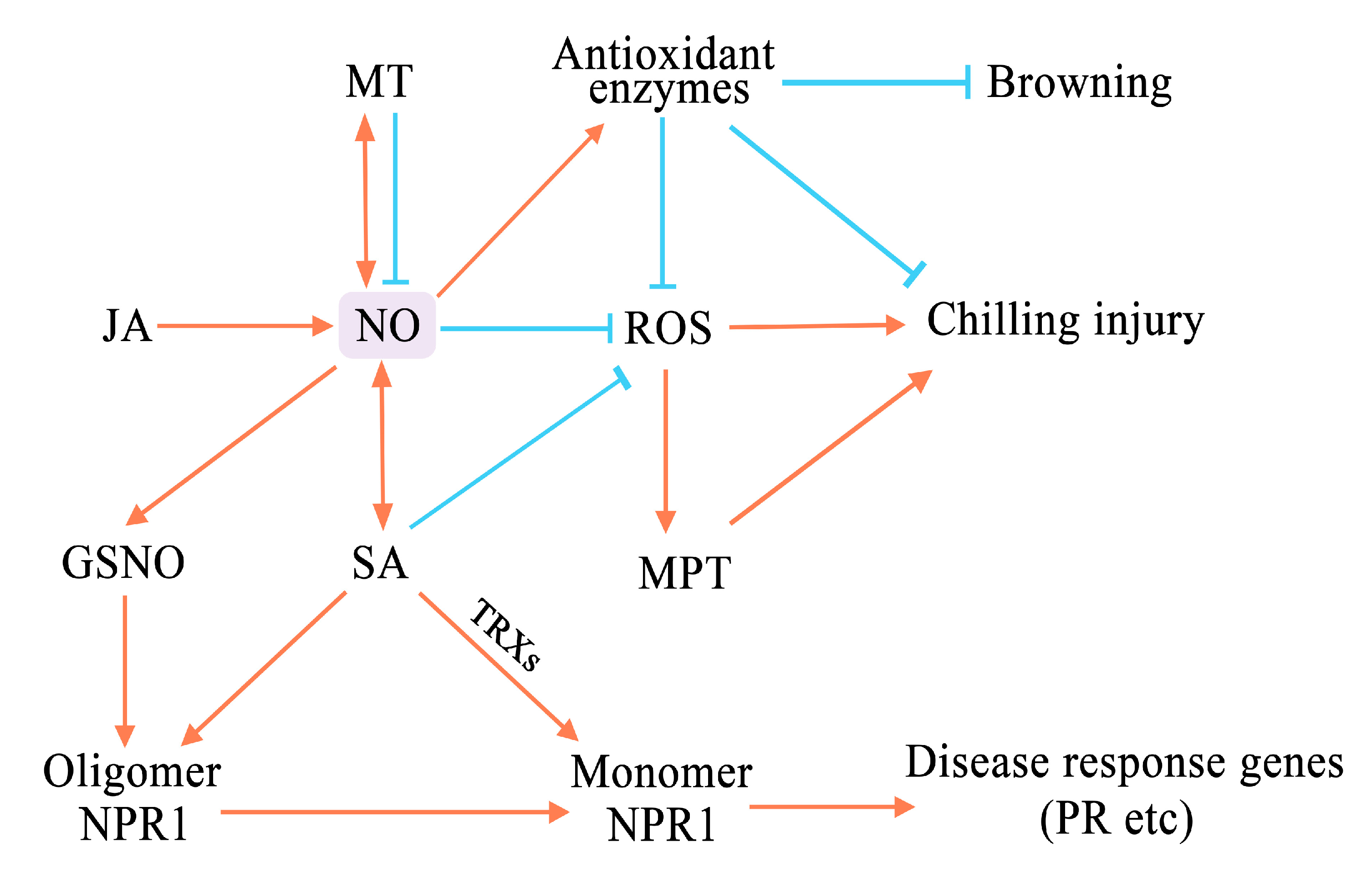
7. Crosstalk between NO and Phytohormones in Fruits and Vegetables
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, T.; Qin, G.Z.; Tian, S.P. Regulatory network of fruit ripening: Current understanding and future challenges. New Phytol. 2020, 228, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, Y.; Nicolas, P.; Fernandez-Pozo, N.; Ma, Q.Y.; Evanich, D.J.; Shi, Y.N.; Xu, Y.M.; Zheng, Y.; Snyder, S.I.; Martin, L.B.B.; et al. High-resolution spatiotemporal transcriptome mapping of tomato fruit development and ripening. Nat. Commun. 2018, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Foresi, N.; Mayta, M.L.; Lodeyro, A.F.; Scuffi, D.; Correa-Aragunde, N.; Garcia-Mata, C.; Casalongue, C.; Carrillo, N.; Lamattina, L. Expression of the tetrahydrofolate-dependent nitric oxide synthase from the green alga Ostreococcus tauri increases tolerance to abiotic stresses and influences stomatal development in Arabidopsis. Plant J. 2015, 82, 806–821. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.V.; Harper, J.E. The Conversion of Nitrite to Nitrogen Oxide(s) by the Constitutive NAD(P)H-Nitrate Reductase Enzyme from Soybean. Plant Physiol. 1988, 88, 389–395. [Google Scholar] [CrossRef]
- Yamasaki, H.; Sakihama, Y. Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: In vitro evidence for the NR-dependent formation of active nitrogen species. Febs Lett. 2000, 468, 89–92. [Google Scholar] [CrossRef]
- Salgado, I.; Martinez, M.C.; Oliveira, H.C.; Frungillo, L. Nitric oxide signaling and homeostasis in plants: A focus on nitrate reductase and S-nitrosoglutathione reductase in stress-related responses. Braz. J. Bot. 2013, 36, 89–98. [Google Scholar] [CrossRef]
- He, H.Y.; He, L.F. Crosstalk between melatonin and nitric oxide in plant development and stress responses. Physiol. Plant. 2020, 170, 218–226. [Google Scholar] [CrossRef]
- Rockel, P.; Strube, F.; Rockel, A.; Wildt, J.; Kaiser, W.M. Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J. Exp. Bot. 2002, 53, 103. [Google Scholar] [CrossRef]
- Bethke, P.C.; Jones, B. Apoplastic Synthesis of Nitric Oxide by Plant Tissues. Plant Cell. 2004, 16, 332–341. [Google Scholar] [CrossRef]
- Moreau, M.; Lindermayr, C.; Durner, J.; Klessig, D.F. NO synthesis and signaling in plants—Where do we stand? Physiol. Plant. 2010, 38, 372–383. [Google Scholar] [CrossRef]
- Santolini, J.; André, F.; Jeandroz, S.; Wendehenne, D. Nitric oxide synthase in plants: Where do we stand? Nitric Oxide 2017, 63, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Alber, N.A.; Sivanesan, H.; Vanlerberghe, G.C. The occurrence and control of nitric oxide generation by the plant mitochondrial electron transport chain. Plant Cell Environ. 2017, 40, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.J.; Igamberdiev, A.U.; Manjunatha, G.; Segu, S.; Moran, J.F.; Neelawarne, B.; Bauwe, H.; Kaiser, W.M. The emerging roles of nitric oxide (NO) in plant mitochondria. Plant Sci. 2011, 181, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.K.; Prommer, J.; Watanabe, M. Endogenous nitric oxide generation in protoplast chloroplasts. Plant Cell Rep. 2013, 32, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Praveen, K.; Kumar, T.R.; Nand, S.P. Sodium nitroprusside-mediated alleviation of iron deficiency and modulation of antioxidant responses in maize plants. Aob Plants 2010, 2010, plq002. [Google Scholar]
- Mukherjee, S. Insights into nitric oxide-melatonin crosstalk and N-nitrosomelatonin functioning in plants. J. Exp. Bot. 2019, 70, 6035–6047. [Google Scholar] [CrossRef]
- Grozeff, G.; Alegre, M.L.; Senn, M.E.; Chaves, A.R.; Bartoli, C.G. Combination of nitric oxide and 1-MCP on postharvest life of the blueberry (Vaccinium spp.) fruit. Postharvest Biol. Technol. 2017, 133, 72–80. [Google Scholar] [CrossRef]
- Steelbeart, C.; Alegre, M.L.; Bahima, J.V.; Senn, M.E.; Grozeff, G. Nitric oxide improves the effect of 1-methylcyclopropene extending the tomato (Lycopersicum esculentum L.) fruit postharvest life. Sci. Hortic. 2019, 255, 193–201. [Google Scholar] [CrossRef]
- Shi, K.K.; Liu, Z.C.; Wang, J.W.; Zhu, S.H.; Huang, D.D. Nitric oxide modulates sugar metabolism and maintains the quality of red raspberry during storage. Sci. Hortic. 2019, 256, 108611. [Google Scholar] [CrossRef]
- Chaki, M.; Paz Morales, A.; Ruiz, C.; Begara-Morales, J.C.; Barroso, J.B.; Corpas, F.J.; Palma, J.M. Ripening of pepper (Capsicum annuum) fruit is characterized by an enhancement of protein tyrosine nitration. Ann. Bot. 2015, 116, 637–647. [Google Scholar] [CrossRef]
- Gonzalez-Gordo, S.; Bautista, R.; Claros, M.G.; Caas, A.; Palma, J.M.; Corpas, F.J. Nitric oxide-dependent regulation of sweet pepper fruit ripening. J. Exp. Bot. 2019, 70, 4557–4570. [Google Scholar] [CrossRef]
- Rodriguez-Ruiza, M.; Mateos, R.M.; Codesido, V.; Corpas, F.J.; Palma, J.M. Characterization of the galactono-1,4-lactone dehydrogenase from pepper fruits and its modulation in the ascorbate biosynthesis. Role of nitric oxide. Redox Biol. 2019, 12, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.P. Effect of Nitric Oxide on Ethylene Synthesis and Softening of Banana Fruit Slice during Ripening. J. Agric. Food Chem. 2009, 13, 5799–5804. [Google Scholar] [CrossRef] [PubMed]
- Li, X.P.; Wu, B.; Guo, Q.; Wang, J.D.; Zhang, P.; Chen, W.X. Effects of Nitric Oxide on Postharvest Quality and Soluble Sugar Content in Papaya Fruit during Ripening. J. Food Process. Preserv. 2014, 38, 1–9. [Google Scholar] [CrossRef]
- Hao, Y.Q.; Chen, F.H.; Wu, G.B.; Gao, W.Y. Impact of Postharvest Nitric Oxide Treatment on Lignin Biosynthesis-Related Genes in Wax Apple (Syzygium samarangense) Fruit. J. Agric. Food Chem. 2016, 64, 8483–8490. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, Y.Y.; Liu, C.; Chen, L.; Ma, J.F.; Sheng, J.P.; Shen, L. Inhibition of nitric oxide synthesis delayed mature-green tomato fruits ripening induced by inhibition of ethylene. Sci. Hortic. 2016, 211, 95–101. [Google Scholar] [CrossRef]
- Zhu, L.Q.; Du, H.Y.; Wang, W.; Zhang, W.; Shen, Y.G.; Wan, C.P.; Chen, J.Y. Synergistic effect of nitric oxide with hydrogen sulfide on inhibition of ripening and softening of peach fruits during storage. Sci. Hortic. 2019, 256, 108591. [Google Scholar] [CrossRef]
- Qi, X.H.; Ji, Z.J.; Lin, C.; Li, S.F.; Liu, J.; Kan, J.; Zhang, M.; Jin, C.H.; Qian, C.L. Nitric oxide alleviates lignification and softening of water bamboo ( zizania latifolia ) shoots during postharvest storage. Food Chem. 2020, 332, 127416. [Google Scholar] [CrossRef]
- Zaharah, S.S.; Singh, Z. Mode of action of nitric oxide in inhibiting ethylene biosynthesis and fruit softening during ripening and cool storage of ‘Kensington Pride’ mango. Postharvest Biol. Technol. 2011, 62, 258–266. [Google Scholar] [CrossRef]
- Deng, L.L.; Pan, X.Q.; Chen, L.; Lin, S.; Sheng, J.P. Effects of preharvest nitric oxide treatment on ethylene biosynthesis and soluble sugars metabolism in ‘Golden Delicious’ apples. Postharvest Biol. Technol. 2013, 84, 9–15. [Google Scholar] [CrossRef]
- Zhu, S.H.; Jie, Z. Effect of nitric oxide on ethylene production in strawberry fruit during storage. Food Chem. 2007, 100, 1517–1522. [Google Scholar] [CrossRef]
- Wu, F.H.; Yang, H.; Chang, Y.; Cheng, J.; Bai, S.; Yin, J. Effects of nitric oxide on reactive oxygen species and antioxidant capacity in Chinese Bayberry during storage. Sci. Hortic. 2012, 135, 106–111. [Google Scholar] [CrossRef]
- De Paepe, A.; Van Der Straeten, D. Ethylene biosynthesis and signaling: An overview. Vitam. Horm. 2005, 72, 399–430. [Google Scholar] [PubMed]
- Manjunatha, G.; Lokesh, V.; Neelwarne, B. Nitric oxide in fruit ripening: Trends and opportunities. Biotechnol. Adv. 2010, 28, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Rafael, Z.; Marta, R.; Patricia, J.; Grazieli, B.P.; Sonia, C.S.A.; Claudia, M.F.; Eduardo, P.; Jose, M.P.; Corpas, F.J.; Magdalena, R.; et al. Multifaceted roles of nitric oxide in tomato fruit ripening: NO-induced metabolic rewiring and consequences for fruit quality traits. J. Exp. Bot. 2020, 3, 3. [Google Scholar]
- Zhu, S.; Liu, M.; Zhou, J. Inhibition by nitric oxide of ethylene biosynthesis and lipoxygenase activity in peach fruit during storage. Postharvest Biol. Technol. 2006, 42, 41–48. [Google Scholar] [CrossRef]
- Ye, J.B.; Chen, F.H.; Wu, G.B. Effect of nitric oxide on physiology and quality of postharvest wax apple(Syzygium samarangense Merr et Perry) Fruits. J. Jim Univ. 2012, 17, 180–185. [Google Scholar]
- Wang, B.; Li, Z.; Han, Z.; Xue, S.; Prusky, D. Effects of nitric oxide treatment on lignin biosynthesis and texture properties at wound sites of muskmelons. Food Chem. 2021, 362, 130193. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, C.; Wu, F.; Cheng, J. Effect of nitric oxide on browning and lignification of peeled bamboo shoots. Postharvest Biol. Technol. 2010, 57, 72–76. [Google Scholar] [CrossRef]
- Zhang, S.Q. Effects of nitric oxide on post-harvest physiology and quality of green asparagus. Chin. Agric. Sci. Bull. 2010, 26, 77–81. [Google Scholar] [CrossRef]
- Ren, Y.F.; He, J.; Liu, H.; Liu, G.; Ren, X. Nitric oxide alleviates deterioration and preserves antioxidant properties in ‘Tainong’ mango fruit during ripening. Hortic. Environ. Biotechnol. 2017, 58, 27–37. [Google Scholar] [CrossRef]
- Zheng, X.; Hu, B.; Song, L.; Jie, P.; Liu, M. Changes in quality and defense resistance of kiwifruit in response to nitric oxide treatment during storage at room temperature. Sci. Hortic. 2017, 222, 187–192. [Google Scholar] [CrossRef]
- Wang, Y.S.; Luo, Z.; Khan, Z.U.; Mao, L.; Ying, T. Effect of nitric oxide on energy metabolism in postharvest banana fruit in response to chilling stress. Postharvest Biol. Technol. 2015, 108, 21–27. [Google Scholar] [CrossRef]
- Wu, B.; Guo, Q.; Li, Q.; Ha, Y.; Chen, W. Impact of postharvest nitric oxide treatment on antioxidant enzymes and related genes in banana fruit in response to chilling tolerance. Postharvest Biol. Technol. 2014, 92, 157–163. [Google Scholar] [CrossRef]
- Zhang, T.; Che, F.B.; Zhang, H.; Pan, Y.; Xu, M.Q.; Ban, Q.Y.; Han, Y.; Rao, J.P. Effect of nitric oxide treatment on chilling injury, antioxidant enzymes and expression of the CmCBF1 and CmCBF3 genes in cold-stored Hami melon (Cucumis melo L) fruit. Postharvest Biol. Technol. 2017, 127, 88–98. [Google Scholar] [CrossRef]
- Jing, G.; Zhou, J.; Zhu, S. Effects of nitric oxide on mitochondrial oxidative defence in postharvest peach fruits. J. Sci. Food Agric. 2016, 96, 1997–2003. [Google Scholar] [CrossRef]
- Venkatachalam, K. Exogenous nitric oxide treatment impacts antioxidant response and alleviates chilling injuries in longkong pericarp. Sci. Hortic. 2018, 237, 311–317. [Google Scholar] [CrossRef]
- Rehman, M.; Singh, Z.; Khurshid, T. Nitric oxide fumigation alleviates chilling injury and regulates fruit quality in sweet orange stored at different cold temperatures. Aust. J. Crop Sci. 2019, 13, 1975–1982. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, J.; Chen, Y.; Wei, J.; Wu, B. Nitric oxide treatment maintains postharvest quality of table grapes by mitigation of oxidative damage. Postharvest Biol. Technol. 2019, 152, 9–18. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox Regulation in Photosynthetic Organisms: Signaling, Acclimation, and Practical Implications. Antioxid. Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Correa-Aragunde, N.; Foresi, N.; Lamattina, L. Nitric oxide is a ubiquitous signal for maintaining redox balance in plant cells: Regulation of ascorbate peroxidase as a case study. J. Exp. Bot. 2015, 66, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.J.; Dong, J.; Zhang, M.; Xu, X.; Sun, L. Cold-induced endogenous nitric oxide generation plays a role in chilling tolerance of loquat fruit during postharvest storage. Postharvest Biol. Technol. 2012, 65, 5–12. [Google Scholar] [CrossRef]
- Hu, H.Q.; Zhou, Z.B.; Sun, X.X.; Zhang, Z.H.; Meng, Q.H. Protective Effect of Nitric Oxide (NO) against Oxidative Damage in Larix gmelinii Seedlings under Ultraviolet-B Irradiation. Forests 2016, 7, 1999–4907. [Google Scholar] [CrossRef]
- Tossi, V.; Amenta, M.; Lamattina, L.; Cassia, R. Retraction: Nitric oxide enhances plant ultraviolet-B protection up-regulating gene expression of the phenylpropanoid biosynthetic pathway (vol 34, pg 909, 2011). Plant Cell Environ. 2018, 41, 704. [Google Scholar] [CrossRef]
- Liu, H.; Song, L.L.; You, Y.L.; Li, Y.B.; Duan, X.W.; Jiang, Y.M.; Joyce, D.C.; Ashraf, M.; Lu, W.J. Cold storage duration affects litchi fruit quality, membrane permeability, enzyme activities and energy charge during shelf time at ambient temperature. Postharvest Biol. Technol. 2011, 60, 24–30. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, C.; Cheng, S.; Wei, B.; Liu, X.; Ji, S. Changes in energy metabolism accompanying pitting in blueberries stored at low temperature. Food Chem. 2014, 164, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Hong, Z.; Lei, W.; Shan, T.M.; Zheng, Y.H. Oxalic acid alleviates chilling injury in peach fruit by regulating energy metabolism and fatty acid contents. Food Chem. 2014, 161, 87–93. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Chen, T.; Li, B.Q.; Qin, G.Z.; Tian, S.P. Molecular basis of pathogenesis of postharvest pathogenic Fungi and control strategy in fruits: Progress and prospect. Mol. Hortic. 2021, 1, 2. [Google Scholar] [CrossRef]
- Khan, M.N.; Mobin, M.; Mohammad, F.; Corpas, F.J. Nitric Oxide Action in Abiotic Stress Responses in Plants; Springer International Publishing: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Yu, Z.F.; Cao, J.X.; Zhu, S.H.; Zhang, L.L.; Peng, Y.; Shi, J.Y. Exogenous nitric oxide enhances disease resistance by nitrosylation and inhibition of s-nitrosoglutathione reductase in peach fruit. Front. Plant Sci. 2020, 11, 543. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M. Exogenous application of nitric oxide promotes growth and oxidative defense system in highly boron stressed tomato plants bearing fruit. Sci. Hortic. 2015, 185, 43–47. [Google Scholar] [CrossRef]
- Shi, J.Y.; Liu, N.; Gu, R.X.; Zhu, L.Q.; Zhang, C.; Wang, Q.G.; Lei, Z.H.; Liu, Y.Y.; Ren, J.Y. Signals induced by exogenous nitric oxide and their role in controlling brown rot disease caused by Monilinia fructicola in postharvest peach fruit. J. Gen. Plant Pathol. 2015, 81, 68–76. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Li, S.M.; Zeng, K.F. Exogenous nitric oxide-induced postharvest disease resistance in citrus fruit to Colletotrichum gloeosporioides. J. Sci. Food Agric. 2016, 96, 505–512. [Google Scholar] [CrossRef]
- Hu, M.J.; Zhu, Y.Y.; Liu, G.S.; Gao, Z.Y.; Zhang, Z.K. Inhibition on anthracnose and induction of defense response by nitric oxide in pitaya fruit. Sci. Hortic. 2019, 245, 224–230. [Google Scholar] [CrossRef]
- Hu, M.; Yang, D.; Huber, D.J.; Jiang, Y.; Li, M.; Gao, Z.; Zhang, Z. Reduction of postharvest anthracnose and enhancement of disease resistance in ripening mango fruit by nitric oxide treatment. Postharvest Biol. Technol. 2014, 97, 115–122. [Google Scholar] [CrossRef]
- Yan, B.W.; Zhang, Z.; Zhang, P.; Zhu, X.; Jing, Y.Y.; Wei, J.; Wu, B. Nitric oxide enhances resistance against black spot disease in muskmelon and the possible mechanisms involved. Sci. Hortic. 2019, 256, 108650. [Google Scholar] [CrossRef]
- Li, G.J.; Zhu, S.H.; Wu, W.X.; Zhang, C.; Peng, Y.; Wang, Q.G.; Shi, J.Y. Exogenous nitric oxide induces disease resistance against Monilinia fructicola through activating the phenylpropanoid pathway in peach fruit. J. Sci. Food Agric. 2016, 97, 3030–3038. [Google Scholar] [CrossRef]
- Dong, J.F.; Zhang, M.; Lu, L.; Sun, L.N.; Xu, M.J. Nitric oxide fumigation stimulates flavonoid and phenolic accumulation and enhances antioxidant activity of mushroom. Food Chem. 2012, 135, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Y.; Yang, Y.; Liu, C.; Chen, L.; Sheng, J.P.; Shen, L. Inhibition of SlMPK1, SlMPK2, and SlMPK3 Disrupts Defense Signaling Pathways and Enhances Tomato Fruit Susceptibility to Botrytis cinerea. J. Agric. Food Chem. 2015, 63, 5509–5517. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, S. MAPK Cascades in Plant Disease Resistance Signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef]
- Zheng, Y.; Hong, H.; Chen, L.; Li, J.; Shen, L. LeMAPK1, LeMAPK2, and LeMAPK3 are associated with nitric oxide-induced defense response against Botrytis cinerea in the Lycopersicon esculentum fruit. J. Agric. Food Chem. 2014, 62, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.B. Nitric oxide fumigation for control of western flower thrips and its safety to postharvest quality of fresh fruit and vegetables. J. Asia-Pac. Entomol. 2016, 19, 1191–1195. [Google Scholar] [CrossRef]
- Yang, X.B.; Liu, Y.B. Residual analysis of nitric oxide fumigation on fresh fruit and vegetables. Postharvest Biol. Technol. 2017, 132, 105–108. [Google Scholar] [CrossRef]
- Huque, R.; Wills, R.; Pristijono, P.; Golding, J.B. Effect of nitric oxide (NO) and associated control treatments on the metabolism of fresh-cut apple slices in relation to development of surface browning. Postharvest Biol. Technol. 2013, 78, 16–23. [Google Scholar] [CrossRef]
- Pristijono, P.; Wills, R.; Golding, J.B. Inhibition of browning on the surface of apple slices by short term exposure to nitric oxide (NO) gas. Postharvest Biol. Technol. 2006, 42, 256–259. [Google Scholar] [CrossRef]
- Wills, R.B.H.; Pristijono, P.; Golding, J.B. Browning on the surface of cut lettuce slices inhibited by short term exposure to nitric oxide (NO). Food Chem. 2008, 107, 1387–1392. [Google Scholar] [CrossRef]
- Zhu, L.Q.; Zhou, J.; Zhu, S.H.; Guo, L.H. Inhibition of browning on the surface of peach slices by short-term exposure to nitric oxide and ascorbic acid. Food Chem. 2009, 144, 174–179. [Google Scholar]
- Shi, J.Y.; Li, J.X.; Zhu, S.H.; Zhou, J. Browning inhibition on fresh-cut chestnut kernel by exogenous nitric oxide. Int. J. Food Sci. Technol. 2011, 46, 944–950. [Google Scholar] [CrossRef]
- Huque, R.; Wills, R.B.H.; Golding, J.B. Nitric oxide inhibits cut-surface browning in four lettuce types. J. Hortic. Sci. Biotechnol. 2011, 86, 97–100. [Google Scholar] [CrossRef]
- Golding, J.B.; Huque, R.; Pristijono, P.; Wills, R. Efficacy of NO treatment to inhibit browning on fresh cut lettuce types. Acta Hortic. 2013, 1012, 933–938. [Google Scholar] [CrossRef]
- Steffens, C.A.; Santana, G.; Amarante, C.; Antonovviski, J.L.; Fenili, C.L. Treatment with nitric oxide in controlled atmosphere storage to preserve the quality of ‘Laetitia’ plums. Lwt-Food Sci. Technol. 2022, 158, 113033. [Google Scholar] [CrossRef]
- Barman, K.; Siddiqui, M.W.; Patel, V.B.; Prasad, M. Nitric oxide reduces pericarp browning and preserves bioactive antioxidants in litchi. Sci. Hortic. 2014, 171, 71–77. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Kakavand, F.; Rabiei, V.; Zaare-Nahandi, F.; Razavi, F. γ-Aminobutyric acid and nitric oxide treatments preserve sensory and nutritional quality of cornelian cherry fruits during postharvest cold storage by delaying softening and enhancing phenols accumulation. Sci. Hortic. 2019, 246, 812–817. [Google Scholar] [CrossRef]
- Soegiarto, L.; Wills, R. Short term fumigation with nitric oxide gas in air to extend the postharvest life of broccoli, green bean, and bok choy. HortTechnology 2004, 14, 538–540. [Google Scholar] [CrossRef]
- Alici, E.; Arabaci, G. Determination of SOD, POD, PPO and cat enzyme activities in Rumex obtusifolius L. Annu. Res. Rev. Biol. 2016, 11, 1–7. [Google Scholar] [CrossRef]
- Wu, Z.H.; Dong, C.H.; Wei, J.; Guo, L.M.; Meng, Y.N.; Wu, B.; Chen, J.L. A transcriptional study of the effects of nitric oxide on rachis browning in table grapes cv. Thompson Seedless. Postharvest Biol. Technol. 2021, 175, 111471. [Google Scholar] [CrossRef]
- Liu, Y.B.; Yang, X.B. Nitric oxide as a new fumigant for post-harvest pest control. In Proceedings of the Entomological Society of America Meeting, Portland, OR, USA, 16–19 November 2014. [Google Scholar]
- Pristijono, P.; Wills, R.; Golding, J.B. Use of the nitric oxide-donor compound, diethylenetriamine-nitric oxide (DETANO), as an inhibitor of browning in apple slices. J. Hortic. Sci. Biotechnol. 2008, 83, 555–558. [Google Scholar] [CrossRef]
- Wills, R.; Pristijono, P.; Golding, J.B. Nitric Oxide and Postharvest Stress of Fruits, Vegetables and Ornamentals; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 221–238. [Google Scholar]
- Tan, D.X.; Reiter, R.J. Mitochondria: The birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2019, 2, 44–66. [Google Scholar] [CrossRef]
- Corpas, F.J.; Freschi, L.; Rodriguez-Ruiz, M.; Mioto, P.T.; Gonzalez-Gordo, S.; Palma, J.M. Nitro-oxidative metabolism during fruit ripening. J. Exp. Bot. 2018, 69, 3449–3463. [Google Scholar] [CrossRef]
- Liu, J.L.; Yang, J.; Zhang, H.Q.; Cong, L.; Zhai, R.; Yang, C.Q.; Wang, Z.Z.; Ma, F.W.; Xu, L.F. Melatonin Inhibits Ethylene Synthesis via Nitric Oxide Regulation to Delay Postharvest Senescence in Pears. J. Agric. Food Chem. 2019, 67, 2279–2288. [Google Scholar] [CrossRef]
- Radhika, V.; Kost, C.; Boland, W.; Heil, M.; Rahman, A. The Role of Jasmonates in Floral Nectar Secretion. PLoS ONE 2010, 5, e9265. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Dai, C. Jasmonic acid is involved in the signaling pathway for fungal endophyte-induced volatile oil accumulation of Atractylodes lancea plantlets. BMC Plant Biol. 2012, 12, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Stettmaier, K.; Michel, C.; Hutzler, P.; Mueller, M.J.; Durner, J. Nitric oxide is induced by wounding and influences jasmonic acid signaling in Arabidopsis thaliana. Planta 2004, 218, 938–946. [Google Scholar]
- Wen, W.J.; Wu, J.Y. Nitric oxide is involved in methyl jasmonate-induced defense responses and secondary metabolism activities of Taxus cells. Plant Cell Physiol. 2005, 46, 923–930. [Google Scholar]
- Girija, A.; Devakumar, L.J.P.S.; Vijayanathan, M.; Vasudevan, S.E. Nitric oxide as a bioactive molecule in the regulation of chalcone synthase during jasmonic acid mediated defense signaling in ginger. Plant Cell Tissue Organ Cult. 2017, 128, 715–721. [Google Scholar] [CrossRef]
- Liu, Y.F.; Yang, X.X.; Zhu, S.J.; Wang, Y.Q. Postharvest application of MeJA and NO reduced chilling injury in cucumber (Cucumis sativus) through inhibition of H2O2 accumulation. Postharvest Biol. Technol. 2016, 119, 77–83. [Google Scholar] [CrossRef]
- Caamal-Chan, M.G.; Souza-Perera, R.; Zuniga-Aguilar, J.J. Systemic induction of a Capsicum chinense nitrate reductase by the infection with Phytophthora capsici and defence phytohormones. Plant Physiol. Biochem. 2011, 49, 1238–1243. [Google Scholar] [CrossRef]
- Gu, R.X.; Zhu, S.H.; Zhou, J.; Liu, N.; Shi, J.Y. Inhibition on brown rot disease and induction of defence response in harvested peach fruit by nitric oxide solution. Eur. J. Plant Pathol. 2014, 139, 363–372. [Google Scholar] [CrossRef]
- Tada, Y.; Spoel, S.H.; Pajerowska-Mukhtar, K.; Mou, Z.L.; Song, J.Q.; Wang, C.; Zuo, J.R.; Dong, X.N. Plant Immunity Requires Conformational Charges of NPR1 via S-Nitrosylation and Thioredoxins. Science 2008, 321, 952–956. [Google Scholar] [CrossRef]
- Dokhanieh, A.Y.; Aghdam, M.S.; Fard, J.R.; Hassanpour, H. Postharvest salicylic acid treatment enhances antioxidant potential of cornelian cherry fruit. Sci. Hortic. 2013, 154, 31–36. [Google Scholar] [CrossRef]
- Oliveira, H.C.; Gomes, B.C.R.; Pelegrino, M.T.; Seabra, A.B. Nitric oxide-releasing chitosan nanoparticles alleviate the effects of salt stress in maize plants. Nitric Oxide-Biol. Chem. 2016, 61, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; He, K.W.; Shen, Z.Q.; Zhang, G.Y.; Yu, Y.Q.; Hu, J.M. Nitric Oxide (NO)-Releasing Macromolecules: Rational Design and Biomedical Applications. Front. Chem. 2019, 7, 530. [Google Scholar] [CrossRef] [PubMed]

| Fruits | Best Treatment | Effects | References |
|---|---|---|---|
| Blueberry (Blue Cuinex, Blue Chip and Misty) | Blue Cuinex: 1 μL L−1 1-MCP + 1 mM GSNO Misty: 1 μL L−1 1-MCP Blue Chip: Not affected by treatment. | Maintained higher firmness, malic acid, citric acid, ascorbic acid, and glutathione contents for 14 d at 4 °C. | [17] |
| Tomato (Elpida) | 1 mM GSNO + 0.5 μL L−1 1-MCP | Delayed fruit softening, reduced the ETH synthesis significantly. | [18] |
| Red raspberry (Rubus idaeus L.) | 15 μM NO solution for 2 min (immersed in) | Reduced ETH production, respiratory intensity, ROS contents and increased the contents of flavonoids, anthocyanin, rutin, influenced metabolism of sugars. | [19] |
| Sweet pepper | 160 μM (5 ppm) NO gas for 1 h | Delayed the ripening of fruit, decreased lipid peroxidation, and increased antioxidant capacity, ascorbate content. | [20,21,22] |
| Banana (Brazil) | 5 mM SNP solution | Reduced ETH production, inhibited degreening of the peel, and delayed softening of the pulp. Inhibited the activity of ACO. | [23] |
| Papaya (Sui you 2) | 60 mL L−1 NO fumigated for 3 h | Suppressed ETH formation and respiratory rate (CO2 levels), reduced weight loss, maintained firmness, and delayed changes in peel color and soluble solid contents during 20 d of storage. | [24] |
| Wax apple (Syzygium samarangense) | 10 μL L−1 NO fumigated for 2 h | Lower rate of weight loss, a softening index, and loss of firmness during storage. Decreased total lignin content. | [25] |
| Tomato (Lichun) | 0.1 mM L-NAME solutions for 0.5 min | Decreased endogenous ETH release and delayed the breaker stage of fruits. | [26] |
| Peach (Dahong) | 15 μL L−1 NO + 20 μL L−1 H2S fumigated for 20 min | Inhibited ripening of peach fruits, reduced softening related enzymes activities, ETH production, ACC content, ACC synthase, and oxidase activities. | [27] |
| Water bamboo shoots (Zizania latifolia) | 30 μL L−1 NO fumigated for 4 h | Suppressed the softening and lignification effectively. | [28] |
| Fruits | Best Treatment | Effects | References |
|---|---|---|---|
| Mango (Kensington Pride) | 10, 20, 40 μL L−1 NO fumigated for 2 h | Reduced the chilling injury index, retarded color development, softening, and delayed fruit ripening and maintained quality during storage at 5 °C for 2 and 4 weeks. | [29] |
| Banana (Brazil) | 0.05 mM SNP solution for 5 min (10 kPa) | Inhibited the development of chilling injury during storage at 7 °C for 20 d. The contents of ATP and energy charge were higher. The activities of enzymes involved in energy metabolism were markedly enhanced. | [43] |
| Banana (Brazil) | 60 μL L−1 NO gas for 3 h | Reduced chilling injury during storage at 7 °C for 15 d. Reduced increases in electrolyte leakage and malondialdehyde content. Postponed the degradation of chlorophyll. | [43,44] |
| Hami melon (86-1) | 60 mL L−1 NO for 3 h | Decreased the chilling injury index and chilling injury incidence during storage at 1 °C. Reduced the increases in membrane permeability and malondialdehyde (MDA), H2O2 content. Inhibited O2•− production rates. Sustained higher activity of SOD, POD, CAT, and APX in the rind. | [45] |
| Peach (Feicheng) | 15 μM NO solutions for 0.5 h | Delayed the decrease of mitochondrial permeability transition, promoted a more stable internal medium in mitochondria. | [46] |
| Longkong (Griff) | 30 mM SNP solution for 20 min | Controlled the chilling injury index, electrolytic leakage and regulated the production of MDA, O2•−, and H2O2. | [47] |
| Sweet orange (Midknight Valencia and Lane Late) | 10 μL L−1 NO fumigated for 2 h | Reduced chilling injury, weight loss, total sugars, and vitamin C in both Midknight Valencia and Lane Late during storage for 90 d at 4 °C and 7 °C. Weight losses 7 °C were higher than 4 °C. | [48] |
| Table grape (Munage) | 300 μL L−1 NO fumigated for 2 h | Increased the activities of antioxidant enzymes; alleviated ROS accumulation and membrane lipid peroxidation during storage at 0 °C for 60 d. | [49] |
| Fruits | Disease | Best Treatment | Effects | References |
|---|---|---|---|---|
| Tomato (Target NF1) | Boron toxicity (B) | 0.1 mM NO as a foliar spray | Overcame the deleterious effects of B toxicity on tomato fruit yield and whole plant biomass by reducing the concentrations of B, MDA, EL (electrolyte leakage), and H2O2 in the leaves. | [62] |
| Peach (Feicheng) | Monilinia fructicola | 15 μmol L−1 NO solution for 10 min | Inhibited postharvest peach brown rot caused by M. fructicola. | [63] |
| Citrus (Valencia) | Colletotrichum gloeosporioides | 50 μmol L−1 SNP for 10 min | Had a positive effect on enhancing resistance against postharvest anthracnose and delayed the ripening and senescence during storage at 20 °C. | [64] |
| Pitaya (Baiyulong) | Colletotrichum gloeosporioides | 0.1 mM SNP for 8 min | Inhibited the lesion expansion on pathogen-inoculated pitaya fruit during storage and reduced the natural disease incidence and index of pitaya fruit stored at 25 °C. | [65] |
| Mango (Guifei) | Colletotrichum gloeosporioides | 0.1 mM SNP for 5 min | Suppressed lesion development on mango fruit inoculated with C. gloeosporioides and reduced natural anthracnose incidence during stored at 25 °C. | [66] |
| Kiwifruit (Bruno) | 0.2 mM SNP for 10 min | Reduced diseases incidence; delayed the increase in soluble solid content; increased the activities phenylalanine PAL, POD; elevated the level of total phenolics, flavonoids, and lignin. | [42] | |
| Muskmelon (Xizhoumi 25) | Alternaria alternata | 60 μL L−1 NO fumigated for 3 h | The lesion diameters and lesion depths were decreased. | [67] |
| Fruits | Best Treatment | Effects | References |
|---|---|---|---|
| Chestnut kernel (fresh-cut) | 5 μM NO solutions for 10 min | Delayed browning; increased the content of catechin, chlorogenic acid, syringic acid, phloretic acid, and ferulic acid but inhibited that of tannic acid during storage at 20 °C. | [79] |
| Cut lettuce slices (Green Oak, Green Coral, Baby Cos, and Butter) | 500 mg L−1 DETANO or SNP for 5 min | Inhibited cut-face browning during storage at 0 °C. | [80,81] |
| Fresh-cut apple slices (Granny Smith) | 10 mg L−1 DETANO solution for 5 min | Delayed development of surface browning during storage at 5 °C; resulted in a lower level of total phenols; inhibited PPO activity, reduced ion leakage, rate of respiration. | [75] |
| Litchi | 2.0 mM SNP for 5 min | Reduced pericarp browning, weight loss, MDA content; increased total phenolics, antioxidant capacity; extended shelf life up to 8 d storage at ambient condition. | [83] |
| Peeled bamboo shoots | 0.5 mM SNP for 1 h | Inhibited activities of PPO, POD, and PAL and maintained high total phenol contents, thus delaying external browning during storage at 10 °C for 10 d. | [39] |
| Table grape (Munage) | 300 μL L−1 NO gas fumigation for 2 h | Reduced pericarp browning and disease incidence for 60 d at 0 °C. | [49] |
| Cornelian cherry (Cornus mas) | 0.5 mM SNP for 20 min | Reduced browning index. | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, T.; Tao, N.; Yan, T.; Wang, Q.; Li, Q. Nitric Oxide Is Essential to Keep the Postharvest Quality of Fruits and Vegetables. Horticulturae 2023, 9, 135. https://doi.org/10.3390/horticulturae9020135
Liu Y, Chen T, Tao N, Yan T, Wang Q, Li Q. Nitric Oxide Is Essential to Keep the Postharvest Quality of Fruits and Vegetables. Horticulturae. 2023; 9(2):135. https://doi.org/10.3390/horticulturae9020135
Chicago/Turabian StyleLiu, Yuhan, Tong Chen, Ning Tao, Ting Yan, Qingguo Wang, and Qingqing Li. 2023. "Nitric Oxide Is Essential to Keep the Postharvest Quality of Fruits and Vegetables" Horticulturae 9, no. 2: 135. https://doi.org/10.3390/horticulturae9020135
APA StyleLiu, Y., Chen, T., Tao, N., Yan, T., Wang, Q., & Li, Q. (2023). Nitric Oxide Is Essential to Keep the Postharvest Quality of Fruits and Vegetables. Horticulturae, 9(2), 135. https://doi.org/10.3390/horticulturae9020135






