Abstract
The in vitro growth of the pollen tube (PT), an object of comprehensive and intensive research, is a model for studying the mechanisms of sexual reproduction in higher plants. We have studied the potential role of reactive oxygen species (ROS) in the in vitro germination and growth maintenance of the petunia (Petunia hybrida E. Vilm.) male gametophyte. The exogenous treatment with H2O2 influences the PT germination and polar growth in vitro. The addition of H2O2 to culture medium increases both the percentage of pollen grain germination and the PT length in the case of long cultivation, but inhibits both processes during the first hour of cultivation. This suggests that endogenous ROS play a decisive role in the early stages of pollen germination, with the sensitivity to endogenous ROS emerging later over the course of their growth. The addition of diphenylene iodonium chloride (DPI), a NADPH oxidase inhibitor, considerably decreases both the germination and the growth of the petunia male gametophyte at low concentrations (0.1 μM), and completely arrests the growth at high concentrations (1 μM). ROS are necessary for polar growth of the petunia male gametophyte; they are secreted in the early stages of pollen grain activation and are further localized to the initiation of the PT, mainly in the PT apical part, during polar growth, as confirmed with the help of intravital fluorescence microscopy.
1. Introduction
The understanding of basic processes in plant sexual reproduction opens up potentially new opportunities for solving various applied problems, for example, in the agricultural production of seed crops, as well as for the clarification of the key principles in the signal transduction of plant cells. Current studies of the sexual reproduction of plants are focused on the signals between cells based on the interaction between a small family of receptor-like kinases and their coreceptors and ligands (for review, see [1]). The known subsequent signaling responses comprise the production of reactive oxygen species (ROS), the generation of specific calcium signatures, and alterations in the cell wall.
The male gametophyte of angiosperms is the key stage in the life cycle, because it provides the delivery of immobile sperm cells to the egg cell. Recent data have shown that ROS are involved in regulating polarity and growth in tip-growing cells in plants [2,3,4,5]. Arabidopsis root hairs exhibit high apical levels of ROS, which are proposed to modulate root hair tip growth by activating a Ca2+ channel [2]. ROS produced by a NADPH oxidase are localized with a tip-high gradient in pollen tubes [4,6,7].
Some data suggest the involvement of ROS in pollination and fertilization [8,9,10], which has advanced our understanding of different pollination stages, including the interaction of pollen with the stigma, the PT with the transmitting tissues of the style, and the PT with the female gametophyte. However, there are still many gaps in our understanding of the role of ROS as signaling molecules in pollen–pistil interaction. ROS accumulate in different compartments of the plant cell, for example, in the cytosol, chloroplasts, mitochondria, and peroxisomes (for review, see [11,12]).
The aim of the present study is to investigate the effect of ROS on petunia male gametophyte germination in vitro and on the maintenance of growth. We have previously demonstrated that in vitro and in vivo PG germination is accompanied by a considerable change in the level of the endogenous phytohormones 3-indolyl acetic acid (IAA), abscisic acid (ABA), gibberellins (GA3), and cytokinins, and is sensitive to exogenous plant growth regulators [13,14,15,16,17,18]. In particular, we have shown that exogenous IAA, ABA, and GA3 are able to stimulate pollen germination and PG growth, while cytokinins inhibit these processes at any concentrations.
A hyperpolarizing effect of IAA was simulated with hydrogen peroxide; moreover, the shift in membrane potential induced by H2O2 was inhibited by the same agents that suppressed the IAA-induced hyperpolarization of the pollen plasmalemma. We inferred that the IAA-induced hyperpolarization of the plasma membrane in petunia male gametophyte was determined by an increase in the electrogenic activity of the ATP-dependent pump in the presence of this phytohormone, and assumed that the effect of IAA was mediated by a transient increase in the level of cytosolic Ca2+ and ROS formation [16,19,20].
It is still unclear how redox regulation is accomplished in the progamic phase of fertilization in angiosperms. Our study, focused on this most relevant issue, has demonstrated the involvement of ROS in the germination of PGs and the maintenance of PT growth under in vitro conditions; it shows that hydrogen peroxide is a necessary component for pollen germination and PT growth, accelerating the germination, and most likely facilitating successful reproduction.
2. Materials and Methods
2.1. Research Object
The object of our study is the male gametophyte (pollen and germinating PTs) of the self-compatible (SC) and self-incompatible (SI) petunia (P. hybrida E. Vilm.) clones from laboratory collection. Micropropagation of SC and SI petunia clones was performed in tubes on agar-solidified Murashige and Skoog basal medium without plant growth regulations in a WLR-351H (Sanyo, Tokyo, Japan) climate chamber under 25/23 (day/night) ± 1 °C, with fluorescent light (65 µmol m−2 s−1) during the long-day photoperiod (16 h light/8 h dark). Rooted microshoots were adapted to the soil conditions and grown in the boxes with soil (Agrobalt, St Petersburg, Russia) under natural illumination in the greenhouse conditions at 22–25 °C (day) and 18–19 °C (night) and 60–70% humidity.
2.2. Pollen Cultivation
Freshly harvested pollen from SC and SI clones was cultivated for 3 h in a thermostat at a temperature of 25 °C. Pollen samples (2 mg) were placed into a 15 mL flask with 2 mL of the medium containing 0.3 M sucrose and 1.6 mM H3BO3; H2O2 and NADPH oxidase inhibitor, and DPI (diphenylene iodonium chloride; Sigma), which was added to culture medium immediately before pollen. The following variants were used for pollen cultivation: H2O2 at a concentration of 1, 5, or 10 μM and DPI at a concentration of 0.5 μM, 1 μM, 0.1 mM, or 1 mM (the H2O2 concentration was adjusted with culture medium). These ROS and DPI concentrations were used based on previously performed experiments [19].
Results were recorded on an hourly basis during 3 h of pollen cultivation. We monitored every hour of cultivation in order to monitor the dynamics of PT growth and investigate the time dependence of ROS exposure on PT germination and PT growth. The degree of germination was assessed based on the number of germinated pollen grains randomly selected and examined in four microscope (Zeiss Axioplan, Carl Zeiss, Jena, Germany) fields (n = 200). The PT lengths were measured using AxioVision 4.8 (Carl Zeiss, Germany) software.
2.3. Detection of ROS Generation (Intravital Fluorescence Microscopy)
During the cultivation in nutrient medium, pollen was sampled after 30 min, 1 h, 2 h, and 3 h of germination. PTs were harvested via filtration though a net (mesh, <10 µm) and transferred to 25 nM) carboxy-H2DFFDA aqueous solution (Thermo Fisher Scientific, Waltham, MA, USA) for intravital ROS visualization in cells after a 30 min incubation. The PTs were then washed three times with sterile water. A drop of the water with pollen was placed on a glass slide, sealed with a cover glass, and examined under an Olympus BX51 fluorescence microscope (Olympus Corporation, Tokyo, Japan) at a magnification of ×10 at a wavelength of 490 nm. Images were captured using a Color View II digital camera (Soft Imaging System, Munster, Germany). At least 200 pollen tubes were examined in each variant of the experiment.
2.4. Statistical Processing
The experiments were performed in three to five replicates with three recordings in each. The data in the figures are shown as the means and their standard errors. The significance of differences was estimated with Student’s t-test at p ≤ 0.05.
3. Results and Discussion
3.1. Effect of Hydrogen Peroxide on the In Vitro Germination Rate of Pollen Grains of Self-Compatible and Self-Incompatible Petunia Clones
In our work, we used hydrogen peroxide because it is the most stable ROS and, presumably, the most important in terms of physiological activity [21,22]. The following effects were observed when adding hydrogen peroxide at different concentrations to the culture medium (Figure 1).
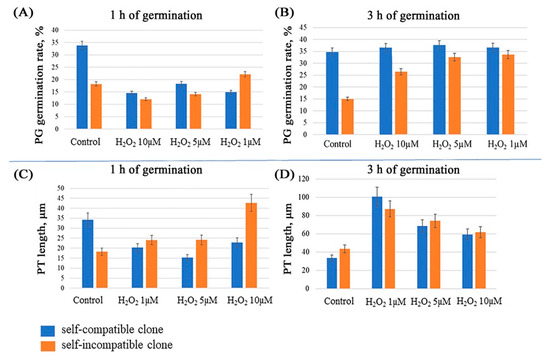
Figure 1.
Effects of hydrogen peroxide on in vitro germination rate and pollen tube length of self-compatible and self-incompatible petunia clones. The rate (%) of PG germination after (A) 1 h and (C) 3 h of germination and the lengths (μm) of PTs after (B) 1 h and (D) 3 h of germination.
The SC clone in the control medium displayed the highest percent rate (34%) of germinated pollen after 1 h of in vitro cultivation (Figure 1A) versus the SI clone, with the rate of germinated PGs not exceeding 20%. After the addition of hydrogen peroxide to the medium, the rate of germinated PGs of SC clone after 1 h of cultivation is 4% higher as compared with the SI clone for two H2O2 concentrations (10 μM); however, this increase is statistically insignificant.
This experiment demonstrates that the addition of hydrogen peroxide to the medium has a positive effect on the percent rate of PG germination in the case of longer pollen cultivation. This is evident in Figure 1B, where all characteristics of the treated pollen are higher as compared with the control. This is rather insignificant for the SC pollen, but displays an almost two-fold increase for SI.
A low germination rate has been characteristic of the SI clone PGs in all our long-term experiments [23]. However, PGs effectively germinate and further grow in the pistil-transmitting tissues of SC clones, and reach the ovary, followed by fertilization and seed setting in the case of cross-pollination in vivo. The PGs of most plant species are able themselves to activate and support the regulatory network that controls polar growth in the absence of the signals produced by the female gametophyte and sporophyte [24]. Correspondingly, PGs often successfully germinate and implement the program of polar cell growth in nutrient medium in vitro. For this purpose, the nutrient medium in the majority of cases must contain boric acid and a source of heterotrophic nutrition, usually sucrose. The composition of medium is additionally optimized to reach a larger germination rate and a higher growth rate. Naturally, in vitro PT growth does not completely match the in vivo processes [4]. However, this experimental model is actively and beneficially used when studying the regular patterns in the polar directed cell growth.
The patterns of the PT growth are detailed in numerous reviews [2,25]; some reviews consider various aspects in more detail, such as the ionic regulation of growth [5,26]. The identification of intracellular signaling systems of the male gametophyte has considerably advanced; in particular, the important roles of Rop GTPases [1,27,28], the intracellular Ca2+ signaling network [29], and anion transport [30] have been discovered. The role of ROS in the processes underlying polar growth is also actively debated in the relevant literature [9].
3.2. Effect of Hydrogen Peroxide on the Pollen Tube Length of Self-Compatible and Self-Incompatible Petunia Clones Cultivated In Vitro
The growth patterns of the PTs on culture medium (0.4 M sucrose and 1.6 mM H3BO3) supplemented with H2O2 at a concentration of 1, 5, or 10 μM differ in a statistically significant manner according to the PT length, depending on the exposure (Figure 1C,D).
Similar to the influence of H2O2 on the percent rate of PG germination, we did not observe any strong inhibitory effect on the PT length. By the first hour of cultivation, the differences in the PT growth displayed by two clones are evident (Figure 1C). In the case of the SC clone, the PT growth at all H2O2 concentrations is inhibited. However, the opposite effect—the stimulation of PT growth—is observed in the case of the SI clone; moreover, the lowest concentration causes the highest increase.
At 3 h of germination, the length of PTs of all treated pollen exceeds the control by 40% (Figure 1D). The maximum result was attained for the SC pollen cultivated in the medium with 1 mM H2O2.
Analysis of the results suggests that the patterns are similar to the data on the percent rate of PG germination; namely, a stimulatory effect of the tested H2O2 concentrations in a long cultivation is evident for both the length of treated PTs and the percent rate of germination.
The experiments by Podolyan et al. [31] demonstrate the stimulation of the lily PT growth after treatment with H2O2. The same effect was observed by Maksimov et al. [12] in the PTs of gymnosperms. Smirnova et al. [32] demonstrated the stimulation of tobacco pollen germination by H2O2 at low concentrations, whereas the high concentrations slowed down this process but did not decrease pollen viability. The authors explain that this effect is due to the fact that H2O2 and hydroxyl radical (•OH) can regulate the germination of tobacco pollen by changing the mechanical properties of pollen intine (inner layer of the pollen wall). The •OH radical caused excessive intine loosening over the entire PG surface, thereby interfering with the induction of polar growth. On the contrary, H2O2 compacted the wall, thereby preventing the PT from emerging. Presumably, this is the cause underlying the PG germination during the first hours of cultivation.
3.3. Effect of DPI on the In Vitro Germination of the P. hydrida Male Gametophyte
It is known that DPI inhibits NADPH oxidase, which is regarded as the main ROS producer in cells. The effect of DPI on the percent rate of PG germination was assessed using four concentrations, namely 0.5 μM, 1 μM, 0.1 mM, and 1 mM. High DPI concentrations (0.1 and 1 mM) completely arrested PG germination, while PGs still succeeded in germinating at lower concentrations (0.5 and 1 μM; Table 1).

Table 1.
Effect of DPI on the germination percent rate of SC and SI petunia clones in in vitro culture.
The cultivation of PGs in the medium containing DPI at two concentrations (0.5 and 1 μM) revealed a distinct dependence. DPI at a concentration of 1 μM caused a very strong inhibitory effect on the PG germination rate of both SC and SI clones. In all cases, the germination rate decreased approximately two-fold, and this pattern was retained over the entire period of PG germination. A DPI concentration of 0.5 μM had a less dramatic effect, although it also decreased the rate of PG germination for both clones, in most cases.
3.4. Effect of DPI on the In Vitro Growth of P. hydrida Male Gametophyte
The experiment when DPI was added to the medium during petunia pollen cultivation revealed a decrease in the PT lengths, similar to the inhibition of PG germination percent (Table 2).

Table 2.
Effect of DPI on the pollen tube length of SC and SI petunia clones in in vitro culture.
The addition of DPI at a concentration of 1 μM to the medium for PT cultivation caused inhibition of the length of the germinating male gametophyte. The PT length considerably varied in each experiment, and the differences between PT lengths in the variants were not always statistically significant.
Earlier papers [12,31,32] demonstrate that DPI at high concentrations (0.1 mM) blocks the PG germination of both angiosperms and gymnosperms. Lassing et al. [33] discovered decreased K+ currents in the PTs of the Arabidopsis mutants with NADPH oxidase deficiency; the same effect was observed when treating non-mutant plants with DPI. Podolyan et al. [31] propose a putative mechanism of ion zoning and the sensitivity of ion transport to ROS. ROS are able to modulate the activity of cation channels and, as a consequence, the efficiency and rate of PT germination.
As is assumed, Ca2+ release and the subsequent inhibition of H+-ATPase in the subapical PT region can mediate the efficiency of H2O2. Thus, DPI can simulate K+- and Ca2+-conducting channels, although with somewhat lower efficiency [31].
It is assumed that ROS generation is determined by NADPH oxidase activity on the membrane. According to our data, the two main phytohormones that stimulate PG germination and PT growth are IAA and ABA. Our data are interpretable in the view that ROS are necessary and mediate the pathways mediated by IAA and ABA as in the other plant systems [34,35,36,37]. In our experimental system, we can assume that IAA induces the activation of NADPH oxidase, responsible for ROS generation, which is presumably caused by an increase in the Ca2+ cytosolic level, when Ca2+ arrives from extracellular medium at the stage of IAA sensing by pollen grain cells. Note that the Ca2+-dependent NADPH oxidase activation is a well-known specific feature of this enzyme in eukaryotic cells [38].
Our results demonstrate that IAA induces hyperpolarization of the plasmalemma in the germinating male gametophyte, which is inhibited by EGTA and verapamil, the agents preventing the input of extracellular calcium to cells. Of particular interest is the fact that a hyperpolarizing effect of IAA is also blocked by DPI, an inhibitor of NADPH oxidase, and, in contrast, is simulated by the addition of hydrogen peroxide. Note that H2O2 shifts the membrane potential, which is inhibited by the same agents as IAA, namely orthovanadate [20].
3.5. Detection of ROS Generation
Figure 2 shows the ROS staining in the male gametophyte of petunia. The petunia PGs secrete ROS as early as the beginning of activation (Figure 2A–C). Bright fluorescence of the viable PGs (red arrows) is observed, whereas the fluorescence of nonviable PGs is very weak, and almost invisible (white arrows). Maksimov et al. [12] demonstrated that ROS were also secreted in the pollen of blue spruce at the early activation stage.
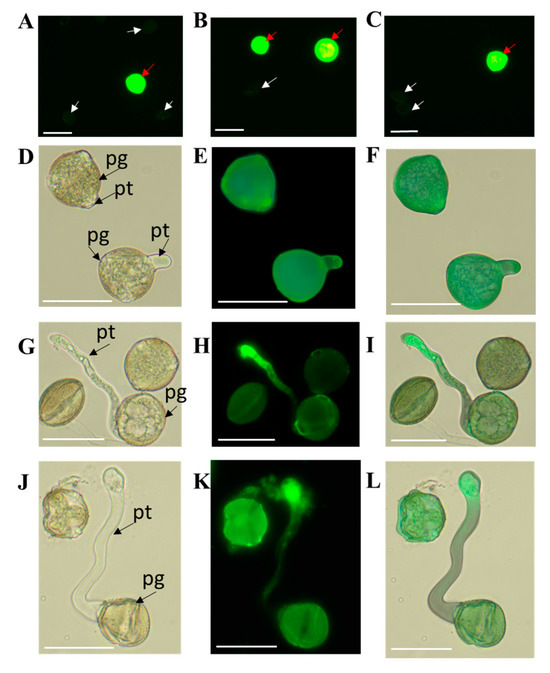
Figure 2.
Distribution of ROS in the pollen and pollen tubes grown in vitro. (A–C) Petunia pollen grains after 30 min of germination. Bright fluorescence of viable pollen grains (red arrows) and very weak, almost invisible fluorescence of nonviable PGs (white arrows). (D–F) Petunia PGs after 1 h of germination. Fluorescence of overall PG and the pollen tube apex. (G–I) Male gametophyte of a petunia self-compatible clone after 3 h of germination. Bright fluorescence of the PT apex. (J–L) Male gametophyte of a petunia self-compatible clone after 3 h of germination. Bright fluorescence of the PT apex. (A,D,G,J) Light-field microscopy; (B,E,H,K) fluorescence microscopy, staining with carboxy-H2DFFDA aqueous solution (wavelength, 490 nm); and (C,F,I,L) overlay of light and fluorescence microscopy images (pg, pollen grain and pt, pollen tube; bar = 50 µm).
According to relatively recent data from Maksimov et al. [12], endogenous ROS, including the species produced by NADPH oxidase, are necessary for the germination of spruce pollen, and regulate the membrane potential in PTs, while H2O2 is nonuniformly distributed along PT. The ROS location in PT initials and PTs themselves suggests H2O2 accumulates in the PT apex. In our experiments, we also observed bright fluorescence in the PT apexes after 1 h (Figure 2D–F) and 3 h (Figure 2G–L) of in vitro germination. The PG rather brightly fluoresces at the moment of hydration and the first hour of germination; then, the bright PG fluorescence disappears after 3 h of germination, while the bright fluorescence at the PT apex remains. Cardenas et al. [39] observed both the apical and subapical ROS accumulation in the (Lilium formosanum) PTs.
Speranza et al. [40] demonstrated that Chinese gooseberry PTs released hydrogen peroxide into culture medium. Presumably, this explains certain green fluorescence near the PT apexes in Figure 2H,K.
According to published data [41], ROS can be involved in the regulation of PT integrity via the metabolism of the cell wall components such as pectin and callose.
It is known that the stigma exudate contains ROS [9,42,43] synthesized on the stigma of various flowering plants. The moderate activity of some enzymes that regulate ROS, in particular, peroxidases, has been shown. In line with the modern view of the exudate as the medium of pollen–stigma interaction, the authors postulate that one of the functions of ROS on the stigma is the support and/or stimulation of pollen germination.
4. Conclusions
ROS have already been detected during the process of hydration and the beginning of PG germination. Subsequently, ROS accumulation is detected in the PT initials. With further growth of the PT, ROS become distributed unevenly in the PT. Additionally, inhibition of ROS generation by DPI negatively affects the PG germination and PT growth, while the addition of H2O2 to the culture medium has a positive effect on these processes, especially during long-term (3 h) cultivation. These facts convincingly demonstrate that ROS are a necessary component in regulating the polar growth of the male gametophyte; however, ROS targets (in particular, those of hydrogen peroxide) in the growing PT are still unclear.
Author Contributions
Conceptualization, E.V.Z.; performed the experiments E.V.Z., Y.Y.G. and T.P.K.; writing and original draft preparation, E.V.Z. and M.R.K.; approved the final manuscript for publication, and agreed to be accountable for all aspects of the manuscript, M.R.K.; Funding Acquisition, M.R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This article was funded by the Russian Scientific Foundation, grant No. 22-24-01148, via the research project “Role of the cytoskeleton in the S-RNase-based self-incompatibility under normal and stressful conditions in vitro and in vivo in a model of transgenic petunia plants (Petunia hybrida L.) with lifetime identification of actin and tubulin”.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baillie, A.L.; Sloan, J.; Qu, L.-J.; Smith, L.M. Signalling between the Sexes during Pollen Tube Reception. Trends Plant Sci. 2023, 1–12. [Google Scholar] [CrossRef]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D.G.; et al. Reactive Oxygen Species Produced by NADPH Oxidase Regulate Plant Cell Growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef]
- Monshausen, G.B.; Bibikova, T.N.; Messerli, M.A.; Shi, C.; Gilroy, S. Oscillations in Extracellular pH and Reactive Oxygen Species Modulate Tip Growth of Arabidopsis Root Hairs. Proc. Natl. Acad. Sci. USA 2007, 104, 20996–21001. [Google Scholar] [CrossRef]
- Potocký, M.; Jones, M.A.; Bezvoda, R.; Smirnoff, N.; Žárský, V. Reactive Oxygen Species Produced by NADPH Oxidase are Involved in Pollen Tube Growth. New Phytol. 2007, 174, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.M.; Brownlee, C.; Bothwell, J.H. A Tip-High, Ca2+-Interdependent, Reactive Oxygen Species Gradient is Associated with Polarized Growth in Fucus Serratus Zygotes. Planta 2008, 227, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-L.; Wu, J.; Xu, G.-H.; Gao, Y.B.; Chen, G.; Wu, J.-Y.; Wu, H.Q.; Zhang, S.-L. S-RNase Disrupts Tip-Localized Reactive Oxygen Species and Induces Nuclear DNA Degradation in Incompatible Pollen Tubes of Pyrus pyrifolia. J. Cell Sci. 2010, 123, 4301–4309. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, K.A.; Bancroft, J.; Bosch, M.; Ings, J.; Smirnoff, N.; Franklin-Tong, V.E. Reactive oxygen species and nitric oxide mediate actin reorganization and programmed cell death in the self-incompatibility response of papaver. Plant Physiol. 2011, 156, 404–416. [Google Scholar] [CrossRef]
- Breygina, M.M.; Klimenko, E.; Podolyan, A.; Voronkov, A. Dynamics of Pollen Activation and the Role of H+-ATPase in Pollen Germination in Blue Spruce (Picea pungens). Plants 2020, 9, 1760. [Google Scholar] [CrossRef] [PubMed]
- Breygina, M.; Schekaleva, O.; Klimenko, E.; Luneva, O. The Balance between Different ROS on Tobacco Stigma during Flowering and Its Role in Pollen Germination. Plants 2022, 11, 993. [Google Scholar] [CrossRef]
- Breygina, M.; Voronkov, A.; Galin, I.; Akhiyarova, G.; Polevova, S.; Klimenko, E.; Ivanov, I.; Kudoyarova, G. Dynamics of Endogenous Levels and Subcellular Localization of ABA and Cytokinins during Pollen Germination in Spruce and Tobacco. Protoplasma 2023, 260, 237–248. [Google Scholar] [CrossRef]
- Mignolet-Spruyt, L.; Xu, E.; Idänheimo, N.; Hoeberichts, F.A.; Mühlenbock, P.; Brosché, M.; Van Breusegem, F.; Kangasjärvi, J. Spreading the News: Subcellular and Organellar Reactive Oxygen Species Production and Signalling. J. Exp. Bot. 2016, 67, 3831–3844. [Google Scholar] [CrossRef] [PubMed]
- Maksimov, N.; Evmenyeva, A.; Breygina, M.; Yermakov, I. The Role of Reactive Oxygen Species in Pollen Germination in Picea pungens (Blue Spruce). Plant Reprod. 2018, 31, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Andreev, I.M.; Timofeeva, G.V.; Minkina, Y.V.; Kovaleva, L.V. Effects of Exogenous Phytohormones on Intracellular pH of Petunia hybrida Pollen Grains. Russ. J. Plant Physiol. 2007, 54, 626–632. [Google Scholar] [CrossRef]
- Kovaleva, L.; Zakharova, E. Hormonal Status of the Pollen-Pistil System at the Progamic Phase of Fertilization after Compatible and Incompatible Pollination in Petunia hybrida L. Sex. Plant Reprod. 2003, 16, 191–196. [Google Scholar] [CrossRef]
- Kovaleva, L.V.; Voronkov, A.S.; Zakharova, E.V. Role of Auxin and Cytokinin in the Regulation of the Actin Cytoskeleton in the in Vitro Germinating Male Gametophyte of Petunia. Russ. J. Plant Physiol. 2015, 62, 179–186. [Google Scholar] [CrossRef]
- Kovaleva, L.V.; Zakharova, E.V.; Voronkov, A.S.; Timofeeva, G.V.; Andreev, I.M. Role of Abscisic Acid and Ethylene in the Control of Water Transport-Driving Forces in Germinating Petunia Male Gametophyte. Russ. J. Plant Physiol. 2017, 64, 782–793. [Google Scholar] [CrossRef]
- Zakharova, E.V.; Khaliluev, M.R.; Kovaleva, L.V. Hormonal Signaling in the Progamic Phase of Fertilization in Plants. Horticulturae 2022, 8, 365. [Google Scholar] [CrossRef]
- Zakharova, E.; Khanina, T.; Knyazev, A.; Milyukova, N.; Kovaleva, L.V. Hormonal Signaling during dPCD: Cytokinin as the Determinant of RNase-Based Self-Incompatibility in Solanaceae. Biomolecules 2023, 13, 1033. [Google Scholar] [CrossRef]
- Voronkov, A.S.; Andreev, I.M.; Timofeeva, G.V.; Kovaleva, L.V. Electrogenic Activity of Plasma Membrane H+-ATPase in Germinating Male Gametophyte of Petunia and Its Stimulation by Exogenous Auxin: Mediatory Role of Calcium and Reactive Oxygen Species. Russ. J. Plant Physiol. 2010, 57, 401–407. [Google Scholar] [CrossRef]
- Kovaleva, L.V.; Voronkov, A.S.; Zakharova, E.V.; Minkina, Y.V.; Timofeeva, G.V.; Andreev, I.M. Exogenous IAA and ABA Stimulate Germination of Petunia Male Gametophyte by Activating Ca2+-Dependent K+-Channels and by Modulating the Activity of Plasmalemma H+-ATPase and Actin Cytoskeleton. Russ. J. Dev. Biol. 2016, 47, 109–121. [Google Scholar] [CrossRef]
- Hiscock, S.; Bright, J.; McInnis, S.M.; Desikan, R.; Hancock, J.T. Signaling on the Stigma. Plant Signal. Behav. 2007, 2, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Breygina, M.; Klimenko, E. ROS and Ions in Cell Signaling during Sexual Plant Reproduction. Int. J. Mol. Sci. 2020, 21, 9476. [Google Scholar] [CrossRef]
- Kovaleva, L.V.; Zakharova, E.V.; Minkina, Y.V.; Timofeeva, G.V.; Andreev, I.M. Germination and In Vitro Growth of Petunia Male Gametophyte Are Affected by Exogenous Hormones and Involve the Changes in the Endogenous Hormone Level. Russ. J. Plant Physiol. 2005, 52, 521–526. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, Z. Rapid Tip Growth: Insights from Pollen Tubes. Semin. Cell Dev. Biol. 2011, 22, 816–824. [Google Scholar] [CrossRef]
- Hepler, P.K.; Winship, L.J. The Pollen Tube Clear Zone: Clues to the Mechanism of Polarized Growth. J. Integr. Plant Biol. 2015, 57, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Hepler, P.K.; Lovy-Wheeler, A.; McKenna, S.T.; Kunkel, J.G. Ions and Pollen Tube Growth. In The Pollen Tube: A Cellular and Molecular Perspective; Malhó, R., Ed.; Plant Cell Monographs; Springer: Berlin/Heidelberg, Germany, 2006; pp. 47–69. ISBN 978-3-540-34276-2. [Google Scholar]
- Ge, Z.; Cheung, A.Y.; Qu, L.-J. Pollen Tube Integrity Regulation in Flowering Plants: Insights from Molecular Assemblies on the Pollen Tube Surface. New Phytol. 2019, 222, 687–693. [Google Scholar] [CrossRef]
- 28. Feiguelman, G; Fu, Y.; Yalovsky, S. ROP GTPases structure-function and signaling pathways. Plant Physiol. 2018, 176, 57–79. [CrossRef]
- Wudick, M.M.; Feijó, J.A. At the Intersection: Merging Ca2+ and ROS Signaling Pathways in Pollen. Mol. Plant 2014, 7, 1595–1597. [Google Scholar] [CrossRef]
- Gutermuth, T.; Lassig, R.; Portes, M.-T.; Maierhofer, T.; Romeis, T.; Borst, J.-W.; Hedrich, R.; Feijó, J.A.; Konrad, K.R. Pollen Tube Growth Regulation by Free Anions Depends on the Interaction between the Anion Channel SLAH3 and Calcium-Dependent Protein Kinases CPK2 and CPK20. Plant Cell 2013, 25, 4525–4543. [Google Scholar] [CrossRef]
- Podolyan, A.; Maksimov, N.; Breygina, M. Redox-Regulation of Ion Homeostasis in Growing Lily Pollen Tubes. J. Plant Physiol. 2019, 243, 153050. [Google Scholar] [CrossRef]
- Smirnova, A.V.; Matveyeva, N.P.; Yermakov, I.P. Reactive Oxygen Species Are Involved in Regulation of Pollen Wall Cytomechanics. Plant Biol. 2014, 16, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Lassig, R.; Gutermuth, T.; Bey, T.D.; Konrad, K.R.; Romeis, T. Pollen Tube NAD(P)H Oxidases Act as a Speed Control to Dampen Growth Rate Oscillations during Polarized Cell Growth. Plant J. 2014, 78, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Postiglione, A.E.; Muday, G.K. The Role of ROS Homeostasis in ABA-Induced Guard Cell Signaling. Front. Plant Sci. 2020, 11, 968. [Google Scholar] [CrossRef] [PubMed]
- Mir, A.R.; Siddiqui, H.; Alam, P.; Hayat, S. Foliar Spray of Auxin/IAA Modulates Photosynthesis, Elemental Composition, ROS Localization and Antioxidant Machinery to Promote Growth of Brassica Juncea. Physiol. Mol. Biol. Plants 2020, 26, 2503–2520. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, S.; Zhang, Q.; Cui, M.; Zhao, M.; Li, N.; Wang, S.; Wu, R.; Zhang, L.; Cao, Y.; et al. The Interaction of ABA and ROS in Plant Growth and Stress Resistances. Front. Plant Sci. 2022, 13, 1050132. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Yuan, S.; Xie, B.; Li, Q.; Wang, Q.; Shao, M. IAA Plays an Important Role in Alkaline Stress Tolerance by Modulating Root Development and ROS Detoxifying Systems in Rice Plants. Int. J. Mol. Sci. 2022, 23, 14817. [Google Scholar] [CrossRef]
- Bánfi, B.; Tirone, F.; Durussel, I.; Knisz, J.; Moskwa, P.; Molnár, G.Z.; Krause, K.-H.; Cox, J.A. Mechanism of Ca2+ Activation of the NADPH Oxidase 5 (NOX5). J. Biol. Chem. 2004, 279, 18583–18591. [Google Scholar] [CrossRef]
- Cárdenas, L.; McKenna, S.T.; Kunkel, J.G.; Hepler, P.K. NAD(P)H Oscillates in Pollen Tubes and Is Correlated with Tip Growth. Plant Physiol. 2006, 142, 1460–1468. [Google Scholar] [CrossRef]
- Speranza, A.; Crinelli, R.; Scoccianti, V.; Geitmann, A. Reactive Oxygen Species Are Involved in Pollen Tube Initiation in Kiwifruit. Plant Biol. 2012, 14, 64–76. [Google Scholar] [CrossRef]
- Feng, H.; Liu, C.; Fu, R.; Zhang, M.; Li, H.; Shen, L.; Wei, Q.; Sun, X.; Xu, L.; Ni, B.; et al. Lorelei-Like Gpi-Anchored Proteins 2/3 Regulate Pollen Tube Growth as Chaperones and Coreceptors for Anxur/Bups Receptor Kinases in Arabidopsis. Mol. Plant 2019, 12, 1612–1623. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Ju, Y.; Kessler, S.A. Reactive Oxygen Species as Mediators of Gametophyte Development and Double Fertilization in Flowering Plants. Front. Plant Sci. 2020, 11, 1199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.J.; Zhang, X.S.; Gao, X.-Q. ROS in the Male–Female Interactions During Pollination: Function and Regulation. Front. Plant Sci. 2020, 11, 177. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).