Abstract
The piperamides profile of Piper nigrum cultivated in Costa Rica was studied using ultra-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight high-resolution mass spectrometry (UPLC-ESI-QTOF-HRMS) on enriched-piperamides extracts. A total of 31 different piperamides were identified, 24 of them with a methylenedioxyphenyl moiety, including piperine and nine other compounds with the characteristic piperidine ring, as well as guineensine, retrofractamide B, and eight other piperamides with an N-isobutyl group. In addition, piperyline and two other compounds with a pyrrolidine ring, as well as piperflaviflorine B, holding a N-2-methylbutyl chain, were characterized. In turn, pellitorine and six other piperamides exhibiting a long olefinic chain instead of the methylenedioxyphenyl group were also tentatively identified. In addition, quantification was performed using UPLC coupled with a diode array detector (UPLC-DAD), with 15 piperamides being quantified, including piperine, piperyline, piperanine, and piperloguminine with values within the range of previous reports, while results obtained for guineensine (276.5–421.0 mg/100 g dry material) and pellitorine (414.4–725.0 mg/100 g dry material) were higher than those reported in the literature. Additionally, preparative and semi-preparative high-performance liquid chromatography (HPLC) separations allowed to isolate, besides piperine, four other piperamides, which were identified through HRMS, 1H and 13C nuclear magnetic resonance (NMR). These included retrofractamide B, guineensine, pellitorine, and (2E,4E,12Z)-N-isobutyl-octadeca-2,4,12-trienamide, with yields of 134.0 mg/100 g dry material, 209.7 mg/100 g dry material, 361.8 mg/100 g dry material and 467.0 mg/100 g dry material, respectively, with all these values higher than those reported in previous studies in the literature. The findings constitute the first report of such a number and diversity of compounds in P. nigrum cultivated in Costa Rica.
Keywords:
Piper nigrum; black pepper; UPLC ESI-MS; piperamides; piperine; guineensine; pellitorine; retrofractamide B 1. Introduction
Black pepper (Piper nigrum) is a fruit cultivated worldwide because of its commercial interest, holding a high position in total production among the main fruit crops used as spices worldwide [1]. The International Trade Center (ITC) in Geneva, Switzerland, estimates the current trade in spices at 400,000–450,000 metric tons with a total value of US USD 1.5–2 billion annually. With an annual growth rate of 3.6% in quantity and 8.4% in value in spices, pepper contributes 34% of total trade in spices [2].
With a practice spanning thousands of years, ayurvedic practitioners have long prescribed black pepper, as it was a key component in many ayurvedic remedies in India and likely enhanced the effectiveness of other compounds in traditional herbal medicines [3]. Likewise, its positive influence on the bioavailability of drugs and nutrients has been reported, increasing intestinal absorption and regulating metabolism and transport [4]. Recent reports have described that piperine enhances the solubility of natural metabolites and drugs through the formation of multicomponent organic materials (MOMs), for instance, forming eutectic mixtures with drugs and naturally occurring compounds and boosting their solubility, dissolution rate, and wettability while decreasing contact angle [5].
Reviews on the use of P. nigrum highlighted the in vivo neuroprotective and hypoglycemic effects presenting a potential for further research, especially to investigate its capacity to inhibit acetylcholinesterase and butyrylcholinesterase, two enzymes critically implicated in Alzheimer’s and Parkinson’s disorders, but also α-amylase, α-glucosidase, and lipase enzymes [6] as well as other neuroprotective properties reducing caffeine-induced effects on mice hyperlocomotion [7].
In turn, extracts derived from Piper nigrum have exhibited notable antibacterial efficacy against a spectrum of microorganisms, including Candida albicans, Escherichia coli, various Aspergillus species, diverse Bacillus strains, various Pseudomonas variants, several Staphylococcus strains, and Salmonella species, showing its broad antibacterial effects [8]. It was also observed that P. nigrum extracts restored the antioxidant profile and normalized levels of urea and creatinine and the renal histological architecture after the induction of renal toxicity in rats via monosodic glutamate [9]. In addition, immunomodulatory functions related to the modulation of cellular energy metabolism have been reported [10].
Piperine, the most abundant amide in P. nigrum, exerts anti-inflammatory, neuroprotective, immunomodulatory, cardioprotective, and anticancer effects [11], for instance, including inhibition of growth and differentiation of cancer cells through various pathways, including ROS (reactive oxygen species) generation, caspase-mediated apoptosis, the inhibition of cell migration, the suppression of oncogene expression, and the enhancement of mitochondrial-mediated pathways [12].
Other amides from Piper spp. have gained interest because of their bioactive properties, such as inhibitors of cellular uptake of the endocannabinoid anandamide. Structure–activity studies emphasize the significance of the alkyl chain length in molecules like guineensine, which inhibits endocannabinoid uptake across various cell lines independently of fatty acid hydrolase (FAAH) [13] and exhibits significant neuro-pharmacological interactions with sigma receptors, 5-hydroxy-tryptamine 2A (5-HT2A) receptors, dopamine transporters (DATs), and 5-hydroxy-tryptamine 2B (5-HT2B) receptors when tested against 45 central nervous system (CNS)-related receptors, channels, and transporters [14].
Piperine also inhibits enzymes like cytochrome P450 3A4 (CYP3A4), which in turn affect drug interactions. For instance, it impacts how the organism processes such drugs as nevirapine, carbamazepine, midazolam, diclofenac sodium, and glimepiride because of CYP enzyme inhibition [15]. With these drugs, botanical interaction is important to highlight since such properties can pose a challenge in terms of public safety concerns, and therefore, quality assessment on the characterization and quantification of metabolites on raw materials and final goods based on P. nigrum is of particular interest.
In turn, pellitorine inhibits lipid accumulation during adipogenesis in 3T3 Swiss albino cell line (3T3-L1) cells and protects vascular barrier integrity by reducing hyperpermeability, cell adhesion molecule (CAM) expression, and leukocyte adhesion and migration [16,17]. Meanwhile, retrofractamide B, along with guinensine and N-isobutyl di and trienamides, has shown analgesic gastroprotective effects in experimental ethanol- and indomethacin-induced gastric lesions in rats [18].
Based on these important reports of pharmacological effects and potential applications, interest in developing or incorporating black pepper extracts or piperamides in final products in the dietary supplements and drug industry is expected to continue to grow, and the secondary metabolic profile can be used as a tool to provide relevant markers for predicting future pharmacological applications. Hence, the aim of this study was the characterization of piperamides of commercial black pepper cultivated in Costa Rica via UPLC-ESI-QTOF-HRMS, as well as their quantification using UPLC-DAD and further isolation of different piperamides of interest because of their bioactive properties.
2. Materials and Methods
2.1. Plant Material
P. nigrum commercialized fruits were acquired in a dry state from one of the main local Costa Rican distributors, corresponding to different producers from the northern part of the country, and harvested in the peak season. Namely, samples Pn-d and Pn-f pertained to the San Carlos region, and Pn-j pertained to the Guatuso region. Furthermore, Pn-d, Pn-j, and Pn-f were harvested in the months of December, January, and February, respectively. These regions have different altitudes, corresponding to 40 masl (meters above sea level) for Guatuso and 266 masl for San Carlos, as well as different temperatures and rainfall. Considering the harvest month for each sample, the annual average temperature was 27.2 °C for Pni-j and 25.8 °C for Pn-d and Pn-f, while the annual average rainfall was 411.6, 182.5, and 395 mm for Pn-d, Pn-j, and Pn-f, respectively. The samples were kept in sealed containers at −20 °C.
2.2. Reagents and Solvents
Reagents such as piperine (97%) and formic acid (99%) were provided by Sigma-Aldrich (St. Louis, MO, USA). Solvents of ACS (American Chemical Society, Washington, DC, USA) and HPLC grade, such as acetonitrile (99.8%), ethyl acetate (99.7%), ethyl ether (99%), methanol (99.8%), and water (100%), were purchased from Baker (Center Valley, PA, USA), while deuterated chloroform (99.8%) and deuterated methanol (98.8%) were acquired from Sigma-Aldrich (St. Louis, MO, USA). Calibration and lock spray kits from Waters (Milford, MA, USA) were used to prepare sodium formate and leucine enkephalin solutions.
2.3. Piperamide Extracts from P. nigrum Fruits
P. nigrum dry fruits were extracted in a Dionex™ ASE™ 150 Accelerated Solvent Extractor (Thermo Scientific™, Walthman, MA, USA) using ethyl acetate as a solvent in a 34 mL cell at room temperature. The extract was washed with water, and then, the organic solvent was eliminated under vacuum to obtain a piperamides-rich extract.
2.4. UPLC-QTOF-ESI MS Analysis
Measurements were performed using a Xevo G2-XS QTOF (Waters, UK) coupled with an AQUITY H Class UPLC system with a quaternary pump. ESI source parameters were set to a capillary voltage of 2 kV, sampling cone of 20 eV, source temperature of 150 °C, source offset of 10 °C, desolvation temperature of 450 °C, cone gas flow of 0 L/h, and desolvation gas flow of 900 L/h. The measurement was performed in MSe high-resolution positive mode using an acquisition mass range from 100 m/z to 1000 m/z and a scan rate of 0.5 s. Instrument calibration was performed in the mass range of the measurement with sodium formate. Lock mass correction was applied directly to the measurement using leucine enkephalin infusion measured each 30 s during the run. The data were analyzed using MassLynx V4.2 software from Waters. A 1 µL sample was injected with a flow of 0.5 mL/min using a Luna C18 column (150 mm × 4.6 mm id × 4 µm; Phenomenex, Torrance, CA, USA) at 30 °C and a chromatographic gradient starting at 40% B increasing to 100% B at 30 min; the gradient was held for 7 min, and then, the column was equilibrated for 5 min to initial conditions. Solvents used in the mobile phase were A water with 0.1% formic acid and B acetonitrile with 0.1% formic acid.
2.5. UPLC-DAD Quantification of Piperamides
For quantification purposes, measurements were performed using a method previously reported in the literature [19] with some modifications for optimization. Briefly, a calibration curve of piperine was performed between 10 and 1000 mg/L using a UPLC-DAD coupled with a simple quadrupole MSQ instrument (ThermoFisher Scientific, San Jose, CA, USA) and a Synergi Polar RP column (150 mm × 4.6 mm id × 4 µm; Phenomenex, Torrance, CA, USA). Mobile phases A and B consisted of a combination of 0.1% formic acid in water (v/v) and 0.1% formic acid in acetonitrile (v/v), respectively. The linear gradient was from 40% to 70% B (v/v) at 17 min to 100% B at 24 min and held at 100% B to 27 min. The PDA acquisition was set at 260 nm and 340 nm. P. nigrum extract samples were measured under the same conditions. Results are expressed as mg of piperine equivalents and as a percentage of each piperamide in the extract composition, calculated by considering the integrated area for each peak as a percentage of the sum of all areas.
2.6. Preparative and Semipreparative HPLC for Piperamides Isolation
A total of 10 mL of cold ethyl ether was added to the pepper extract, stirred to dissolve completely, concentrated in a rotary evaporator at 20 °C to half the volume, and left to rest in a cold bath for 15 min. The remaining solid was separated via vacuum filtration and allowed to air dry. The solid obtained constituted piperine (PIP), while the filtrate (SBN) was used for subsequent purifications.
The SBN extract was dissolved at 1 g/mL in methanol, and 200 μL were injected using a preparative column (Phenomenex Luna C12(2) 250 × 21.2 mm, 5 μm) with elution at 7.0 mL/min using water (A), methanol (B) and acetonitrile (C). The gradient started with 55% B and 35% C and was maintained for 8 min, then increased to 50% B and 25% C at 10 min, maintained constant until 13 min, and then increased to 60% B and 40% C at 18 min and remained constant until 30 min. Fractions were collected and evaporated to dryness and then analyzed via UPLC-MS and NMR
Fraction 4 was subjected to a subsequent semipreparative process with a Synergi Polar-RP column (150 × 10 mm id, 4 μm; Phenomenex, Torrance, CA) using water (A) and acetonitrile (B) with a gradient from 50% B to 62.4% B in 7 min, 70% B at 13 min and up to 80% B at 20 min with a flow rate of 3.8 mL/min. Fractions were collected and evaporated to dryness and then analyzed via UPLC-MS and NMR.
2.7. Nuclear Magnetic Resonance Analysis
Analyses were performed with 5 mg of each compound dissolved in 0.75 mL of deuterated chloroform or deuterated methanol. Measurements were performed in a Bruker Ascend 400 MHz instrument, and chemical shifts (δ) are reported in ppm relative to internal tetramethylsilane (TMS, δ = 0.0 ppm) as standard.
2.8. Statistical Analysis
One-way analysis of variance (ANOVA), followed by Tukey’s post hoc test, was applied to piperamides quantification values, and differences were considered significant at p < 0.05.
3. Results and Discussion
3.1. Profile by UPLC-QTOF-ESI MS Analysis of Piperamides
The UPLC-QTOF-ESI MS analysis could identify 31 piperamides in the extracts from P. nigrum commercial dry fruits, prepared as described in the Materials and Methods section, from the three different commercial dry samples, namely Pn-d, Pn-j, and Pn-f. Extracts were obtained as oleoresins with an average yield of 8.62%. Table 1 and Figure 1 summarize the results of the piperamides identification analysis.

Table 1.
Profile of piperamides identified by UPLC-QTOF-ESI-MS analysis for P. nigrum samples.
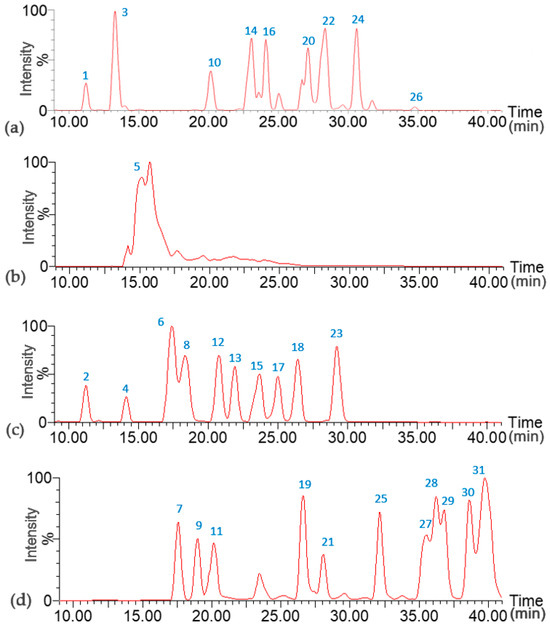
Figure 1.
UPLC-ESI-QTOF-HRMS extracted ion chromatograms of piperamides from Piper nigrum cultivated in Costa Rica. [M + H]+: (a) 274, 276, 330, 356, 358, 384, 398, 412, 426; (b) 286; (c) 272, 288, 314, 312, 340, 342, 344, 368, 370, 396; (d) 326, 328, 224, 386, 382, 332, 334, 332, 336, 346, 362. Peak numbers correspond to assignation as described in Table 1.
As stated, each chromatogram shows the corresponding extracted [M + H]+ ion for the respective compounds listed in Table 1 and discussed in this section. The first group of piperamides has a characteristic methylenedioxyphenyl moiety and an isobutyl bonded to the amide group (Figure 2). These compounds were identified by the characteristic fragments of [M + H − 73]+ due to the loss of the isobutylamine and [M + H − 101]+ from the loss of the amide group along with the isobutyl chain. Both fragmentations could occur with an additional CH2O loss from the methylenedioxy group cleavage, which generated the corresponding fragments at [M + H − 103]+ and [M + H − 131]+. An additional fragment at 135 Da was due to the rupture of the central chain to obtain a methylbenzodioxole group [20,21]. Finally, an ion at 161 Da was present because of the propenylbenzodioxole fragment [22].
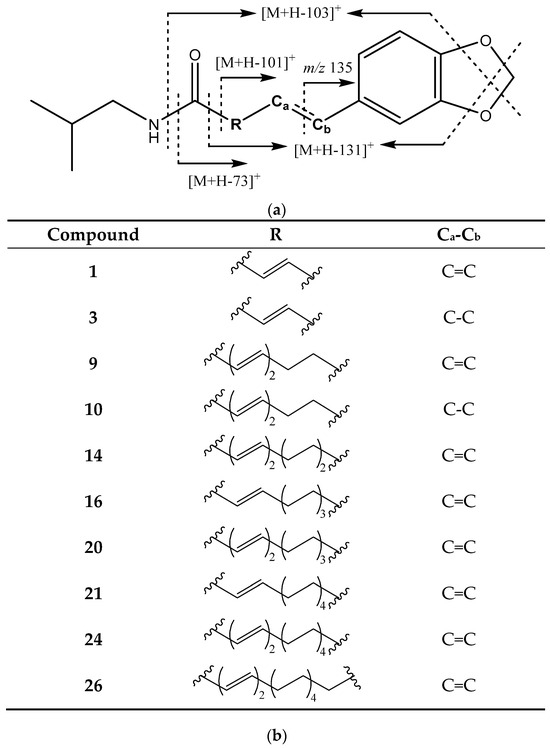
Figure 2.
(a) Structure and main fragments for piperamides with a methylenedioxyphenyl moiety and an isobutyl amine substituent. (b) R chain and Ca-Cb bonding for each piperamide in this group.
For instance, peaks 1 and 3 (Figure 1a) correspond to piperloguminine and dihydropiperlonguminine, respectively. Peak 1 shows fragments at 201 Da [M + H − 73]+, 173 Da [M + H − 101]+, and 135 Da, while peak 3 has fragments at 173 Da [M + H − 101]+, 145 Da [M + H − 131]+, and 135 Da. In turn, peak 9 (Figure 1a) corresponds to retrofractamide A, and peak 14 (Figure 1d) corresponds to retrofractamide B, both with fragments at [M + H − 73]+, [M + H − 101]+, 161 Da, and 135 Da. Peak 10 (Figure 1a) is identified as pipercallosine and shows fragments 135 Da and [M + H − 101]+ at 229 Da, while peak 16 (Figure 1a) corresponds to piperchabamide D with fragments at 285 Da [M + H − 73]+, 227 Da [M + H − 131]+, and 135 Da.
In turn, peak 20 (Figure 1a), identified as guineensine, shows fragments [M + H − 73]+ at 311 Da and [M + H − 101]+ at 283 Da, plus the two fragments of benzodioxole moiety corresponding to 161 and 135 Da. In peak 21 (Figure 1d), corresponding to piperflaviflorine A, fragments are present at 313 Da [M + H − 73]+ and 135 Da, while peak 24 (Figure 1a) corresponds to brachistamide B and exhibits fragments at 339 Da [M + H − 73]+, 311 Da [M + H − 101]+, 161 Da, and 135 Da. The last compound in this group is peak 26 (Figure 1a), assigned to brachistamide D, with a characteristic main fragment at 135 Da [20,22,23,24,25,26,27].
When the side chain is a pyrrolidine ring instead of the isobutyl group (Figure 3), the fragments produced are [M + H − 71]+ from the loss of this pirrolydine moiety and [M + H − 99]+ from the loss of pyrrolidine along with the carbonyl group. Additional loss of CH2O (30 Da) from the methylenedioxy group results, in these cases, in the loss of [M + H − 101]+ and [M + H − 129]+, respectively [20,21].
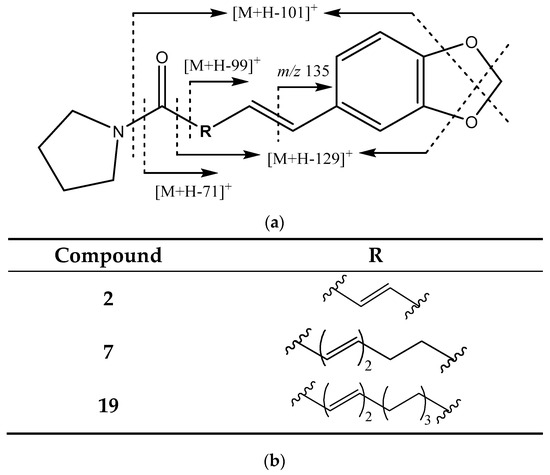
Figure 3.
(a) Structure and main fragments for piperamides with a methylenedioxyphenyl moiety and a pyrrolidine cycle. (b) R chain for each piperamide in this group.
This pattern led to the identification of peak 2 (Figure 1c) as piperyline, showing fragments at [M + H − 71]+ (201 Da), [M + H − 101]+ (171 Da), [M + H − 129]+ (143 Da), and 135 Da. In turn, peak 7 (Figure 1d) corresponds to (2E,4E,8E)-Piperamide-C9:3) and exhibits fragments at 227 Da [M + H − 99]+ and 197 Da [M + H − 129]+; peak 19 (Figure 1d), assigned to brachiamide A, presents fragments at 311 Da [M + H − 71]+, 283 Da [M + H − 99]+, 161 Da, and 135 Da [8,28,29].
The side chain can also be a piperidine ring. In this group of compounds, the loss of piperidine moeity gives the fragment at [M + H − 85]+, while the loss of the amide produces the ion at [M + H − 113]+. In both cases, an additional loss of CH2O generates the fragments [M + H − 115]+ and [M + H − 143]+ (Figure 4) [20,21].
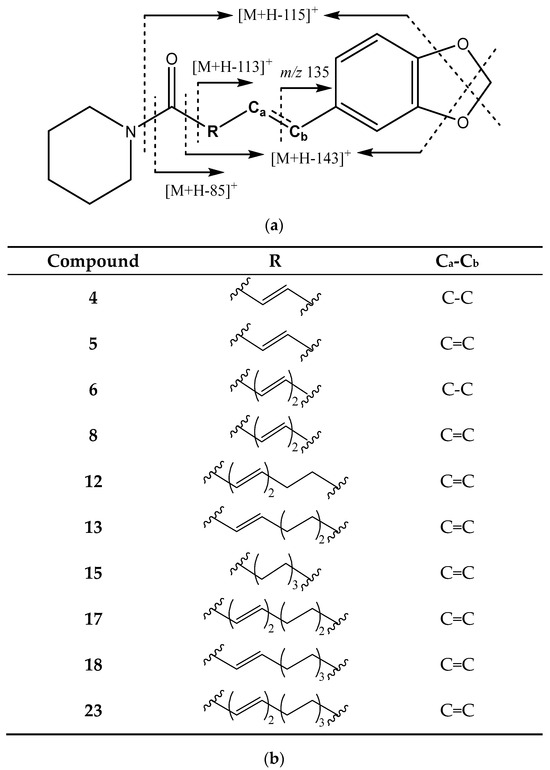
Figure 4.
(a) Structure and main fragments for piperamides with a methylenedioxyphenyl moiety and a piperidine ring. (b) R chain and Ca-Cb bonding for each piperamide in this group.
Peak 4 (Figure 1c) corresponds to piperanine and is identified by fragments at 203 Da [M + H − 85]+, 175 Da [M + H − 113]+, 145 Da [M + H − 143]+, as well as 161 Da and 135 Da. Peak 5 (Figure 1b), identified as piperine, holds fragments at 201 Da [M + H − 85]+, 171 Da [M + H − 115]+, 143 Da [M + H − 143]+, and 135 Da. In turn, peak 6 (Figure 1c), assigned to piperdardine, exhibits fragments at 199 Da [M + H − 115]+ and 161 Da, while peak 8 (Figure 1c) corresponds to piperettine and has fragments at 227 Da [M + H − 85]+, 199 Da [M + H − 113]+, and 169 Da [M + H − 143]+. Furthermore, peak 12 (Figure 1c), dehydropipernonaline, shows fragments [M + H − 85]+ at 255 Da, [M + H − 113]+ at 227 Da, 161 Da, and 135 Da and an additional fragment at 179 Da from the loss of the propenylbenzodioxole moiety [8,19,20,23,28,30,31].
Peak 13 (Figure 1c) corresponds to pipernonaline, with fragments at 229 Da [M + H − 113]+, 227 Da [M + H − 115]+, 199 Da [M + H − 143]+, 161 Da, and 135 Da. The main fragments of peak 15 (Figure 1c), identified as piperolein B, are shown at 201 Da [M + H − 143]+ and 135 Da, while peak 17 (Figure 1c), piperundecalidine, exhibits fragments at 255 Da [M + H − 113]+ and 135 Da. Peak 18 (Figure 1c), assigned to piperchabamide B, has fragments at 285 Da [M + H − 85]+, 161 Da, and 135 Da, while the last compound in this group, peak 23 (Figure 1c), corresponds to piperchabamide C and holds fragments at 311 Da [M + H − 85]+, 283 Da [M + H − 113]+, 161 Da, and 135 Da [8,19,20,23,28,30,31].
On the other hand, peak 22 (Figure 1a), identified as piperflaviflorine B, possesses a different fragmentation pattern (Figure 5), with fragments at 311 Da ([M + H − 87]+) and 283 Da ([M + H − 115]+), suggesting the presence of a 2-methylbutyl group in the amide, as previously described in the literature [32], and fragments at 161 Da and 135 Da are characteristic of the methylenedioxyphenyl moiety [32].
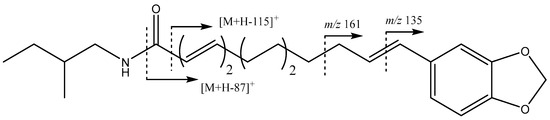
Figure 5.
Structure and main fragments for piperflaviflorine B 22.
The remaining group of peaks lacks the methylenedioxyphenyl group in their structures (Figure 6). Peak 11 (Figure 1d), assigned to pellitorine, shows fragments of an aliphatic unsaturated chain at 208 Da [M + H − CH4]+, 168 Da [M + H − C4H8]+, and 154 Da [M + H − C5H10]+ [29,33]. Peaks 25 and 28 (Figure 1d) were identified as isomers 1-(octadeca-2E,4E,12/13Z-trienoyl)pyrrolidine, with fragments at 261 Da [M + H − 71]+ and 233 Da [M + H − 99]+ from the loss of pyrrolidine and pyrrolidine amide, respectively, and an additional fragment at 304 Da [M + H − C2H4]+ [28,34], not showing fragments at 135 Da or 161 Da or the additional loss of 30 Da of CH2O from the methylenedioxyphenyl group.
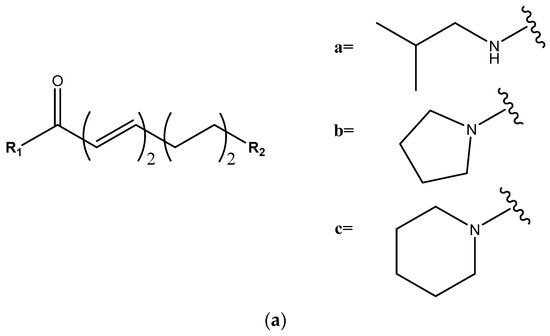
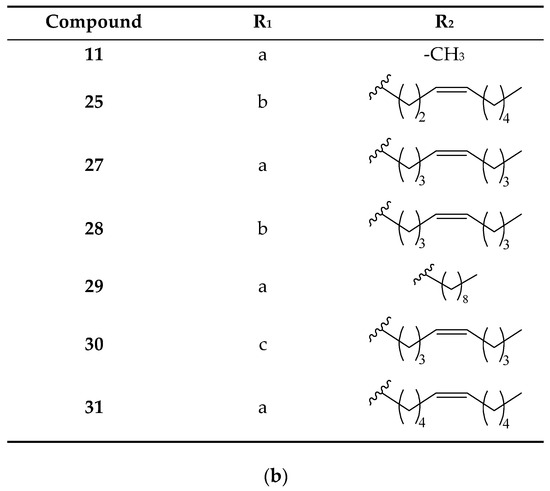
Figure 6.
(a) Structure for piperamides with an aliphatic unsaturated olefinic chain. (b) R1 and R2 substituents for each piperamide in this group.
The fragment at [M + H − 73]+ originated from the loss of isobutylamine, indicating the presence of an isobutyl lateral chain in peak 27 (Figure 1d) (2E,4E,13Z)-N-isobutyl-2,4,13-octadecatrienamide, peak 29 (Figure 1d) (2E,4E)-N-Isobutyl-2,4-octadecadienamide), and peak 31 (Figure 1d) (2E,4E,14Z)-N-Isobutyl-2,4,14-eicosatrienamide. Other observed fragments correspond to the rupture of the olefinic chain as [M + H − CH4]+ in peak 29, [M + H − C2H4]+ in peak 27, and [M + H − C4H8]+ in the three described peaks [28,35,36].
Finally, peak 30 (Figure 1d), assigned to 1-(octadeca-2E,4E,13Z-trienoyl)piperidine, had a fragment pertaining to the aliphatic unsaturated chain at [M + H − C2H4]+ and another at [M + H − 85]+ from the loss of piperidine [20,35].
3.2. Piperamides Quantification
The quantification of piperamides was carried out via UPLC-DAD with respect to a calibration curve of a piperine standard, with results expressed as mg of piperine equivalents (PE) per 100 g of dry sample and as a percentage of each piperamide in the extract composition, as described in the Materials and Methods section. Results are summarized in Table 2.

Table 2.
Piperamides content in samples of Piper nigrum cultivated in Costa Rica.
Regarding total piperamide contents, the Pn-j sample is the one with the highest value of 8514 mg PE/100 g dry material, which is 30% and 40% higher than the other two samples, Pn-f and Pn-d, respectively. This is in agreement with local producers’ information that samples growing under less-rainfall conditions (Pn-j) are richer in contents than samples under higher-rainfall conditions (Pn-d and Pn-f). However, further studies would be needed, along with information on soil and other cultivation conditions, to evaluate their relationships with secondary metabolite content. With respect to the individual piperamides, piperine was the most abundant, which is in agreement with the literature [19,24,29], constituting between 44–54% of total piperamides, followed by (2E,4E,13Z)-N-isobutyl-octadeca-2,4,13-trienamide, ranging from 8.9 to 12.2%, and pellitorine, with values between 8.1 and 9.6%.
Compared with the literature, our values for piperine are within the range reported in previous studies, varying from 3320 to 5220 mg/100 g dry material [19,24,29]. With respect to the other piperamides, piperanine and piperlonguminine results are within the range that was reported in the literature, indicating values of 0.8–1.1% and 0.5–1.9% of total piperamides, respectively [19], while no quantification results were reported in the literature for (2E,4E,13Z)-N-isobutyl-octadeca-2,4,13-trienamide, which is the piperamide with the second highest value in our study. In turn, results obtained for guineensine and pellitorine in Costa Rican black pepper dry fruits are higher than those reported by previous studies in the literature, which range between 0.76–1.18% and between 1.22–3.34%, respectively [24,29].
3.3. Semipreparative HPLC Analysis
Once piperine 5 was isolated from P. nigrum piperamides extracts through the procedure described in the Materials and Methods section, the remaining extract (SBN) was used for subsequent piperamides isolation through preparative and semipreparative HPLC separation. The solid isolated piperine 5 showed a molecular ion and fragmentation similar to the ones described in Section 3.1, and the HPLC-DAD analysis (Figure S1, Supplementary Materials) indicated 93% purity. 1H and 13C NMR analyses summarized in Figure S2 and Table S1 align with previous reports from the literature [28].
Main signals in the 1H-NMR spectrum (Figure S2a) correspond to the three aromatic protons (δ 6.88–7.15 ppm) with the characteristic 1,3, 4-substitution coupling pattern and to four other protons in the olefinic region (δ 6.66–7.19 ppm), showing coupling constants (J = 14.5–15.6 Hz) consistent with trans alkene bonds. 13C-NMR (Figure S2b) shows ten signals between δ 101.56 and 148.06 ppm corresponding to the aromatic and olefinic protons and a signal at low field (δ 165.01 ppm) that corresponds to the carbonyl group. An additional carbon is observed at δ 109.0 ppm that, along with the singlet at δ 5.90 ppm in the 1H-NMR spectra, corresponds to the methylendioxy group. In the aliphatic region, there are no methyl groups, and two carbons at δ 40–50 ppm (13C-NMR), along with the corresponding protons at δ 3.49 ppm (1H-NMR), are explained by the bonding with the nitrogen in the piperidine ring.
HPLC preparative separation performed in the SBN remaining extract allowed us to isolate guineensine in fraction 8 and (2E,4E,12Z)-N-isobutyl-octadeca-2,4,12-trienamide in fraction 12, whose structures were confirmed by HRMS and NMR. In fact, fraction 8 showed a molecular ion and fragmentation consistent with guineensine 20 (Figure 7), as explained in Section 3.1. Figures S3 and S4 show the HPLC-DAD chromatogram and the 1H and 13C NMR spectra. Table S2 summarizes the shifts and coupling constants for these spectra.
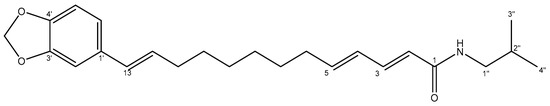
Figure 7.
Structure of isolated guineensine 20.
In fact, all signals shown in Table S2 and Figure S4 are coincident with the ones reported for guineensine in the literature [23]. For instance, the 13C-NMR spectrum (Figure S4b) shows peaks at δ 166.7 ppm, corresponding to the carbonyl moiety, and at δ 100.9 ppm, characteristic of the methylenedioxy group, along with the singlet at δ 5.95 ppm in the 1H-NMR spectrum (Figure S4a). In addition, the 13C-NMR shows twelve signals in the unsaturated region, consistent with the aromatic ring (δ 105.4–147.9 ppm) that, along with the respective signals in the 1H-NMR (δ 6.71–6.91 ppm), correspond to the coupling pattern for the 1,3,4 tri-substituted benzene ring.
The additional signals at δ 121.6, 128.3, 129.4, 141.5, and 143.3 ppm in the 13C-NMR, along with the signals at δ 5.77, 5.99–6.15, 6.30, and 7.80 ppm in the 1H-NMR, correspond to the olefinic signals with J = 15.0 and 15.8 Hz, indicating trans double bonds. Other representative signals are observed in the 13C-NMR at δ 47.0 ppm that, along with the signal at δ 3.16 ppm (1H-NMR), agree with C-N bonding and one important methyl signal at δ 20.1 ppm that, along a doublet at δ 0.92 ppm (1H-NMR), corresponds to an isobutyl group in the lateral chain of the amide.
Guineensine 20 was isolated with a yield of 209.7 mg/100 g dry black pepper, which is higher than reported in the literature. For instance, Nicolussi et al. [13] reported for P. nigrum an extraction with chloroform followed by column chromatography and preparative HPLC fractionation, obtaining 12.2 mg of purified guineensine (2.17 mg/100 g dry black pepper). On the other hand, Ramesh et al. [29] analyzed a methanolic extract of P. nigrum by HPTLC, obtaining 51 mg/100 g dry black pepper, while Rao et al. [24] obtained 48.9 mg/100 g dry black pepper for a methanolic extract analyzed by HPLC. Therefore, our results are higher than those in these reports, as well as the values obtained for guineensine isolated from Piper longum [13].
This result is important because of the different reported bioactive properties of guineensine, for instance, inhibiting endocannabinoid uptake [13] and significant neuro-pharmacological interactions when tested against CNS-related receptors, channels, and transporters [14]. In drug design studies for neuropathic pain regulation, bioactivity results obtained through quantitative structure–activity relationship (QSAR) model predictions found an association between bioactivity strength and chain length and molecular insights suggesting that the inhibitory mechanism may be related to the ability to initiate unstable vibrations in fatty acid hydrolase (FAAH)-like anandamide transporter [37]. Furthermore, guineensine’s bioactivity goes beyond neuromodulation, having the ability to bind to and block the activities of two dual targets of secreted proteins and membrane-bound enzymes with high specificity from microbial pathogens [38].
Along with guineensine, (2E,4E,12Z)-N-isobutyl-octadeca-2,4,12-trienamide 25 was isolated in fraction 12 and characterized using HRMS and NMR. The molecular ion [M + H]+ at 334 Da and fragmentation were consistent with the description (Section 3.1) for this trienamide 25 (Figure 8), which was obtained with a yield of 467.0 mg/100 g dry material. Figures S5 and S6 show the UPLC-DAD chromatogram and the 1H and 13C NMR spectra, and Table S3 summarizes the shifts and coupling constants for these spectra.
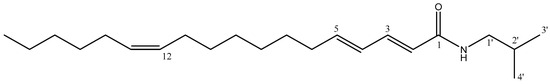
Figure 8.
Structure of isolated (2E,4E,12Z)-N-isobutyl-octadeca-2,4,12-trienamide 25.
The signals shown in Table S3 and Figure S6 for the 1H and 13C-NMR spectra are consistent with previous reports [23]. For instance, in the 13C-NMR (Figure S6b), the carbonyl signal is observed at δ 166.4 ppm, and a total of six olefinic carbons are present (δ 121.7–143.3 ppm), while in the 1H-NMR (Figure S6a), the signals between δ 5.36 and 7.21 ppm correspond to the six olefinic protons. Furthermore, the different coupling constants for four of them (J = 14.9–15.0 Hz) indicate their trans alkene bonding, while the other two olefinic protons (δ 5.36 ppm) have a low coupling constant, indicating a cis double bond. The isobutyl chain can be observed similarly as described for the previous compound, with a signal at δ 46.9 ppm (13C-NMR) corresponding to the C-1′ bonded to the nitrogen, along with the signal at δ 3.18 ppm (1H-NMR), and the two equivalent methyl groups at 20.1 ppm (13C-NMR), along with a doublet signal at 0.91 ppm (1H-NMR).
The isolation of this molecule is relevant because of previous reports on biological activities from similar compounds. For instance, (2E,4E,14Z)-N-isobutyleicosa-2,4,14-trienamide from P. longum exerted significant inhibitory effects on phenylephrine-induced mesenteric artery vasoconstriction [39], while another trienamide, 2E,4E,14Z)-N-(4-methylpentyl)octadeca-2,4,12-trienamide, identified in P. nigrum, exhibited important activity against larvae of Aedes aegypti, as determined by the World Health Organization (WHO) methodology [40]. In sensory studies, 1-(octadeca-2E,4E,13Z-trienyl)piperidine, among other similar compounds, displayed both a pungent impression and a long-lasting tingling sensation at higher concentrations [28]. Furthermore, some dienamides and trienamides from the Piper genus have exhibited hepatoprotective activities on tumor necrosis factor (TNF-α)-induced cytotoxicity in L929 cells [41].
Fraction 4 from the aforementioned preparative HPLC consisted of a mixture of two main compounds; therefore, a semi-preparative HPLC separation was performed, as described in Section 2.7, resulting in the isolation of these two compounds, identified as pellitorine 11 and retrofractamide B 14 through HRMS and NMR analyses. In fact, the molecular ion [M + H]+ at 224 Da and fragmentation were consistent with the description (Section 3.1) for pellitorine 11 (Figure 9), which was isolated with a yield of 361.8 mg/100 g dry material. Figures S7 and S8 show the UPLC-DAD chromatogram and the 1H and 13C NMR spectra.
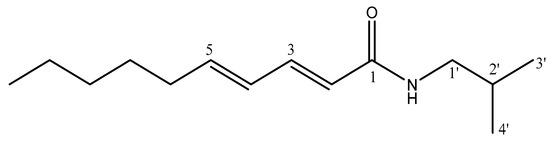
Figure 9.
Structure of isolated pellitorine 11.
1H and 13C NMR data shown in Table S3 and Figure S8 are consistent with literature [23]. For instance, 13C-NMR (Figure S8b) shows a signal at δ 167.8 ppm consistent with the carbonyl group and four signals at δ 121.6–142.6 ppm from olefinic carbons with their corresponding protons at δ 5.77–7.21 ppm in the 1H-NMR (Figure S8a) with coupling constants of J = 14.9–15.0 Hz, indicating trans alkene bonding. In addition, the signals at δ 46.6 and δ 19.1 ppm (13C-NMR), along with the doublets at δ 3.08 and 0.94 ppm (1H-NMR), are attributed to C-1′ and the two equivalent methyl groups, characteristic of the isobutyl moiety.
These results are relevant because of pellitorine’s different reported bioactivities. For instance, its anti-inflammatory effects, along with piperine, were reported to inhibit the production of inflammatory factors and to reduce oxidative damage, attenuating the production of ROS [42]. In fact, in vitro and in mouse model studies proved promising for severe vascular inflammatory diseases, like sepsis and septic shock, because of pellitorine inhibiting the release of the high-mobility group box 1 (HMGB1) triggered by lipopolysaccharide and cecal ligation and puncture [43]. Previous pharmacokinetic tests found that pellitorine could cross the colorectal adenocarcinoma (Caco-2) cells monolayer and the rat gut when administered orally, and in the case of intravenous administration in mice, pellitorine also showed rapid penetration of the blood–brain barrier into the brain parenchyma, supporting the potential use of Anacyclus pyrethrum extract, containing pellitorine, in treating central nervous system diseases [44]. Furthermore, pellitorine has been found to display strong cytotoxicity against human leukemic cell line 60 (HL60) and Michigan Cancer Foundation-7 (MCF-7) cancer cells [45] as well as to exhibit anticoagulant effects in mice [46].
Finally, the molecular ion [M + H]+ at 256 Da and fragmentation were consistent with the description (Section 3.1) for retrofractamide B 14 (Figure 10), which was obtained with a yield of 134.0 mg/100 g dry material. Figures S9 and S10 show the UPLC-DAD chromatogram and the 1H and 13C NMR spectra.
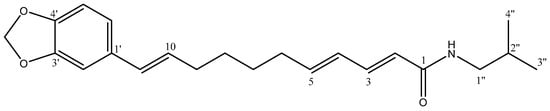
Figure 10.
Structure of isolated retrofractamide B 14.
The data in Table S2 and Figure S10 for the 1H and 13C-NMR spectra of retrofractamide B 14 are consistent with those in the previous literature [23]. The 13C-NMR spectrum (Figure S10b) shows a signal at δ 167.8 ppm, corresponding to the carbonyl group, and another at δ 100.8 ppm, which is consistent with the methylenedioxy carbon. In addition, twelve signals in the double-bond region are observed (δ 100.8–148.0 ppm), which correspond to the 1,3,4 tri-substituted aromatic ring and the three double bonds. Finally, eight aliphatic carbons, including one signal at δ 46.6 ppm from C-1’’ bonded to the nitrogen and two methyl groups at δ 19.1 ppm from the isobutyl moiety, are observed. With respect to 1H-NMR signals (Figure S10a), the singlet at δ 5.92 ppm corresponds to the methylenedioxy protons, and signals at δ 6.73, 6.78, and 6.92 ppm pertain to the aromatic ring. In addition, the coupling constants for the olefinic protons (J = 15.1–15.8 Hz) indicate the trans double bonds. Finally, the doublets at δ 3.08 ppm and δ 0.94 ppm correspond to H-1″ and the two equivalent methyl groups from the isobutyl chain, respectively.
Several studies have reported different biological activities for retrofractamide B. For instance, some authors proposed that besides piperine, retrofractamide B can be responsible for the transient receptor potential vanilloid 1 (TRPV1) activation [47,48]. TRPV1 has become an attractive target for many pathologies, as its activation has roles in the pathogenesis of chronic inflammatory skin diseases, thermogenesis [48], diabetes, and bladder and lung function [49]. Retrofractamide B has been found to significantly increase the accumulation of triglyceride levels and to promote adipogenesis of 3T3-L1 cells [50]. In turn, P. nigrum extract containing retrofractamide Bm, along with piperine, pellitorine, and guineensine, has been reported to improve cell viability in neurotoxin 6-hydroxydopamine-induced SK-N-SH and SH-SY5Y (human neuroblastoma cell lines), showing the characteristics of amide alkaloids and their neuroprotective activities [51].
Compared with the literature, no previous isolation yield has been reported for the (2E,4E,12Z)-N-isobutyl-octadeca-2,4,12-trienamide, and in the case of pellitorine and retrofractamide B, our results yield higher values than those reported by previous studies on Piper nigrum from Korea [52,53] and India [54]. Therefore, these results, along with the high yields obtained for the (2E,4E,12Z)-N-isobutyl-octadeca-2,4,12-trienamide and guineensine, indicate the interest in further phytochemical and bioactivity studies of these Costa Rican species extracts and their isolated piperamides.
4. Conclusions
The analysis of piperamides-enriched extracts from P. nigrum commercial dry fruits cultivated in Costa Rica using the UPLC-QTOF-ESI MS technique allowed us to characterize a total of 31 different piperamides with and without a methylenedioxyphenyl moiety in their structures and with different N-substituents, including piperidine, pyrrolidine, isobutyl, and 2-methylbutyl groups. UPLC-DAD analyses enabled the quantification of 15 piperamides with values within the range reported in previous studies, such as the ones from piperine, piperyline, piperanine, and piperlonguminine, or higher than those from the literature, such as those obtained for guineensine and pellitorine. Furthermore, besides piperine, four other piperamides, namely retrofractamide B, pellitorine, (2E,4E,12Z)-N-isobutyl-octadeca-2,4,12-trienamide, and guineensine, were isolated with excellent yields, higher than literature findings. These results, along with the presence of diverse piperamides, which are reported for the first time for P. nigrum cultivated in Costa Rica, suggest the potential of these extracts for further research. Hence, structure–activity relationship studies can contribute to the increase in knowledge on fruits as a source of dietary compounds and their associated bioactivities with potential health benefits.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9121323/s1, Figure S1: HPLC-DAD chromatogram for isolated piperine 5; Figure S2: (a) 1H-RMN (b) 13C-RMN from isolated piperine 5; Table S1: 1H and 13C NMR shifts for isolated piperine 5; Figure S3: HPLC-DAD chromatogram for isolated guineensine 20; Figure S4: (a) 1H-RMN (b) 13C-RMN from isolated guineensine 20. Table S2: Shifts for 1H and 13C NMR analysis of guineensine 20 and retrofractamide B 14; Figure S5: UPLC-DAD chromatogram from isolated (2E,4E,12Z)-N-isobutyl-octadeca-2,4,12-trienamide 25; Figure S6: (a) 1H-RMN (b) 13C-RMN from isolated (2E,4E,12Z)-N-isobutyl-octadeca-2,4,12-trienamide 25; Table S3: Shifts for 1H and 13C NMR analysis of pellitorine 11 and (2E,4E,12Z)-N-isobutyl-octadeca-2,4,12-trienamide 25; Figure S7: UPLC-DAD chromatogram from isolated pellitorine 11; Figure S8: (a) 1H-RMN (b) 13C-RMN from isolated pellitorine 11; Figure S9: UPLC-DAD chromatogram from isolated retrofractamide B 14; Figure S10: (a) 1H-RMN (b) 13C-RMN from isolated retrofractamide B 14.
Author Contributions
Conceptualization, L.F.V.-H. and M.N.-H.; methodology, M.N.-H., A.M.A.-S. and L.F.V.-H.; formal analysis, L.F.V.-H., A.S.-K.; investigation, L.F.V.-H., M.N.-H., A.M.A.-S. and L.D.A.-C.; resources, M.N.-H., A.S.-K. and A.M.A.-S., data curation, L.F.V.-H., M.N.-H., A.M.A.-S. and A.S.-K.; writing—original draft preparation, L.F.V.-H., A.S.-K. and L.D.A.-C.; writing—review and editing, M.N.-H. and A.M.A.-S.; supervision, M.N.-H. and A.M.A.-S.; project administration, M.N.-H.; funding acquisition, M.N.-H. and A.M.A.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially funded by grants from the University of Costa Rica (115-B6-163, 115-C0-001, 115-C3-005, ED-2033).
Data Availability Statement
The data from this study is in the manuscript and the corresponding supplementary material.
Acknowledgments
Authors thank the University of Costa Rica (UCR), the National Laboratory of Nanotechnology (LANOTEC) and the Costa Rica Institute of Technology (TEC).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant Capacity of 26 Spice Extracts and Characterization of Their Phenolic Constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.P. The Geography of Black Pepper (Piper nigrum): The “King” of Spices—Volume 1; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-52864-5. [Google Scholar]
- Srinivasan, K. Black Pepper (Piper nigrum) and Its Bioactive Compound, Piperine. In Molecular Targets and Therapeutic Uses of Spices; World Scientific: Singapore, 2009; pp. 25–64. ISBN 978-981-283-790-5. [Google Scholar]
- Katarina, S.; Darinka, A.G.; Aleksandar, C.; Tatjana, R.; Bojana, V.; Mustafa, A. Piperine: Old Spice and New Nutraceutical? Curr. Pharm. Des. 2019, 25, 1729–1739. [Google Scholar] [CrossRef]
- Wilhelm-Romero, K.; Quirós-Fallas, M.I.; Vega-Baudrit, J.R.; Guillén-Girón, T.; Vargas-Huertas, F.; Navarro-Hoyos, M.; Araya-Sibaja, A.M. Evaluation of Piperine as Natural Coformer for Eutectics Preparation of Drugs Used in the Treatment of Cardiovascular Diseases. AAPS PharmSciTech 2022, 23, 127. [Google Scholar] [CrossRef] [PubMed]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A Systematic Review on Black Pepper (Piper nigrum L.): From Folk Uses to Pharmacological Applications. Crit. Rev. Food Sci. Nutr. 2019, 59, S210–S243. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Jung, J.; Kim, M.; Lee, C.; Cho, S.; Um, M. Effect of Black Pepper (Piper nigrum) Extract on Caffeine-Induced Sleep Disruption and Excitation in Mice. Nutrients 2022, 14, 2249. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef] [PubMed]
- Onyesife, C.O.; Chukwuma, I.F.; Okagu, I.U.; Ndefo, J.C.; Amujiri, N.A.; Ogugua, V.N. Nephroprotective Effects of Piper nigrum Extracts against Monosodium Glutamate-Induced Renal Toxicity in Rats. Sci. Afr. 2023, 19, e01453. [Google Scholar] [CrossRef]
- Lasso, P.; Rojas, L.; Arévalo, C.; Urueña, C.; Murillo, N.; Nossa, P.; Sandoval, T.; Chitiva, L.C.; Barreto, A.; Costa, G.M.; et al. Piper nigrum Extract Suppresses Tumor Growth and Enhances the Antitumor Immune Response in Murine Models of Breast Cancer and Melanoma. Cancer Immunol. Immunother. 2023, 72, 3279–3292. [Google Scholar] [CrossRef]
- Turrini, E.; Sestili, P.; Fimognari, C. Overview of the Anticancer Potential of the “King of Spices” Piper nigrum and Its Main Constituent Piperine. Toxins 2020, 12, 747. [Google Scholar] [CrossRef]
- Mitra, S.; Anand, U.; Jha, N.K.; Shekhawat, M.S.; Saha, S.C.; Nongdam, P.; Rengasamy, K.R.R.; Proćków, J.; Dey, A. Anticancer Applications and Pharmacological Properties of Piperidine and Piperine: A Comprehensive Review on Molecular Mechanisms and Therapeutic Perspectives. Front. Pharmacol. 2022, 12, 772418. [Google Scholar] [CrossRef]
- Nicolussi, S.; Viveros-Paredes, J.M.; Gachet, M.S.; Rau, M.; Flores-Soto, M.E.; Blunder, M.; Gertsch, J. Guineensine is a novel inhibitor of endocannabinoid uptake showing cannabimimetic behavioral effects in BALB/c mice. Pharmacol. Res. 2014, 80, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Reynoso-Moreno, I.; Najar-Guerrero, I.; Escareño, N.; Flores-Soto, M.E.; Gertsch, J.; Viveros-Paredes, J.M. An Endocannabinoid Uptake Inhibitor from Black Pepper Exerts Pronounced Anti-Inflammatory Effects in Mice. J. Agric. Food Chem. 2017, 65, 9435–9442. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Jain, S.; Jangid, R.; Sharma, M.K. Cytochrome P450 and P-Gp Mediated Herb–Drug Interactions of Some Common Indian Herbs. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2022; Volume 72, pp. 225–258. ISBN 978-0-12-823944-5. [Google Scholar]
- Lieder, B.; Zaunschirm, M.; Holik, A.-K.; Ley, J.P.; Hans, J.; Krammer, G.E.; Somoza, V. The Alkamide Trans-Pellitorine Targets PPARγ via TRPV1 and TRPA1 to Reduce Lipid Accumulation in Developing 3T3-L1 Adipocytes. Front. Pharmacol. 2017, 8, 316. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ku, S.-K.; Min, B.-W.; Lee, S.; Jee, J.-G.; Kim, J.A.; Bae, J.-S. Vascular Barrier Protective Effects of Pellitorine in LPS-Induced Inflammation In Vitro and In Vivo. Fitoterapia 2014, 92, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Matsuda, H.; Yamaguchi, I.; Pongpiriyadacha, Y.; Yoshikawa, M. New Amides and Gastroprotective Constituents from the Fruit of Piper chaba. Planta Med. 2004, 70, 152–159. [Google Scholar] [CrossRef]
- Friedman, M.; Levin, C.E.; Lee, S.U.; Lee, J.S.; Ohnisi-Kameyama, M.; Kozukue, N. Analysis by HPLC and LC/MS of pungent piperamides in commercial black, white, green, and red whole and ground peppercorns. J. Agric. Food Chem. 2008, 56, 3028–3036. [Google Scholar] [CrossRef]
- Li, K.; Fan, Y.; Wang, H.; Fu, Q.; Jin, Y.; Liang, X. Qualitative and quantitative analysis of an alkaloid fraction from Piper longum L. using ultra-high performance liquid chromatography-diode array detector-electrospray ionization mass spectrometry. J. Pharm. Biomed. Anal. 2015, 109, 28–35. [Google Scholar] [CrossRef]
- Chithra, S.; Jasim, B.; Anisha, C.; Mathew, J.; Radhakrishnan, E.K. LC-MS/MS Based Identification of Piperine Production by Endophytic Mycosphaerella sp. PF13 from Piper nigrum. Appl. Biochem. Biotechnol. 2014, 173, 30–35. [Google Scholar] [CrossRef]
- Zaugg, J.; Baburin, I.; Strommer, B.; Kim, H.J.; Hering, S.; Hamburger, M. HPLC-based activity profiling: Discovery of piperine as a positive GABAA receptor modulator targeting a benzodiazepine-independent binding site. J. Nat. Prod. 2010, 73, 185–191. [Google Scholar] [CrossRef]
- Li, K.; Zhu, W.; Fu, Q.; Ke, Y.; Jin, Y.; Liang, X. Purification of amide alkaloids from Piper longum L. using preparative two-dimensional normal-phase liquid chromatography reversed-phase liquid chromatography. Analyst 2013, 138, 3313–3320. [Google Scholar] [CrossRef]
- Rao, V.R.S.; Raju, S.S.; Sarma, V.U.; Sabine, F.; Babu, K.H.; Babu, K.S.; Rao, J.M. Simultaneous determination of bioactive compounds in Piper nigrum L. and a species comparison study using HPLC-PDA. Nat. Prod. Res. 2011, 25, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.M.; Jensen, H.R.; Philogène, B.J.R.; Arnason, J.T. A review of Piper spp. (Piperaceae) phytochemistry, insecticidal activity and mode of action. Phytochem. Rev. 2008, 7, 65–75. [Google Scholar] [CrossRef]
- Banerji, A.; Sarkar, M.; Datta, R.; Sengupta, P.; Abraham, K. Amides from Piper brachystachyum and Piper retrofractum. Phytochemistry 2002, 59, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Okwute, S.K.; Egharevba, H.O. Piperine-Type Amides: Review of the Chemical and Biological Characteristics. Int. J. Chem. 2013, 5, 99–122. [Google Scholar] [CrossRef]
- Dawid, C.; Henze, A.; Frank, O.; Glabasnia, A.; Rupp, M.; Büning, K.; Orlikowski, D.; Bader, M.; Hofmann, T. Structural and Sensory Characterization of Key Pungent and Tingling Compounds from Black Pepper (Piper nigrum L.). J. Agric. Food Chem. 2012, 60, 2884–2895. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, B.; Sarma, V.; Kumar, K.; Babu, K.S.; Devi, P.S. Simultaneous Determination of Six Marker Compounds in Piper nigrum L. and Species Comparison Study Using High-Performance Thin-Layer Chromatography–Mass Spectrometry. J. Planar Chromatogr. 2015, 28, 280–286. [Google Scholar] [CrossRef]
- Liu, H.L.; Luo, R.; Chen, X.Q.; Ba, Y.Y.; Zheng, L.; Guo, W.W.; Wu, X. Identification and simultaneous quantification of five alkaloids in Piper longum L. by HPLC-ESI-MSn and UFLC-ESI-MS/MS and their application to Piper nigrum L. Food Chem. 2015, 177, 191–196. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, J.X.; Da-Cunha, E.V.L.; Maria, M.C.; Gray, A.I. Piperdardine, a piperidine alkaloid from Piper tuberculatum. Phytochemistry 1997, 44, 559–561. [Google Scholar] [CrossRef]
- Shi, Y.N.; Liu, F.F.; Jacob, M.R.; Li, X.C.; Zhu, H.T.; Wang, D.; Cheng, R.R.; Yang, C.R.; Xu, M.; Zhang, Y.J. Antifungal Amide Alkaloids from the Aerial Parts of Piper flaviflorum and Piper sarmentosum. Planta Med. 2017, 83, 143–150. [Google Scholar] [CrossRef]
- Scott, I.M.; Puniani, E.; Jensen, H.; Livesey, J.F.; Poveda, L.; Sánchez-Vindas, P.; Durst, T.; Arnason, J.T. Analysis of Piperaceae Germplasm by HPLC and LCMS: A Method for Isolating and Identifying Unsaturated Amides from Piper spp. Extracts. J. Agric. Food Chem. 2005, 53, 1907–1913. [Google Scholar] [CrossRef]
- Da Luz, S.; Yamaguchi, L.; Kato, M.; De Lemos, O.; Xavier, L.; Maia, J.; Ramos, A.; Setzer, W.; Da Silva, J. Secondary Metabolic Profiles of Two Cultivars of Piper nigrum (Black Pepper) Resulting from Infection by Fusarium solani f. sp. Piperis. Int. J. Mol. Sci. 2017, 18, 2434. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Kawabata, M.; Ishida, E.; Akazawa, Y.; Takei, Y.; Nakatani, N. LC-MS Analysis and Structural Determination of New Amides from Javanese Long Pepper (Piper retrofractum). Biosci. Biotechnol. Biochem. 1993, 57, 1329–1333. [Google Scholar] [CrossRef]
- Wei, K.; Li, W.; Koike, K.; Pei, Y.; Chen, Y.; Nikaido, T. New Amide Alkaloids from the Roots of Piper nigrum. J. Nat. Prod. 2004, 67, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Tou, W.I.; Chang, S.-S.; Lee, C.-C.; Chen, C.Y.-C. Drug Design for Neuropathic Pain Regulation from Traditional Chinese Medicine. Sci. Rep. 2013, 3, 844. [Google Scholar] [CrossRef]
- Mgbeahuruike, E.E.; Yrjönen, T.; Vuorela, H.; Holm, Y. Bioactive Compounds from Medicinal Plants: Focus on Piper Species. S. Afr. J. Bot. 2017, 112, 54–69. [Google Scholar] [CrossRef]
- Li, D.; Wang, R.; Cheng, X.; Yang, J.; Yang, Y.; Qu, H.; Li, S.; Lin, S.; Wei, D.; Bai, Y.; et al. Chemical Constituents from the Fruits of Piper longum L. and Their Vascular Relaxation Effect on Rat Mesenteric Arteries. Nat. Prod. Res. 2020, 36, 674–679. [Google Scholar] [CrossRef]
- Siddiqui, B.S.; Gulzar, T.; Mahmood, A.; Begum, S.; Khan, B.; Afshan, F. New Insecticidal Amides from Petroleum Ether Extract of Dried Piper nigrum L. Whole Fruits. Chem. Pharm. Bull. 2004, 52, 1349–1352. [Google Scholar] [CrossRef]
- Matsuda, H.; Ninomiya, K.; Morikawa, T.; Yasuda, D.; Yamaguchi, I.; Yoshikawa, M. Hepatoprotective Amide Constituents from the Fruit of Piper chaba: Structural Requirements, Mode of Action, and New Amides. Bioorg. Med. Chem. 2009, 17, 7313–7323. [Google Scholar] [CrossRef]
- Duan, Z.; Xie, H.; Yu, S.; Wang, S.; Yang, H. Piperine Derived from Piper nigrum L. Inhibits LPS-Induced Inflammatory through the MAPK and NF-κB Signalling Pathways in RAW264.7 Cells. Foods 2022, 11, 2990. [Google Scholar] [CrossRef]
- Ku, S.-K.; Lee, I.-C.; Kim, J.A.; Bae, J.-S. Anti-Septic Effects of Pellitorine in HMGB1-Induced Inflammatory Responses In Vitro and In Vivo. Inflammation 2014, 37, 338–348. [Google Scholar] [CrossRef]
- Veryser, L.; Bracke, N.; Wynendaele, E.; Joshi, T.; Tatke, P.; Taevernier, L.; De Spiegeleer, B. Quantitative In Vitro and In Vivo Evaluation of Intestinal and Blood-Brain Barrier Transport Kinetics of the Plant N-Alkylamide Pellitorine. Biomed Res. Int. 2016, 2016, 5497402. [Google Scholar] [CrossRef] [PubMed]
- Ee, G.C.L.; Lim, C.M.; Rahmani, M.; Shaari, K.; Bong, C.F.J. Pellitorine, a Potential Anti-Cancer Lead Compound against HL60 and MCT-7 Cell Lines and Microbial Transformation of Piperine from Piper nigrum. Molecules 2010, 15, 2398–2404. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.-K.; Lee, I.-C.; Kim, J.A.; Bae, J.-S. Antithrombotic Activities of Pellitorine In Vitro and In Vivo. Fitoterapia 2013, 91, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Panthong, S.; Imai, Y.; Matsuoka, T.; Suzuki, W.; Watanabe, T.; Terada, Y.; Kurohane, K.; Sekiguchi, K.; Ogawa, E.; Endo, Y.; et al. The Role of Piper chaba Hunt. and Its Pure Compound, Piperine, on TRPV1 Activation and Adjuvant Effect. BMC Complement. Med. Ther. 2020, 20, 134. [Google Scholar] [CrossRef] [PubMed]
- Okumura, Y.; Narukawa, M.; Iwasaki, Y.; Ishikawa, A.; Matsuda, H.; Yoshikawa, M.; Watanabe, T. Activation of TRPV1 and TRPA1 by Black Pepper Components. Biosci. Biotechnol. Biochem. 2010, 74, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Brito, R.; Sheth, S.; Mukherjea, D.; Rybak, L.; Ramkumar, V. TRPV1: A Potential Drug Target for Treating Various Diseases. Cells 2014, 3, 517–545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Matsuda, H.; Nakamura, S.; Yoshikawa, M. Effects of Amide Constituents from Pepper on Adipogenesis in 3T3-L1 Cells. Bioorganic Med. Chem. Lett. 2008, 18, 3272–3277. [Google Scholar] [CrossRef]
- Yu, L.; Hu, X.; Xu, R.; Ba, Y.; Chen, X.; Wang, X.; Cao, B.; Wu, X. Amide Alkaloids Characterization and Neuroprotective Properties of Piper nigrum L.: A Comparative Study with Fruits, Pericarp, Stalks and Leaves. Food Chem. 2022, 368, 130832. [Google Scholar] [CrossRef]
- Park, I.-K.; Lee, S.-G.; Shin, S.-C.; Park, J.-D.; Ahn, Y.-J. Larvicidal Activity of Isobutylamides Identified in Piper nigrum Fruits against Three Mosquito Species. J. Agric. Food Chem. 2002, 50, 1866–1870. [Google Scholar] [CrossRef]
- Ngo, Q.T.; Tran, P.T.; Tran, M.H.; Kim, J.A.; Rho, S.S.; Lim, C.-H.; Kim, J.-C.; Woo, M.H.; Choi, J.S.; Lee, J.-H.; et al. Alkaloids from Piper nigrum Exhibit Antiinflammatory Activity via Activating the Nrf2/HO1 Pathway: Alkaloids from Piper nigrum Activate the Nrf2/HO1 Pathway. Phytother. Res. 2017, 31, 663–670. [Google Scholar] [CrossRef]
- Venkat Reddy, S.; Srinivas, P.V.; Praveen, B.; Hara Kishore, K.; China Raju, B.; Suryanarayana Murthy, U.; Madhusudana Rao, J. Antibacterial Constituents from the Berries of Piper nigrum. Phytomedicine 2004, 11, 697–700. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).