Abstract
Current agriculture faces the challenge of producing food with the least interference from the environment. In this sense, the implementation of ecological agricultural practices is essential to obtaining healthy and more sustainable production systems. The objective of this study was to investigate the impact of different inoculation technologies on formulations of bokashi-type biofertilizer and its application as soil and substrate organic amendment. We examined the effects of treatments on the chemical and biological quality of the evaluated formulations, as well as their influence on the agronomic and nutritional characteristics of red-beet and cabbage crops in the field. The metagenomic analysis of the taxonomic profile of the microbiological populations revealed relative abundance of plant growth-promoting rhizobacterial genera, including Azospirillum sp., Rhizobium sp., Bradyrhizobium sp., Burkholderia sp., Paraburkholderia sp., and Paenibacillus sp. in the evaluated formulations. Additionally, no phytopathogenic contaminants were detected among the investigated treatments. The highest yields of field-grown beet crops were obtained from seedlings produced using the following treatments: bokashi biofertilizer + biodynamic preparations P502-P507, bokashi + Chamomilla 12 CH, and bokashi + Carbo vegetabilis 12 CH. The increase in productivity of cabbage plants was higher in the treatment bokashi + Calcarea carbonica 12 CH.
1. Introduction
Catering to the growing global demand for high-quality food brings substantial challenges for modern agriculture [1]. To establish resilient agri-food systems, it is necessary to mitigate the adverse environmental repercussions arising from human activities, which contribute to the degradation and decrease of soil fertility and biodiversity loss. These challenges are particularly pronounced in the face of climate change. Practices that simplify ecological interactions in agroecosystems, such as monoculture, intense soil disturbance, over-reliance on soluble mineral fertilizers, and the utilization of harmful chemical compounds, can interfere with trophic networks and alter the composition of biotic communities. This interference leads to a decline in the diversity of beneficial species within ecosystems, thereby compromising their multifunctional capacities. The integration of ecological agricultural practices with smart management strategies constitutes a pivotal approach to addressing these complex challenges [2,3].
As a general trend, Brazilian soils tend to be characterized by their acidic nature and chemical deficiencies, often displaying a high degree of weathering. Correcting these inherent limitations and fulfilling the nutritional requirements of crops necessitates the strategic application of fertilizers and soil amendments. In 2022, the agricultural sector in Brazil recorded an approximate consumption of 38.2 million tons of fertilizer within the national market, with a substantial portion being imported (33.8 million tons) [4]. This reliance on external sources, combined with uncertainties in the input supply chain, dwindling global mineral reserves, and imbalances in trade dynamics, elevates concerns about the nation’s food security [5]. The horticultural landscape in Brazil comprises 336 thousand establishments dedicated to cultivating over 100 diverse horticultural species across a sprawling area of 1.6 million hectares. This important sector contributes to an annual production of roughly 30 million tons of vegetables while also providing livelihoods for nearly 2.4 million individuals and contributing around BRL 55 billion annually to the national economy [6]. However, Brazilian horticulture faces a spectrum of challenges across various scenarios, encompassing issues related to infrastructure, technology, management, and market access. Among these challenges, the foremost concern lies in the adoption of technologies and practices that lead to nutritional imbalances, soil fertility depletion, and compromised plant health [7].
The meta-analysis underscores that organic food systems across the globe exhibit an average productivity shortfall of 19–25% in comparison to conventional agriculture. This variance hinges on factors such as geographic location, type of agricultural practices, technological approaches, and the prevailing edaphoclimatic conditions [8]. In this context, the significance of biofertilizers as catalysts for heightened plant productivity becomes evident. These bio-based solutions encompass various products or substances, whether liquid or solid-based, that incorporate live microbial compositions [9]. On a global scale, they have contributed to an increase of 11.8% in the yields of vegetables, root crops, and cereals when contrasted with unfertilized controls. Furthermore, biofertilizers have demonstrated their potential to enhance nutrient utilization efficiency by an average of 9.6%, effectively curbing nutrient loss while boosting plant uptake [10,11]. Mirroring their importance, the global biofertilizer market’s value stood at USD 1.81 billion in 2021, with projections indicating a compound annual growth rate of approximately 12% from 2021 to 2028. This trajectory is expected to culminate in a market valuation of about USD 4.5 billion by 2028 [12].
When considering the recycling of organic materials, including agricultural and animal waste, an alternative organic amendment finds acceptance within organic systems: the bokashi-type biofertilizer [13]. Renowned for its capacity to enhance microbial activity, fertility, and the physical-hydric properties of substrates and agricultural soils, the processing system of this biofertilizer consists of a harmonious blend of materials from animal, plant, and mineral sources [14,15,16,17]. Subsequently, the mixture of these components is channeled into creating a windrow, which triggers a controlled process of thermophilic aerobic semi-decomposition. This complex transformation is performed by a diverse community of microorganisms, and the addition of this microbial inoculum to the raw materials reduces the processing time [18,19]. The outcome of this microbiological transformation is a stable and bioavailable compound. To further optimize the efficiency of the bio-oxidation process and enhance the mineralization of organic matter, the application of microbial inoculants and ultra-diluted dynamized preparations is viable [17,20]. This holistic approach elevates the overall efficacy of the process, fostering the conversion of base materials into a nutrient-rich and biologically active substrate [21,22].
The bokashi-type biofertilizer exerts influence on the biofortification of vegetable, which can be observed in increasing nutrient absorption, utilization efficiency and enhances photosynthetic capabilities [23]. Moreover, it demonstrates evidence of its ability to suppress soil-borne pathogens, thus mitigating the presence of phytopathogenic species [24]. The bioactive effects derive from the complex interaction of organic radicals and physicochemical attributes. They synergistically converge with their microbiological richness and the presence of bioactive compounds, such as phytohormones, amino acids, enzyme complexes, and siderophores [19]. This unique composition allows the integration of the biofertilizer in a mixture with the substrate [25], promoting seed germination and the development of high-quality seedlings, in addition to contributing to obtaining satisfactory yields in various crops [26,27]. Bokashi-type formulations show a positive influence on the yield and quality of diverse agricultural crops [28], exhibiting promising outcomes in beet and cabbage cultivation [29,30], as well as tomato [31], lettuce [32], and radish [33].
Elucidating the microbiome composition and nutrient profile in bokashi-type biofertilizer formulations assumes significance for advancing our comprehension of how to optimize its application and its potential uses in agricultural systems. Such characterization offers insights about the precise microbial constituents that will be introduced into the system and unveils the biofertilizer’s inherent potential as a nutrient source [34,35]. Thus, the present study investigated the effect of different inoculation technologies to stabilize the process of aerobic biological transformation of the bokashi-type biofertilizer, as well as the effect of these formulations on the production of beet and cabbage seedlings and subsequent transplantation and cultivation in the field. Our hypothesis is that the use of bokashi-type biofertilizer as a substrate for vegetable seedling production and its application as a soil organic amendment will increase the yields of the test cultures, making it a viable option for production in organic-based systems.
2. Materials and Methods
The bioassay was carried out in the municipality of Lages, Santa Catarina (50°18′10.80″ W; 27°47′31.82″ S), situated at a geometric altitude of 920 m (SIRGAS 2000), during the period of January 2021 to June 2022. The prevailing climate type is Cfb, according to the Köppen classification, indicating a humid subtropical highland climate with mild summers [36]. The experiment was conducted under a controlled greenhouse environment for the production of seedlings, and when they reached the appropriate phenological stage, these seedlings were transplanted into field conditions. This transition to field cultivation followed the principles of the vegetable no-tillage system (SPDH), unfolding in the agroecological experimental area located in the Center of Agroveterinary Sciences (CAV-UDESC).
2.1. Production of Bokashi-Type Biofertilizer Formulations
The base-formulation of the bokashi-type biofertilizer involved the utilization of distinct regional materials in specific proportions (w/w): poultry litter (27.42%), sieved clayey soil (27.42%), rice husk (27.42%), rice bran (1.37%), varvite remineralizer (9.51%), charcoal (1.37%), brown sugar (0.034%), wood ash (1.00%), yeast (0.03%), and water until reaching 60% humidity. From the base recipe of 3 tons, these constituents were mixed and homogenized with the aid of a bucket loader attached to the tractor. With the mixture ready, 200 kg of the compost blend were weighed and separated into eight distinct windrows. These windrowns served as the foundation for the subsequent inoculation of the following bio-based treatments: T1 BBAC: bokashi + 10 mL L−1 of commercial product Bioaction Power® (concentration of 2 × 1011 endospores/L of Bacillus megaterium, B. subtillis, B. amyloquefaciens, and B. pumillus); T2 BEM: bokashi + 10 mL L−1 Efficient Microorganisms (following the methodology [37]); T3 BPPGR: bokashi + commercial product® Biostart Power (concentration of 1 × 108 CFU L−1 of Azospirillum brasilense, Pseudomonas fluorescens, Rhizobium sp., and Saccharomyces cerevisiae); T4 BCH: bokashi + 10 mL L−1 of dynamized ultra-diluted homeopathic preparation Chamomilla 12 CH; T5 BCV: Bokashi + 10 mL L−1 of dynamized ultra-diluted homeopathic preparation Carbo vegetabilis 12 CH; T6 BCC: bokashi + 10 mL L−1 of dynamized ultra-diluted homeopathic preparation Calcarea carbonica 12 CH; T7 BBP: bokashi + biodynamic preparations for compound P502-P507; T8: BCONTROL: bokashi only inoculated with commercial yeast (Saccharomyces cerevisiae). The application of treatments was standardized at a 1% dose of each specific inoculant, diluted in water for dispensation. The biodynamic preparations P502-P507 were obtained through the Biodynamic Association (IBD) and inoculated in the quantity of 0.1 g of each preparation. Each biodynamic preparation was added using the standard biodynamic practice, piercing the compost pile at 0.3 m intervals, and inoculating the preparations along the row. The PB 507 (Valeriana officinalis) was heated in water and sprayed over the entire pile in the late afternoon.
The entire procedure took place in a specialized facility, featuring suitable overhead shielding and flooring. The aerobic semi-decomposition process extended for a duration of 13 days, with manual turning twice a day in the first seven days and with a decrease in temperature, once a day. Throughout the production course of the bokashi-type formulations, continuous monitoring of daily temperature variations in the windrows was performed (six measurements per windrow, via digital thermometer). Subsequent to the biostabilization of the microbiological transformation process, the diverse formulations exhibited suitable characteristics for application, like a pleasant smell, temperatures below ambient levels, and crumbly consistency (Figure 1). For analytical purposes, six sub-samples from each treatment were collected and homogenized to compose the representative work sample. These biofertilizer samples were sent for analysis to the ABC Foundation laboratory (Paraná), where analyses of total macro- and micronutrient content (nitric-perchloric digestion followed by quantification on ICP-OES), fulvic and humic acid concentrations (titrimetry), nitrogen (AOAC—Official Method 993.13—Nitrogen/Total Nitrogen Combustion Method, 2018), organic matter (combustion followed by gravimetry), total organic carbon content (potassium dichromate followed by titrimetry), and density (gravimetry) were carried out. The pH H2O, pH CaCl2 (potentiometry 1:2.5), and electrical conductivity (Ec) analyses were carried out in the Genesis and Mineralogy laboratory at CAV-UDESC. This analytical protocol was conducted in triplicate to ensure robust results.

Figure 1.
Preparation of the bokashi-type biofertilizer base mixture using a loader attached to a tractor (A); aerial view of the experimental windrows, showcasing the distinct formulations of bokashi-type biofertilizer (B); and refined and sifted bokashi-type biofertilizer through a 4 mm mesh screen (C). Lages, Santa Catarina State, Brazil, 2020.
The samples of the biofertilizer formulations were preserved by freezing until they were sent for metagenomic analysis. This analytical process was conducted at the company Lagbio—Metagenomics Analysis and Biotechnology, located in Toledo (PR). In this phase, 0.25 g from each assessed bokashi-type biofertilizer formulation was used for DNA extraction and subsequent sequencing. The metagenomic analysis unfolded following the pipeline described by [38]. To explore the bacterial diversity, sequencing of the V4-V5 region of the 16S rRNA gene was undertaken. The library was sequenced on the Illumina MiSeq platform (Illumina, San Diego, CA, USA), resulting in an average of 55.000 raw reads per sample. Subsequent data analysis was conducted within a Linux environment with the programs R (R core team, 2020), Pavian [39], MGrast [40], and Stamp [41] to summarize the taxonomic information of the database.
2.2. Production of Beet and Cabbage Seedlings in a Greenhouse Environment
This comprehensive assessment encompassed the eight previously detailed treatments, along with an additional control treatment employing solely the commercial substrate Carolina Soil® (CSCONTROL). This phase of the experiment consisted of four replications, arranged in a completely randomized design. The approach to distribute and conduct the bioassay was executed utilizing a “double-blind” methodology, whereby the disclosure of treatments only occurred at the conclusion of the study. Each bokashi-type biofertilizer formulation was sieved through a 4 mm mesh and subsequently blended with the commercial substrate Carolina Soil® in a specific ratio: 40% based on the weight of bokashi and 60% based on the weight of the substrate, for the production of beet (Beta vulgaris) seedlings. A slightly adjusted ratio of 30% (w/w) of bokashi and 70% based on the substrate weight was employed for the cultivation of cabbage (Brassica oleraceae) seedlings.
Beet sowing was carried out on 18 January 2021, employing plastic trays containing 128 cells, with 4 beet glomeruli (seeds) per cell (a total of 60 cells earmarked for evaluation). The beet cultivar chosen was “Katrina—Feltrin”, a variety widely accepted in the region. On the 6th day after sowing (DAS), thinning was performed, retaining one plant per cell. The trays were diligently maintained within the greenhouse environment for a span of 30 days, until their eventual transplanting to the field on 18 February 2021. Similarly, the production of cabbage seedlings was executed following a comparable methodology to that of beet cultivation. In this case, two seeds were sown per cell within a total of 80 cells designated for evaluation (on 1 May 2021). The cabbage variety utilized was “Chato de Quintal—Topseed blue line”. Similar to the beet, on the 6th DAS, seedling thinning was performed, leaving one plant per cell. These trays remained in the greenhouse for a period of 25 days, culminating in their transfer to the field on 26 May 2021. To simulate the floating system, the trays were positioned within plastic basins, and water levels were manually adjusted based on the observed water layer’s height.
2.3. Preparation of the Cultivation Area, Experimental Design, and Transplanting of Cultures to the Field
The experimental field underwent initial subsoiling, followed by harrowing, to prepare the soil for subsequent interventions. Following this, a tanned poultry litter application of 5 Mg ha−1 and a liquid pig manure application of 5 Mg ha−1 were administered across the entire designated area. Subsequently, the cultivation beds were prepared with the assistance of a filler. To ensure the beds’ optimal condition, they were covered with finely crushed biomass sourced from tree pruning. This protective layer was consistently maintained through periodic upkeep. The soil was classified as a Humic Dystrudept [42], and samples were collected for chemical analysis before applying treatments and transplanting cultures (Table 1). The experimental design was a randomized block arrangement featuring four replications, also conducted in the “double-blind” approach. The experiment encompassed the eight aforementioned treatments along with a control treatment (CSCONTROL), where seedlings were cultivated solely with the Carolina Soil® substrate.

Table 1.
Chemical and physical attributes of the Humic Dystrudept before the implementation of the experiment. Agroecological experimental area, CAV-UDESC. Lages, Santa Catarina State, Brazil, 2021.
The study embraced a total of 36 sampling units, each represented by a bed spanning 1.44 m2. A surface application ten days before transplanting, equivalent to 5 Mg ha−1 of the respective bokashi-type biofertilizer that the seedling was produced, was administered in each bed. In the control treatment, 5 Mg ha−1 of tanned poultry litter was applied. A total of 30 beet seedlings were transplanted on 18 February 2021, adhering to a spacing of 0.15 × 0.30 m. On the 40th day post-transplanting, leaf tissue samples were collected from the upper portion of the aerial segment of five healthy plants, aiming for macronutrient foliar analysis. The beet plants were harvested on 7 April 2021, with 10 central plants being harvested from each plot for this parameter evaluation. The beet roots were subjected to a complete washing process and subsequently weighed on an analytical balance to determine the fresh mass of the tuberous roots and productivity extrapolation. The classification of beet roots was carried out according to the Brazilian Horticulture Modernization Program, following the CEAGESP classification standards. Subsequently, they were packed in paper bags and then air-dried to determine the dry mass of tuberous roots. For evaluations related to the tuberous roots of beet, the (a) mean root diameter (RD) was calculated as the average of longitudinal and transverse measurements and facilitated by a digital caliper; (b) root fresh mass (RFM); (c) root yield (kg ha−1); (d) dry mass of tuberous roots (DMR). Productivity was computed by multiplying the average weight of tuberous roots specific to the treatment by the number of plants per hectare.
The assessments concerning the aerial portion of the beet encompassed the following parameters: (a) shoot fresh mass (SFM); (b) shoot dry mass (SDM); and (c) leaf area index (LAI). The determination of the leaf area index was executed utilizing a digital scanner (Licor model 3100). After LAI determination, the samples were introduced into a circulation oven at 65 °C, maintaining them until a constant mass was achieved, thereby determining the dry mass of the aerial part.
Post-beet harvest, six cabbage seedlings were transplanted per bed and positioned with a spacing of 0.80 × 0.50 m (on 26 May 2021). Subsequent fertilization, employing the respective bokashi-type biofertilizer utilized during seedling production, occurred with a 50% reduction in application rate compared to the preceding crop (2.5 Mg ha−1). During the head closure stage of the majority of plants, samples of the aerial part from two representative plants were collected for the purpose of macronutrient analysis. Turning to the analysis of the cabbage’s aerial portion, the following parameters were evaluated: (a) number of leaves (both external and internal); (b) head size; (c) head fresh mass; and (d) productivity (kg ha−1).
2.4. Preparation of Samples of Leaf Tissue of Crops for Chemical Analysis
Plant tissue from both sugar beet and cabbage was ground within an agate mortar and subsequently sifted through a 1 mm mesh. This was followed by sulfuric foliar digestion of 0.2 g of plant tissue for the determination of macronutrients [43]. The calcium (Ca) and magnesium (Mg) concentrations in the plant tissue were quantified using the AA 200 equipment via atomic absorption. Phosphorus (P) and potassium (K) were determined by colorimetry [44] and by flame photometry, respectively. The nitrogen (N) concentration was determined by steam entrainment in Kjeldahl semi-micro equipment and subsequent titration with 0.0125 M H2SO4. Employing the shoot dry mass (SDM) alongside the levels of N, P, K, Ca, and Mg, the cumulative concentrations of these elements within the plant tissue of the evaluated plants were calculated according to Equation (1) [45].
where NAmacro corresponds to the amount of macronutrient accumulated in the plant tissue of the plants and the shoot dry mass (SDM) produced by the tested plants.
NAmacro (mg) = SDM (mg) × Nutrient concentration (%)/100
2.5. Statistical Analysis
The data were submitted to the test for homogeneity of variances by the Ascombe–Tukey test and for normality of residues by the Shapiro–Wilk test. When the assumptions were met, the means were compared by the means multiple comparison test (LSD) at 5% significance. All analyses were performed in the STATGRAPHICS statistical environment (Centurion version XVI.I). A principal component analysis (PCA) and redundancy analysis (RDA) were performed to infer the relationship between the chemical and biological parameters of the bokashi formulations and crop productivity using the Canoco 5.0 software.
3. Results
3.1. Chemical Characterization of Bokashi-Type Biofertilizer Formulations
The physical-chemical properties of bokashi-type biofertilizer formulations are presented in Table 2. The range of fulvic acid values was from 3.79% to 36.43%, with the BPPGR treatment exhibiting the highest fulvic acid levels (36.43%), followed by BEM with 10.24%. Regarding humic acid contents, they ranged from 3.21% (BCONTROL) to 5.85% (BEM). When assessing organic carbon content, a variation of 59.2% was observed when comparing the average content between the BPPGR (12.36%) and BCH (19.68%) treatments. Concerning organic matter (OM) content, a range of 59.2% was observed between the lowest value of 27.19% (BPPGR) and the highest of 43.31% (BCH). In contrast, narrower ranges of variation were observed in the content levels of N, P, K, Na, and S. The bokashi-type biofertilizer formulations are characterized by high levels of micronutrients such as Fe, Mn, Zn, and B. The values of pH H2O and pH CaCl2 are in the range of neutrality. As a general trend, the nutrient content within the bokashi-type biofertilizer formulations was comparable to that observed in commonly employed organic fertilizers. Furthermore, these formulations exhibited nutrient values in line with existing literature reports [46,47,48].

Table 2.
Chemical characterization of the contents of fulvic acids (FA), humic acids (HA), dry matter (DM), total organic carbon (TOC), Organic Matter (OM), nitrogen (N), phosphorus (P), calcium (Ca), magnesium (Mg), sodium (Na), sulfur (S), boron (B), copper (Cu), manganese (Mn), zinc (Zn), density (Ds), pH H2O, pH CaCl2, Ec H2O, Ec CaCl2, and C/N of the evaluated formulations of the bokashi-type biofertilizer. Lages, Santa Catarina State, Brazil, 2021.
Moisture was also monitored and was in the ideal range for the composting process, staying close to 40% of moisture in the windrows at the beginning of the process and ending with a DM content above 83%. According to the data obtained from the C/N ratio, there was a variation from 8.64 (BMPCV) to 13.89 (BCONTROLE), values that point to a net rate of decomposition and mineralization of N quickly in the different formulations evaluated when incorporated into the soil.
3.2. Metagenomic Analysis of Bokashi-Type Biofertilizer Formulations
The highlights of the metagenomic assessment of the sequenced samples are shown in Table 3. These results exhibit a comprehensive characterization of the whole-microbial community of the different formulations through the utilization of high-throughput sequencing.

Table 3.
Metagenome data refer to the number of reads, number of genera and species, and % of bacteria, eukaryotes, and others in the different formulations of the bokashi-type biofertilizer. Sequencing is carried out by the company Lagbio—Metagenomic Analysis and Biotechnology. Toledo, Paraná State, Brazil, 2021.
This omic approach reveals key information on the final taxonomic composition of the microbiome of bokashi-type formulations devised via aerobic metabolism examined in this study. Overall, the community structure analyses performed showed that the microbiome of the different formulations is dominated by species in the Bacteria domain, comprising more than 99% of the sequenced taxonomic profile composed of them. The bacterial community remaining in the different formulations sampled is distributed among more than one thousand and one hundred different genera. The number of reads ranged from 197,097 (BBAC) to 458,348 (BBP), the number of genera from 1187 (BBAC) to 1728 (BBP), and finally the number of species from 4038 (BBAC) to 7286 (BBP). Regarding the bacterial genera present in the assessed treatments, a predominance of the genera Marinobacter sp. (17.9–27.3% DNA g−1) is observed, followed by Halomonas sp. (7.3–9.96% DNA g−1), Galbibacter sp. (2.86–11.58% DNA g−1), and Alcanivorax sp. (5.95–7.28% DNA g−1). In a minor relative proportion, within the Proteobacteria phylum, the genus Bradyrhizobium sp. (0.12–0.39% DNA g−1) is detected, which plays a significant role as an N-fixing inoculant for legume crops like soybeans. The Actinobacteria class was boosted by the increase in the amount of Streptomyces sp. (1.81–2.87% DNA g−1), where this genus of aerobic filamentous bacteria has important saprophytic functions like organic matter decomposition and the production of a wide range of antibiotics, such as streptomycin. It is still possible to verify the presence of the genera of plant-promoting growth rhizobacteria, such as Bacillus sp. (2.06–2.88% DNA g−1), Pseudomonas sp. (2.02–2.96% DNA g−1), Azospirillum sp. (1.42–2.44% DNA g−1), and Rhizobium sp. (0.11–0.17% DNA g−1). It is worth emphasizing that none of the sequenced formulations contained phytopathogenic bacterial genera like Enterobacter sp., Escherichia sp., Klebsiella sp., Citrobacter sp., Salmonella sp., or Clostridium sp. (all below 0.1% DNA g−1). This outcome underscores the efficacy of the aerobic semi-decomposition process in deactivating potential pathogens. This could also suggest the potential absence of biological contaminants within the utilized materials.
3.3. Agronomic Variables of Beet and Cabbage Crops after Transplanting in the Field
The highest value of SFM was observed in beet plants cultivated using the bokashi-type biofertilizer elaborated with the application of Carbo vegetabilis 12 CH (Table 4). The treatment incorporating biodynamic compost preparations (P502-P507) showed the highest values of RFM and RDM (p < 0.05). This was followed by the treatments formulated with the ultra-diluted and dynamized preparations Chamomilla 12 CH (BCH) and Carbo vegetabilis 12 CH. The BCC treatment (bokashi-type biofertilizer + Calcarea carbonica 12 CH) exhibited the highest LAI (p < 0.05).

Table 4.
Effect of bokashi-type biofertilizer formulations on shoot fresh mass (SFM), shoot dry mass (SDM), root fresh mass (RFM), root dry mass (RDM), leaf area index (LAI), mean transversal diameter of tuberous root beet (RD), and commercial classification of “Katrina” variety beet plants in an ecological field-based system. Lages, Santa Catarina State, Brazil, 2021.
When analyzing the RD, the BPB treatment presented the highest value, showing an increase of nearly 12.2% in the tuberous root caliber compared to the CSCONTROL (p < 0.05). An interesting dynamic emerged from the interaction analysis of beet plants derived from BPPGR treatment, wherein a certain antagonistic influence on beet quality and productivity was observed. The BPPGR treatment showed the lowest value for RD and root yield (37.08 t ha−1). In terms of commercial classification, all tuberous roots were categorized as class 2A, featuring diameters greater than 50 mm and less than 90 mm.
Significant increments in root yield of beet plants were observed when they were cultivated with a combination of bokashi-type biofertilizer (40% w/w) + conventional substrate (60% w/w) in relation to those produced only with conventional substrate (Figure 2). The highest yields were observed in the BBP (62.6 t ha−1), BCH (60.2 t ha−1), and BCV (56.3 t ha−1) treatments, corresponding to an increase of 60.9%, 54.7%, and 44.7%, respectively, compared to the CSCONTROL root yield (38.9 t ha−1). Biodynamic preparations (P502-P507) have action on the development, physiology, and resistance to biotic stresses in plants, and in this case, the interaction between PB 502-507 when applied in the production of bokashi-type biofertilizer and the latter as fertilizer for the crop increased the productivity of beet plants [49].
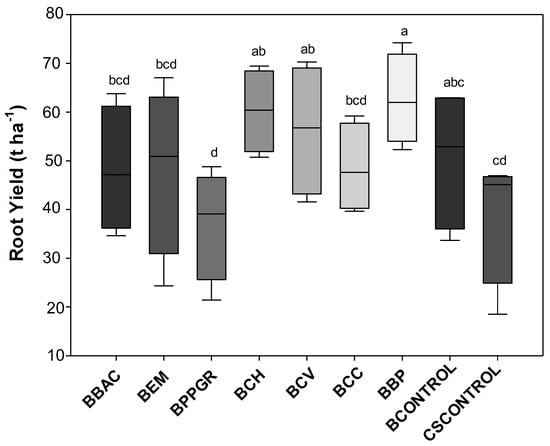
Figure 2.
Root yield (t ha−1) of “Katrina” variety beet plants produced with bokashi-type biofertilizer formulations in an ecological field-based system. Lages, Santa Catarina State, Brazil, 2021. Note 1: CV = 17.81%. Note 2: Means followed by the same letter do not differ from each other by the LSD test (p < 0.05). BBAC = Bokashi + Bacillus megaterium, B. subtillis, B. amyloquefaciens, and B. pumillus (Bioaction Power®); BEM = Bokashi + EM; BPPGR = Bokashi + Azospirillum brasilense, Pseudomonas fluorescens, Rhizobium sp., and Saccharomyces cerevisiae (Biostart Power®); BCH = Bokashi + Chamomilla 12 CH; BCH = Bokashi + Carbo vegetabilis 12 CH; BCC = Bokashi + Calcarea carbonica 12 CH; BBP = Bokashi + biodynamic preparations for compound P502-P507; BCONTROL = Bokashi control (only commercial yeast); CSCONTROL = Carolina Soil substrate®.
Significant differences in the leaf tissue nitrogen (N) content were observed, which aligned within an appropriate range for the crop (Table 5). The BPPGR treatment exhibited the highest content (5.94%), followed by the BBAC (5.3%), BEM (5.29%), and BCH (5.45%) treatments. The lowest N content in the leaf tissue was obtained in the BCONTROL treatment (4.37%), followed by BBP (5.0%) and CSCONTROL (5.01%).

Table 5.
Effect of bokashi-type biofertilizer formulations on the chemical composition of the aerial part of the “Katrina” variety beet plants. Lages, Santa Catarina State, Brazil, 2021.
The highest accumulated N content is attributed to plants cultivated with the substrate + bokashi-type biofertilizer formulated with biodynamic compost preparations P502-P507 (5215.4 mg) and Chamomilla 12 CH (5492.1 mg). Nitrogen assumes a foundational role in essential biomolecules such as proteins, enzymes, chlorophyll, and nucleic acids; in addition, it actively participates in plant hormone synthesis, a fact that justifies its high demand across various crops [50]. Concerning leaf tissue phosphorus (P) levels, the observed concentrations surpass recommended reference values, but no differences were found in the content of P in the leaves between the evaluated treatments. The highest P accumulation was observed in the BBP treatment (p < 0.05). The biodynamic preparation P507, derived from valerian extract (Valeriana officinalis), is linked to processes involving phosphorus (P) during the preparation of the compound [49]. Meanwhile, the highest leaf tissue potassium (K) contents were observed in the treatments CSCONTROL (6.96%), BCONTROL (6.37%), and BCC (6.37%), but no significant effect was verified on the accumulation of K between the evaluated treatments.
The highest average of the fresh mass of cabbage head was obtained by the BCC treatment, a value that expresses an increase near to 147.9% when contrasted with the CSCONTROL (578.1 g), where this presented the lowest average of this variable across the treatments (Table 6). Only the BBAC treatment (884.5 g) was not significant compared to CSCONTROL. The transversal diameter of the cabbage head was significantly affected by bokashi-produced treatments (p < 0.05). The plants cultivated with the bokashi-type biofertilizer elaborated with Calcarea carbonica 12 CH (22.1 cm) and with the biodynamic preparations for compost P502-P507 (21.5 cm) obtained the highest averages for this variable. There were significant differences in the productivity of cabbage by the application of bokashi-type biofertilizer, as shown in the box-plot graph in Figure 3 (p < 0.05). The increase in productivity of cabbage plants was higher in the BCC treatment (35.8 t ha−1), being 148.6% superior to the CSCONTROL (14.4 t ha−1). Only the BBAC treatment (22.1 t ha−1) did not differ from the CSCONTROL treatment. The average value of increase in productivity in relation to the control among treatments BEM, BMPCV, BCH, BCV, BPB, and BCONTROLE was 115.2%.

Table 6.
Mean values of shoot fresh mass (SFM), number of external head leaves (NEL), number of leaves on the head (NLH), head transverse diameter (HTD), and head fresh mass (HFM) of “Chato de Quintal” variety cabbage plants produced with bokashi-type biofertilizer formulations in an ecological field-based system. Lages, Santa Catarina State, Brazil, 2021.
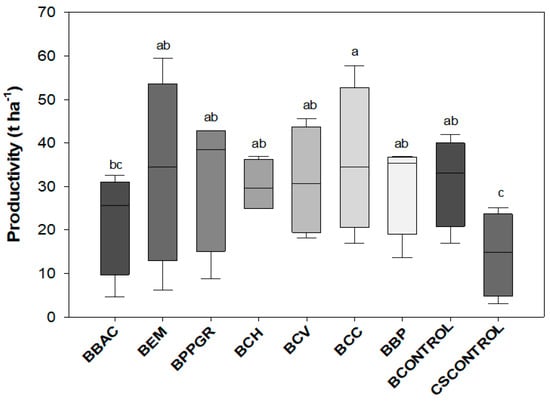
Figure 3.
Mean productivity (t ha−1) of “Chato de Quintal” variety cabbage plants, produced with bokashi-type biofertilizer formulations, in an ecological field-based system. Lages, Santa Catarina State, Brazil, 2021. BBAC = Bokashi + Bacillus megaterium, B. subtillis, B. amyloquefaciens, and B. pumillus (Bioaction Power®); BEM = Bokashi + EM; BPPGR = Bokashi + Azospirillum brasilense, Pseudomonas fluorescens, Rhizobium sp., and Saccharomyces cerevisiae (Biostart Power®); BCH = Bokashi + Chamomilla 12 CH; BCH = Bokashi + Carbo vegetabilis 12 CH; BCC = Bokashi + Calcarea carbonica 12 CH; BBP = Bokashi + biodynamic preparations for compound P502-P507; BCONTROL = Bokashi control (only commercial yeast). CSCONTROL = Carolina Soil substrate®. Means followed by the same letter do not differ from each other by the LSD test (p < 0.05).
A significant difference was verified in the N content of the leaf tissue between the evaluated treatments (Table 7). The CSCONTROL exhibited values in agreement with the N reference, displaying a higher average content (4.05%). As the productivity of CSCONTROL was the lowest among the evaluated treatments, it is possible to verify that this accumulation of N in leaf tissues was not converted into biomass productivity. The highest N accumulation was observed in cabbage plants cultivated with the bokashi-type biofertilizer formulated with Calcarea carbonica 12 CH (p < 0.05), reflecting an average increase of 54% and 102.7% in contrast to treatments involving biological inoculations such as BBAC and BEM, respectively.

Table 7.
Effect of bokashi-type biofertilizer formulations on the chemical composition of the aerial part of the cabbage plant variety “Chato de Quintal”. Lages, Santa Catarina State, Brazil, 2021.
Regarding the levels of P in the leaf tissue, the treatments CSCONTROL (1.8%) and BPPGR (1.7%) had the highest averages, and it is noteworthy that for all treatments, the values of leaf P were higher than the reference values. No significant differences were found in accumulated P content. The average K contents are above the recommended reference values, where BBAC (6.1%) and BPPGR (5.9%) treatments showed an average increase of 41.8% and 37.2% when compared to the control (4.3%). Accumulation of K was also influenced by the applied treatments (p < 0.05), where plants cultivated with bokashi-type biofertilizer + Calcarea carbonica 12 CH (558.2 mg) and BPPGR (542.1 mg) showed an average increase of 87% and 81%, respectively, when compared with K accumulation in the CSCONTROL.
Average foliar Ca levels were found to be lower than the reference values for the cabbage crop in all treatments. The BCC (0.40%) and BBAC (0.40%) treatments had the highest means, with these contents being 73.9% higher than the BCH (0.23%). The highest Ca accumulation was also observed in the treatment employing the ultra-diluted and dynamized homeopathic preparation Calcarea carbonica 12 CH (p < 0.05). The foliar Mg contents were within the appropriate range for the crop, and the treatment produced from the bokashi-type biofertilizer + biodynamic preparations for compost P502-P507 exhibited the highest average (0.30%), but for the accumulation of Mg, the treatment with Calcarea carbonica 12 CH showed the highest Mg accumulation (p < 0.05).
The results of the principal component analysis (PCA) for the beet crop revealed that principal component 1 (CP1) accounted for 47.9% of the data variability, while principal component 2 (CP2) explained 34.9%, totaling 82.8% of the data variability (Figure 4). Along CP2 (Axis 2) on the upper left side, the variables strongly associated with the BBP treatment are root yield, SFM, SDM, RFM, RDM, MTD, LAI, % P, and accumulated P. These variables were also weakly associated with the BCV treatment. On the other hand, the Ca content and accumulation of Ca and % K were more strongly associated with the bokashi + Calcarea carbonica 12 CH. In the PCA analysis for the cabbage crop, CP1 explained 54.1% of the data variability, while CP2 explained 24.3%, totaling 89% of the data variability. Along CP2 (Axis 2) on the upper left side, variables such as the productivity of cabbage plants, SFM, HTD, HFM, and leaf % K content were associated with the BPB, BCONTROL, BPP, and BPPGR treatments. The accumulation of N, Ca, Mg, K, and P was more strongly associated with the BCC treatment, with weaker associations observed for the BCV treatment.
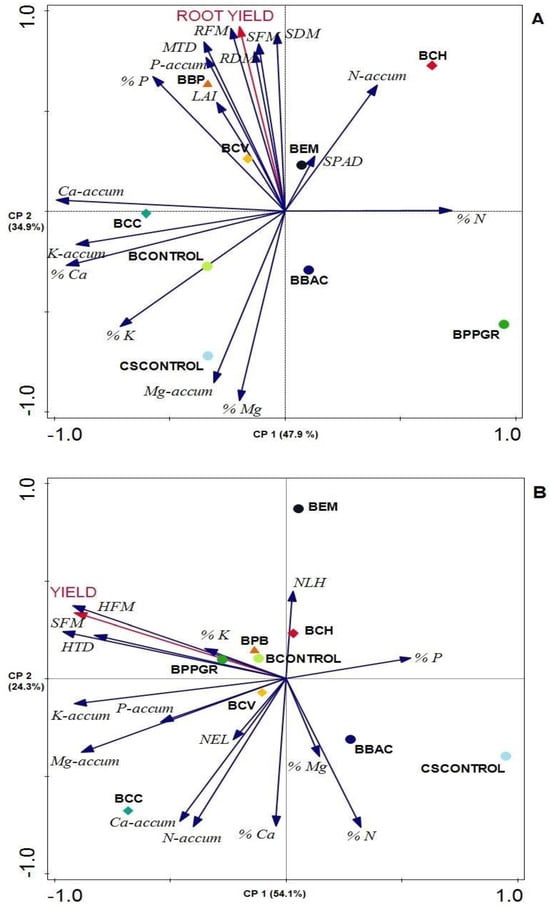
Figure 4.
Relation between Principal Components 1 (CP1) and 2 (CP2) of Principal Component Analysis (PCA) for crop yield and phytometric attributes of (A) beet (Beta vulgaris) and (B) cabbage (Brassica oleracea) produced with different bokashi-type biofertilizer formulations in an ecological field system. BBAC = Bokashi + Bacillus megaterium, B. subtillis, B. amyloquefaciens, and B. pumillus (Bioaction Power®); BEM = Bokashi + EM; BPPGR = Bokashi + Azospirillum brasilense, Pseudomonas fluorescens, Rhizobium sp., and Saccharomyces cerevisiae (Biostart Power®); BCH = Bokashi + Chamomilla 12 CH; BCH = Bokashi + Carbo vegetabilis 12 CH; BCC = Bokashi + Calcarea carbonica 12 CH; BBP = Bokashi + biodynamic preparations for compound P502-P507; BCONTROL = Bokashi control (only commercial yeast); CSCONTROL = Carolina Soil substrate®. (A) Shoot fresh mass (SFM), shoot dry mass (SDM), root fresh mass (RFM), root dry mass (RDM), leaf area index (LAI), mean transversal diameter of tuberous root beet (MTD), N-accum (N accumulated), P-accum (P accumulated), K-accum (K accumulated), Ca-accum (Ca accumulated), and Mg-accum (Mg accumulated). (B) Shoot fresh mass (SFM), number of external head leaves (NEL), number of leaves on the head (NLH), head transverse diameter (HTD), and head fresh mass (HFM).
The RDA examining relationships between chemical and biological parameters of bokashi formulations and plant traits for different treatments, showed that the explained constrained eigen value was 80.8% and 19.1% for the first and second axes, respectively (Figure 5). The variables that contributed most to explaining the variation were, respectively: pH CaCl2 (63.8%), fulvic acids (14.2%), Paraburkholderia sp. (10.4%), Boron (5.5%), Bacillus sp. (4.8%), and potassium (1.2%). The content of B and Bacillus sp. showed a stronger correlation with the BBAC treatment, while pH CaCl2 and fulvic acids were associated with the BPPGR treatment. The BCONTROL, BEM, and BCC treatments were positioned along the same axis on the graph, showing a relationship with potassium content, the presence of the microorganism Paraburkholderia sp., and cabbage crop productivity. The BBP, BCH, and BCV treatments were more closely associated with beet productivity and did not exhibit a relationship with the chemical and biological parameters of their respective bokashi formulations.
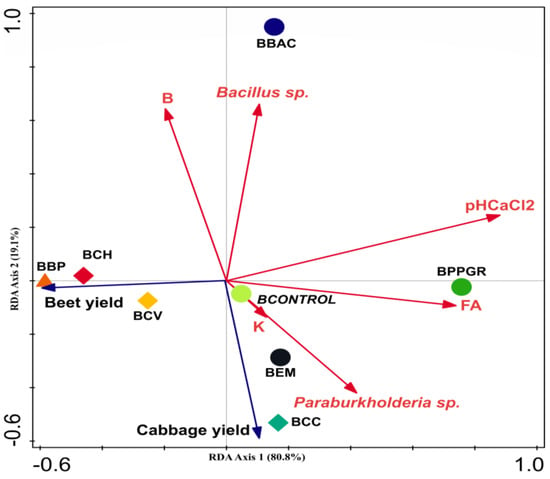
Figure 5.
Biplot diagram of axes 1 and 2 of the redundancy analysis (RDA) outlining interrelationships between bokashi chemical and biological parameters and plant yield obtained from different formulations of bokashi-type biofertilizer treatments. BBAC = Bokashi + Bacillus megaterium, B. subtillis, B. amyloquefaciens, and B. pumillus (Bioaction Power®); BEM = Bokashi + EM; BPPGR = Bokashi + Azospirillum brasilense, Pseudomonas fluorescens, Rhizobium sp., and Saccharomyces cerevisiae (Biostart Power®); BCH = Bokashi + Chamomilla 12 CH; BCH = Bokashi + Carbo vegetabilis 12 CH; BCC = Bokashi + Calcarea carbonica 12 CH; BBP = Bokashi + biodynamic preparations for compound P502-P507; BCONTROL = Bokashi control (only commercial yeast).
4. Discussion
The periodic addition of organic materials as soil amendments in tropical and subtropical regions, exemplified by the context of Brazilian soils, assumes a significant role due to the accelerated rate of organic matter decomposition and the high degree of weathering. These phenomena are attributed to the elevated levels of precipitation and temperatures prevalent in these ecosystems [51]. The application of organic amendments is often used in organic-based management systems, aiming to improve the physical, chemical, and biological attributes of the soil. These amendments serve as a source of both energy and nutrients, actively participating in the chemical reactions and biological cycles of soil microorganisms. This synergistic interaction modulates the dynamic equilibrium within the soil system, which in turn exerts a substantial role on systemic soil fertility and plant health [35]. Organic compounds intended for agricultural use must exhibit characteristics like tolerable odor, a homogeneous blend of the constituents, and desirable agronomic traits such as well-balanced nutrient levels, sanitization, and freedom of impurities and contaminants. Odor-causing organic components are degraded during the bio-oxidation process, while the humification of OM imparts a dark color to the final mature compound. Throughout the entire preparation process of the bokashi-type formulations, no unpleasant odors or excessive moisture were detected.
Within the compost, mineral nutrients like K, Ca, and Mg are mainly present in forms readily assimilable by plants. Nitrogen exists within the compost in both organic and inorganic forms, encompassing ammonium (N–NH4) and nitrate (N–NO3), with the latter being promptly available for plant uptake. The development of humic substances is linked to the degree of compound maturation. Humic acids (HA) and fulvic acids (FA), constituents of the humic fraction, are formed by the action of specialized microorganisms that transform organic waste into humified material [52]. The humic fraction has complex physicochemical properties that are different from the original organic material, where the predominance of HA over FA at the end of composting is indicative of adequate humification of the compost since FA contains elements that are susceptible to rapid degradation [53]. The HA/FA ratio is taken as an indicator of the degree of humification of organic compounds, where higher HA/FA ratios denote more humified composts [54]. A well-matured compost can show a HA/FA ratio ranging from 0.5 to 2.0, although some authors explain that it is challenging to establish a universal value to describe and predict the degree of maturation of compost with distinct compositions [55,56]. Only the treatments involving bokashi-type biofertilizer with Bacillus spp. (HA/FA ratio of 1.3), bokashi + Carbo vegetabilis 12 CH (ratio of 1.0), and bokashi + Calcarea carbonica 12 CH (ratio of 1.22) showed HA/FA ratios higher than 1.0. In the other treatments, there was a predominance of the fulvic acid fraction in relation to the humic acid.
The content of moisture, total organic carbon (TOC), nitrogen, C/N ratio, and pH H2O values within the formulations BBAC, BCH, BCV, BCC, and BCONTROL adhere to the criteria outlined in IN Nº 61 of 8 July 2020, by the Brazilian Ministry of Agriculture, Livestock, and Food Supply (MAPA), thereby classifying them as “Class A” solid compost organic fertilizers. However, the formulations BPPGR (bokashi + commercial product Biostart Power® based on Azospirillum brasilense, Pseudomonas fluorescens, Rhizobium sp., and Saccharomyces cerevisiae), BEM (bokashi + efficient microorganisms, elaborated following Bomfim et al., 2011 methodology [31]), and those containing biodynamic preparations P502-P507 displayed TOC levels below 15% [57]. This observation might be linked to an intensified degradation of carbon during the controlled bio-oxidation process of these specific bokashi-type formulations. This effect could be attributed to the biological stimulation promoted by these treatments, necessitating an increase in the proportions of carbon-rich materials (such as rice husk and coal) to elevate organic carbon content in the final product when employing such inoculations. Overall, the levels of macro and micronutrients reported in the analyzed formulations in our study are consistent with the findings documented in the scientific literature [21].
It is noteworthy that none of the assessed formulations revealed the presence of phytopathogenic bacterial genera through sequencing. This observation suggests that the aerobic semi-decomposition process was effective in inactivating pathogens. There is also the possibility that the materials used were free of biological contaminants. The reduction or elimination of pathogens during the composting process can be attributed to elevated temperatures during the thermophilic phase, the production of antimicrobial compounds, hydrolytic enzyme activity, the synthesis of antibiotics by antagonistic microorganisms (which diminishes the survival of pathogens), or competition with new colonizers [58].
The utilization of microbial communities through bokashi-type biofertilizer, when applied to soil, can effectively stimulate plant growth and exert positive influences on other microbiological populations, including archaea and fungi. These microorganisms have multiple roles in supporting plant development and are recognized as plant probiotics or grow-promoting microorganisms [59,60]. These mechanisms encompass various actions, such as the ability of specific bacteria and fungi to facilitate nutrient uptake for plants. This occurs through processes like nutrient solubilization (e.g., P and K solubilizing bacteria, Zn solubilizing bacteria) and nitrogen fixation [61,62], the synthesis of phytohormones, and the production of compounds with antipathogenic properties, which enhance the plants defense systems against phytopathogens [63].
The observed improvements in plant productivity can be attributed to the presence of bioactive compounds and a balanced blend of macro and micronutrients present in bokashi-type formulations. Certain microorganisms present in bokashi can produce plant growth-promoting substances, such as 3-Phenyllactic acid, which have the potential to enhance plant yield [64]. Nutrients are available in the form of organic chelates, which are bound to organic radicals. This characteristic prevents losses through leaching and volatilization after application [65]. The introduction of exogenous microorganism communities into soil via organic compost (bokashi-type) can enhance the production rate of organic acids, phytohormones, and extracellular enzymes within the system. Moreover, it directly influences the cycling of organic matter, thereby providing essential nutrients to the microbial biomass [66]. Previous research has demonstrated that the application of bokashi can enhance the ability of the microbial community to effectively utilize carbon, resulting in an increased microbial carbon biomass within the substrate. This phenomenon indicates that the nutrients present in the substrate and organic matter are utilized by microorganisms. As these microorganisms die off, the nutrients they have assimilated are gradually released into the environment, thereby becoming available for plant uptake [67,68].
The combination of the bokashi-type biofertilizer with substrates is beneficial for the cultivation of vegetables, as it increases the efficiency of nutrient use by activating enzymes that stimulate the absorption and chelation of certain elements [65]. Through the mineralization process of the organic substances present in these mixtures, several nutrients and organic molecules are released into the soil solution, promoting plant growth [69]. Collectively, these mechanisms contribute to biomass production and can also influence the content of macronutrients within plant tissues. However, the lack of positive outcomes in terms of productivity in the BPPGR treatment (application of the commercial product Biostart Power®, based on the species Azospirillum brasilense, Pseudomonas fluorescens, Rhizobium sp., and Saccharomyces cerevisiae) could be attributed to antagonistic interactions between microbiological communities, which can cause a negative impact by inhibiting and hindering the multiplication of the newly introduced inoculum in the system [70].
Some of the reference studies that applied bokashi to the soil reported an increase in the crop yields of sweet corn [71], tomato [72], Pogostemon cablin [73], and Origanum vulgare [74]. A similar study evaluated the effect of organic fertilizers, organic compost, biodynamic compost, laminar compost with manure, and laminar compost with bokashi on beet production and concluded that all forms of fertilization were satisfactory in terms of nutrient supply and beet production [75]. Several studies with the use of bokashi in different doses and formulations have shown positive results and increased yields of brassicas and other vegetables [76,77,78]. Among the benefits of using organic fertilizers are the increase in the amount and diversity of microflora, which favor the cycling of nutrients, thus improving the chemical fertility conditions of the soil, in addition to acting in the control of pests and diseases such as cruciferous hernia, caused by Plasmodiophora brassicae Wor [79]. The use of 4 Mg ha−1 bokashi biofertilizer resulted in greater vegetative development of cabbage plants, with an average productivity of 80.36 Mg ha−1 [79]. It evaluated the doses of bokashi 0, 2.5, 5.0, 7.5, and 10.0 Mg ha−1 in a test with summer broccoli, and it was possible to verify that the doses of bokashi linearly increased the characteristics of plant height, number of leaves per plant, stem and head diameter, and average head mass up to the maximum dose used, which corresponded to 10 Mg ha−1 [80]. The cabbage crop is responsive to organic fertilization with bokashi, and the application of a dose of 10.0 Mg ha−1 of the biofertilizer resulted in plants with superior characteristics, providing higher crop productivity [30].
Notably, non-residual agricultural practices such as homeopathy and biodynamic preparations offer viable alternatives for farmers. The biodynamic preparations demonstrated an effect on the development of the BRS Margot grapevine, as evidenced by the increased length of branches, CO2 assimilation, RuBisCO enzyme efficiency, chlorophyll content, and a reduction in downy mildew incidence in the vine’s aerial parts [81]. Beyond enhancing the well-being of those engaged in these practices, they exhibit integrated characteristics that focus on the management of agricultural production systems, rendering them sustainable in the long term [82].
5. Conclusions
The analysis of the taxonomic profile of the microbiological populations revealed relative abundance of plant growth-promoting rhizobacterial genera, including Azospirillum sp., Rhizobium sp., Bradyrhizobium sp., Burkholderia sp., Paraburkholderia sp., and Paenibacillus sp., in the evaluated formulations. Additionally, no phytopathogenic contaminants were detected among the investigated treatments.
The highest yields of the beet crop in the field were observed from seedlings produced from the treatments biofertilizer bokashi + biodynamic preparations P502-P507 (BPB), bokashi + Chamomilla 12CH, and bokashi + Carbo vegetabilis 12CH, corresponding to an increase of 60.9%, 54.7%, and 44.7%, respectively, compared to the productivity of the control treatment.
The increase in productivity of cabbage plants was higher in the treatment bokashi + Calcarea carbonica 12CH, being 148.6% higher than the control. Only the BBAC treatment did not differ from the control. The average value of increase in productivity in relation to the control among treatments BEM, BPPGR, BCH, BCV, BBP, and BCONTROLE was 115.2%.
Author Contributions
Conceptualization, G.K., E.S.G., Á.L.M. and J.A.d.A.; methodology, G.K.; software, G.K.; validation, Á.L.M.; formal analysis, G.K.; investigation, G.K. and J.M.d.S.d.S.; writing—original draft preparation, G.K.; writing—review and editing, G.K., Á.L.M. and E.S.G.; supervision, Á.L.M.; project administration, Á.L.M. and G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the ongoing nature of the study and the need to maintain dataset coherence until full compilation.
Acknowledgments
Thanks to Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES for the scholarships granted. We thanks the UDESC for supporting this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ma, H.-K.; Pineda, A.; Van Der Wurff, A.W.G.; Raaijmakers, C.; Bezemer, T.M. Plant Soil feedback Effects on Growth, Defense and susceptibility to a Soil-Borne Disease in a Cut Flower Crop: Species and Functional Group Effects. Front. Plant Sci. 2017, 8, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Thiessen-Mertens, J.R.; Entz, M.H.; Wonneck, M.D. Review: Redesingning Canadian Prairie cropping systems for profitability, sustainability, and resilience. Can. J. Plant Sci. 2015, 95, 1049–1072. [Google Scholar] [CrossRef]
- Tilman, D.; Blazer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- ANDA—Associação Nacional para Difusão de Adubos. Pesquisa Setorial 2022, Macro Indicadores do Mercado de Fertilizantes No Brasil. Available online: http://anda.org.br/pesquisa_setorial/ (accessed on 15 June 2023).
- Metson, G.S.; Macdonald, G.K.; Habermana, D.; Nesme, T.; Bennett, E.M. Feeding the corn belt: Opportunities for phosphorus recycling in U.S. agriculture. Sci. Total Environ. 2016, 542, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- IBGE–Instituto Brasileiro de Geografia e Estatística. Sidra. Censo Agropecuário Brasileiro. 2017. Available online: https://censoagro2017.ibge.gov.br/ (accessed on 15 June 2023).
- Brainer, M.S.C.P. Produção de hortaliças na área de atuação do BNB. Cad. Setorial ETENE 2021, 180, 1–14. [Google Scholar]
- De Ponti, T.; Rijk, B.; Van Ittersum, M.K. The crop yield gap between organic and conventional agriculture. Agric. Syst. 2012, 108, 1–9. [Google Scholar] [CrossRef]
- Al Abboud, M.A.; Ghany, T.A.; Alawlaqi, M.M. Role of biofertilizers in agriculture: A brief review. Mycopath 2013, 11, 95–101. [Google Scholar]
- Rao, K.M.; Singh, K.; Ryingkhun, H.B.K.; Maying, B. Use of bio-fertilizers in vegetable production. Indian Hortic. J. 2014, 4, 73–76. [Google Scholar]
- Schütz, L.; Gattinger, A.; Meier, M.; Müller, A.; Boller, T.; Mäder, P.; Mathimaran, N. Improving crop yield and nutrient use efficiency via biofertilization—A global meta-analysis. Front. Plant Sci. 2018, 8, 2204. [Google Scholar] [CrossRef]
- Biofertilizers Market by Microorganism (Bacillus, Azotobacter, Rhizobium, Azospirillum, VAM, Pseudomonas, and Others), by Type (Phosphate Solubilizers, Nitrogen Fixing, and Others), by Crop Type (Cereals, Pulses & Oilseeds, Fruits & Vegetables, and Others), by Application (Soil Treatment, and Seed Treatment), and by Region—Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2022—2028. 2022. Available online: https://www.zionmarketresearch.com/report/biofertilizers-market (accessed on 14 May 2023).
- FAO. Organización de las Naciones Unidas para la Alimentación y la Agricultura. Aboneras Tipo Bocashi. Colección “Buenas Prácticas”; Programa Extraordinario de Apoyo a la Seguridad Alimentaria y Nutricional (Food Facility) FAO/Unión Europea: Ciudad de Guatemala, Guatemala, 2011; p. 13. [Google Scholar]
- Wijayanto, T.; Zulfikar, M.; Tufaila, M.; Alam, S.M.; Zamrun, M.F. Agricultural wastes based-organic fertilizers (Bokashi) improve the growth and yield of soybean (Glycine max (L.) Merrill). Int. J. Agric. Sci. 2016, 1, 27–32. [Google Scholar]
- Lasmini, S.A.; Nasir, B.; Hayati, N.; Edy, N. Improvement of soil quality using bokashi composting and NPK fertilizer to increase shallot yield on dry land. Aust. J. Crop Sci. 2018, 12, 1743–1749. [Google Scholar] [CrossRef]
- Salisu, M.A.; Sulaiman, Z.; Rus, R.C.; Samad, M.Y.A. Water use efficiency, plant growth and vegetative traits of rubber (Hevea brasiliensis) seedlings grown using different growing media and water levels. Aust. J. Crop Sci. 2020, 14, 1497–1505. [Google Scholar] [CrossRef]
- Jusoh, M.L.; Manaf, L.A.; Latiff, A. Composting of rice straw with effective microorganisms (EM) and its influence on compost quality. J. Environ. Health Sci. Eng. 2013, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, R.; Arora, A.; Shah, R.; Singh, A.; Pranaw, K.; Nain, L. Insights into rapid composting of paddy straw augmented with efficient microorganism consortium. Int. J. Recycl. Org. Waste Agric. 2014, 3, 54. [Google Scholar] [CrossRef]
- Silva, J.W.; Rodríguez, W.; Rosas, G. Caracterización física y química de bokashi y lombricompost y su evaluación agronómica en plantas de maíz. Ing. Amazon. 2015, 7, 5–16. [Google Scholar]
- Mayer, J.; Scheid, S.; Widmer, F.; Fließbach, A.; Oberholzer, H.R. How effective are ‘effective microorganisms® (EM)’? Results from a field study in temperate climate. Appl. Soil Ecol. 2010, 46, 230–239. [Google Scholar] [CrossRef]
- Quiroz, M.; Céspedes, C. Bokashi as an amendment and source of nitrogen in sustainable agricultural systems: A Review. J. Soil Sci. Plant Nutr. 2019, 19, 237–248. [Google Scholar] [CrossRef]
- Hata, F.T.; da Silva, D.C.; Yassunaka-Hata, N.N.; Cancian, M.A.d.Q.; Sanches, I.A.; Poças, C.E.P.; Ventura, M.U.; Spinosa, W.A.; Macedo, R.B. Leafy vegetables’ agronomic variables, nitrate, and bioactive compounds have different responses to bokashi, mineral fertilization, and boiled chicken manure. Horticulturae 2023, 9, 194. [Google Scholar] [CrossRef]
- Christel, D.M. The Use of Bokashi as a Soil Fertility Amendment in Organic Spinach Cultivation. Master’s Thesis, University of Vermont, Burlington, VT, USA, 2017; 162p. [Google Scholar]
- Bernard, E.; Larkin, R.P.; Tavantzis, S.; Erich, M.S.; Alyokhin, A.; Sewell, G.; Lannan, A.; Gross, S.D. Compost, rapeseed rotation, and biocontrol agents significantly impact soil microbial communities in organic and conventional potato production systems. Appl. Soil Ecol. 2012, 52, 29–41. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. Rev. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- de Paula Vicente, N.F.; Marafeli, É.A.M.; de Castro Oliveira, J.A.; Tomita, J.L.C.; Piccoli, R.H. Uma revisão bibliográfica sobre bokashi dos últimos 20 anos. Res. Soc. Dev. 2020, 9, 10. [Google Scholar]
- Olle, M.; Williams, I.H. Effective microorganisms and their influence on vegetable production—A review. J. Hortic. Sci. Biotechnol. 2013, 88, 380–386. [Google Scholar] [CrossRef]
- Olle, M. Review: Bokashi technology as a promising technology for crop production in Europe. J. Hortic. Sci. Biotechnol. 2020, 96, 145–152. [Google Scholar] [CrossRef]
- Silva, N.L.; Lanna, N.B.L.; Cardoso, A.I.I. Doses de Bokashi em cobertura na produção de beterraba. Rev. Agric. Neotrop. 2018, 5, 28–34. [Google Scholar] [CrossRef]
- Xavier, M.C.G.; Santos, C.A.; Costa, E.S.P.; Carmo, M.G.F. Produtividade de repolho em função de doses de bokashi. Rev. De Agric. Neotrop. 2019, 6, 17–22. [Google Scholar] [CrossRef]
- França, F.C.T.; da Silva, E.C.; Pedrosa, M.W.; de Almeida Carlos, L. Adubos orgânicos no cultivo e nutrição mineral de tomateiro. Ambiência 2017, 13, 235–244. [Google Scholar]
- Goulart RG, T.; dos Santos, C.A.; de Oliveira, C.M.; Costa ES, P.; de Oliveira, F.A.; de Andrade, N.F.; do Carmo MG, F. Desempenho agronômico de cultivares de alface sob adubação orgânica em Seropédica—RJ. Rev. Bras. De Agropecuária Sustentável 2018, 8, 66–72. [Google Scholar] [CrossRef]
- Hata, F.T.; Ventura, M.U.; Sousa, V.; Fregonezi, G.A.F. Low-cost organic fertilizations and bioactivator for arugula-radish intercropping. Emir. J. Food Agric. 2019, 31, 773–778. [Google Scholar] [CrossRef]
- Lima, C.E.P.; Fontenelle, M.R.; Silva, L.R.B.; Soares, D.C.; Moita, A.W.; Zandonadi, D.B.; Souza, R.B.; Lopes, C.A. Short-term changes in fertility attributes and soil organic matter caused by the addition of EM Bokashis in two tropical soils. Int. J. Agron. 2015, 2015, 754298. [Google Scholar] [CrossRef]
- Quiroz, M.; Flores, F. Nitrogen availability, maturity and stability of bokashi-type fertilizers elaborated with different feedstocks of animal origin. Arch. Agron. Soil Sci. 2018, 65, 867–875. [Google Scholar] [CrossRef]
- Wrege, M.S.; Steinmetz, S.; Junior, C.R.; Almeida, I.R. Atlas Climático da Região Sul do Brasil: Estados do Paraná, Santa Catarina e Rio Grande do Sul; Brasília DF Embrapa: Pelotas, Brazil, 2012. [Google Scholar]
- Bonfim, F.P.G.; Honório, I.C.G.; Reis, I.L.; Pereira, A.D.J.; Souza, D.D.B. Caderno dos Microrganismos Eficientes (EM): Instruções Práticas Sobre o uso Ecológico e Social do EM; Universidade Federal de Viçosa, Departamento de Fitotecnia: Viçosa, Brazil, 2011. [Google Scholar]
- Breitwieser, F.P.; Lu, J.; Salzberg, S.L. A review of methods and databases for metagenomic classification and assembly. Brief Bioinform. 2017, 20, 1125–1136. [Google Scholar] [CrossRef]
- Breitwieser, F.P.; Salzberg, S.L. Pavian: Interactive analysis of metagenomics data for microbiome studies and pathogen identification. Bioinformatics 2020, 36, 1303–1304. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Paarmann, D.; D’Souza, M.; Olson, R.; Glass, E.M.; Kubal, M.; Paczian, T.; Rodriguez, A.; Stevens, R.; Wilke, A.; et al. The metagenomics RAST server—A public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinform. 2008, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.G. Sistema Brasileiro de Classificação de Solos; EMBRAPA: Brasilia, Brazil, 2018; p. 356. [Google Scholar]
- Tedesco, M.J.; Gianello, C.; Bissani, C.A.; Bohnen, H.; Volkweiss, S.J. Análise de solo Plantas e Outros Materiais, 2nd ed.; Universidade Federal do Rio Grande do Sul, (Boletim Técnico, 5): Porto Alegre, Brazil, 1995. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural Waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Laviola, B.G.; Dias, L.A.S. Teor e acúmulo de nutrientes em folhas e frutos de pinhão-manso. Rev. Bras. De Ciência Do Solo 2008, 32, 1969–1975. [Google Scholar] [CrossRef]
- Leblanc, A.; Cerrato, M.; Vélex, L. Comparación del contenido de nutrientes de Bocashis elaborados con desechos de fincas del trópico húmedo de Costa Rica. Tierra Trop. 2005, 2, 149–159. [Google Scholar]
- Leblanc, H.; Cerrato, M.; Miranda, A.; Valle, G.Y. Determinación de la calidad de abonos orgánicos a través de bioensayos. Tierra Trop. 2007, 3, 97–107. [Google Scholar]
- Trani, P.E.; Terra, M.M.; Tecchio, M.A.; Teixeira LA, J.; Hanasiro, J. Adubação Orgânica de Hortaliças e Frutíferas; Instituto Agronômico de Campinas: Campinas, Brazil, 2013. Available online: http://www.iac.sp.gov.br/imagem_informacoestecnologicas/83.pdf (accessed on 8 June 2023).
- Turinek, M. Biodynamic soil fertility management in fruit crops. In Fruit Crops: Diagnosis and Management of Nutrient Constraints; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 28; pp. 393–400. [Google Scholar]
- Taiz, L.; Zeiger, E. Fisiologia Vegetal, 5th ed.; Artmed: Porto Alegre, Brazil, 2013; p. 954. [Google Scholar]
- Curi, N.; Ker, J.C.; Novais, R.F.; Vidal-Torrado; Schaefer, C.E.G.R. Pedologia: Solos dos Biomas Brasileiros; Sociedade Brasileira de Ciência do Solo: Viçosa, Brazil, 2017. [Google Scholar]
- Garcia-Gómez, A.; Bernal, M.P.; Roig, A. Organic matter fractions involved in degradation and humification processes during composting. Compost. Sci. Util. 2005, 13, 127–135. [Google Scholar] [CrossRef]
- Silva, F.A.M.; Guerrero Lopez, F.; Villas Boas, R.L.; Silva, R.B. Transformação da matéria orgânica em substâncias húmicas durante a compostagem de resíduos vegetais. Rev. Bras. De Agroecol. 2009, 4, 59–66. [Google Scholar]
- Iglesias-Jimenez, E.; Perez-Garcia, V. Determination or maturity indexes for city refuse composts. Agric. Ecosyst. Environ. 1992, 38, 331–343. [Google Scholar] [CrossRef]
- Jodice, R. Parametri chimici e biologici per la valutazione della qualità del compost. In Proceedings of the COMPOST Production and Use International Symposium, S. Michelle all’Adige, Italy, 20–23 June 1989; Volume 20–23, pp. 363–384. [Google Scholar]
- Bernal, M.P.; Paredes, C.; Sánchez-Monedero, M.A.; Cegarra, J. Maturity and stability parameters of composts prepared with a wide range of organic wastes. Bioresour. Technol. 1998, 63, 91–99. [Google Scholar] [CrossRef]
- Brasil. Instrução Normativa Nº 61, 8 de julho de 2020. Estabelece as Regras sobre Definições, Exigências, Especificações, Garantias, Tolerâncias, Registro, Embalagem e Rotulagem dos Fertilizantes Orgânicos e dos Biofertilizantes, Destinados à Agricultura. 2020. Available online: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-agricolas/fertilizantes/legislacao/in-61-de-8-7-2020-organicos-e-biofertilizantes-dou-15-7-20.pdf (accessed on 15 June 2023).
- Kjellberg, K. Supervision of the Sanitary Quality of Composting in the Nordic Countries; TemaNord—Nordic Council of Ministers: Copenhagen, Denmark, 2002; Volume 567. [Google Scholar]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition-current knowledge and future directions. Front Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Malik, A. Microbial Biofertilizers and Micronutrient Availability: The Role of Zinc in Agriculture and Human Health; Springer International Publishing: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd Allah, E.F.; Hashem, A. Phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef] [PubMed]
- Maki, Y.; Soejima, H.; Kitamura, T.; Sugiyama, T.; Sato, T.; Watahiki, M.K.; Yamaguchi, J. 3-Phenyllactic acid, a root-promoting substance isolated from Bokashi fertilizer, exhibits synergistic effects with tryptophan. Plant Biotechnol. 2021, 38, 9–16. [Google Scholar] [CrossRef]
- Siqueira, A.P.P.; Siqueira, M.F.B. Bokashi: Adubo Orgânico Fermentado; Programa Rio Rural: Niterói, Brazil, 2013; Volume 40, pp. 1–16. [Google Scholar]
- Hata, F.T.; Spagnuolo, F.A.; de Paula, M.T.; Moreira, A.A.; Ventura, M.U.; Fregonezi, G.A.F.; de Oliveira, A.L.M. Bokashi compost and biofertilizer increase lettuce agronomic variables in protected cultivation and indicates substrate microbiological changes. Emir. J. Food Agric. 2020, 32, 640–646. [Google Scholar] [CrossRef]
- Scotton, J.C.; da Silva Pereira, J.; Campos, A.A.B.; Pinto, D.F.P.; Costa, W.L.F.; Homma, S.K. Different sources of inoculum to the bokashi provides distinct effects on the soil quality. Braz. J. Sustain. Agric. 2017, 7, 32. [Google Scholar] [CrossRef]
- Santos, F.T.; Ludwig, F.; Costa, L.A.M.; Costa, M.S.S.M. Nutrition and growth of potted gerbera according to mineral and organic fertilizer. Rev. Bras. De Hortic. Ornam. 2015, 21, 251–258. [Google Scholar] [CrossRef]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2015, 80, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Eisenhauer, N.; Scheu, S.; Jousset, A. Increasing antagonistic interactions cause bacterial communities to collapse at high diversity. Ecol. Lett. 2012, 15, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Xu, H. Effects of a microbial inoculant and organic fertilizers on the growth, photosynthesis and yield of sweet corn. J. Crop Prod. 2001, 3, 183–214. [Google Scholar] [CrossRef]
- Xu, H.; Wang, R.; Mridha, A. Effects of organic fertilizers and a microbial inoculant on leaf photosynthesis and fruit yield and quality of tomato plants. J. Crop Prod. 2001, 3, 173–182. [Google Scholar] [CrossRef]
- Zaman, A.; Ahmed, M.; Gogoi, P. Effect of bokashi on plant growth, yield and essential oil quantity and quality in patchouli (Pogostemon cablin Benth.). Biosci. Biotech. Res. Asia 2010, 7, 383–387. [Google Scholar]
- Murillo-Amador, B.; Morales-Prado, L.E.; Troyo-Diéguez, E.; Córdoba-Matson, M.V.; Hernández-Montiel, L.G.; Rueda-Puente, E.O.; Nieto-Garibay, A. Changing environmental conditions and applying organic fertilizers in Origanum vulgare L. Front. Plant Sci. 2015, 6, 549. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, G.P. Adubação Orgânica e Biodinâmica na Produção de Chicória (Cichorium endivia) e de Beterraba (Beta vulgaris), em Sucessão. Master’s Thesis, UNESP, Botucatu, Brazil, 2009. [Google Scholar]
- Ferreira, S.; Assis, R.P.; Souza, R.J.; Gomes, L.A.A. Avaliação da adição de bokashi no cultivo de brócolis Lord Summer. Rev. Agrogeoambiental 2012, 4, 1–6. [Google Scholar] [CrossRef][Green Version]
- Saiter, O.; Oliveira, L.A.A.; Oliveira, E.A.G.; Araujo, D.B. Efeito do Adubo Orgânico Fermentado Bokashi no Desempenho Agronômico do Brócolis Americano; Program Rio Rural: Teresópolis, Brazil, 2016. [Google Scholar]
- Silva, N.L.; Lanna, N.B.L.; Cardoso, A.I.I. Produção de beterraba em função de doses de torta de mamona em cobertura. Hortic. Bras. 2016, 34, 416–421. [Google Scholar] [CrossRef]
- Condé, F.; Oliveira, D.M.; Oliveira, J.E.Z. Incidência e severidade de hérnia das crucíferas (Plasmodiophora brassicae W.) em repolho (Brassica oleracea L. var. capitata) em solo tratado com biofertilizante tipo bokashi. Ciência E Nat. 2017, 39, 7–15. [Google Scholar] [CrossRef]
- Ferreira, S.; Souza, R.J.; Gomes, L.A.A. Produtividade de brócolis de verão com diferentes doses de bokashi. Rev. Agrogeoambiental 2013, 5, 31–38. [Google Scholar] [CrossRef]
- Piva, R.; Botelho, R.V.; de Lima PC, G.; Rambolà, A.D. Desenvolvimento, fisiologia e ocorrência de míldio em videiras cv. BRS Margot tratadas com preparados biodinâmicos. Rev. De Ciências Agrárias 2019, 42, 472–482. [Google Scholar]
- Leite, A.B.; Polli, H.Q. Agricultura orgânica no Brasil com enfoque na agricultura biodinâmica. Interface Tecnológica 2020, 17, 417–430. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).