Micropropagation of Duboisia Species via Shoot Tip Meristem
Abstract
1. Introduction
2. Materials and Methods
2.1. Sterilisation and In Vitro Culture Establishment
2.2. Basal Medium Selection
2.3. Meristem Induction and Multiplication
2.4. Rooting
2.4.1. General Rooting Methods
2.4.2. Agar Medium-Based Rooting
2.4.3. Substrate-Based Rooting
2.5. Acclimatisation
2.6. Data Analysis
3. Results
3.1. Sterilisation and In Vitro Culture Establishment
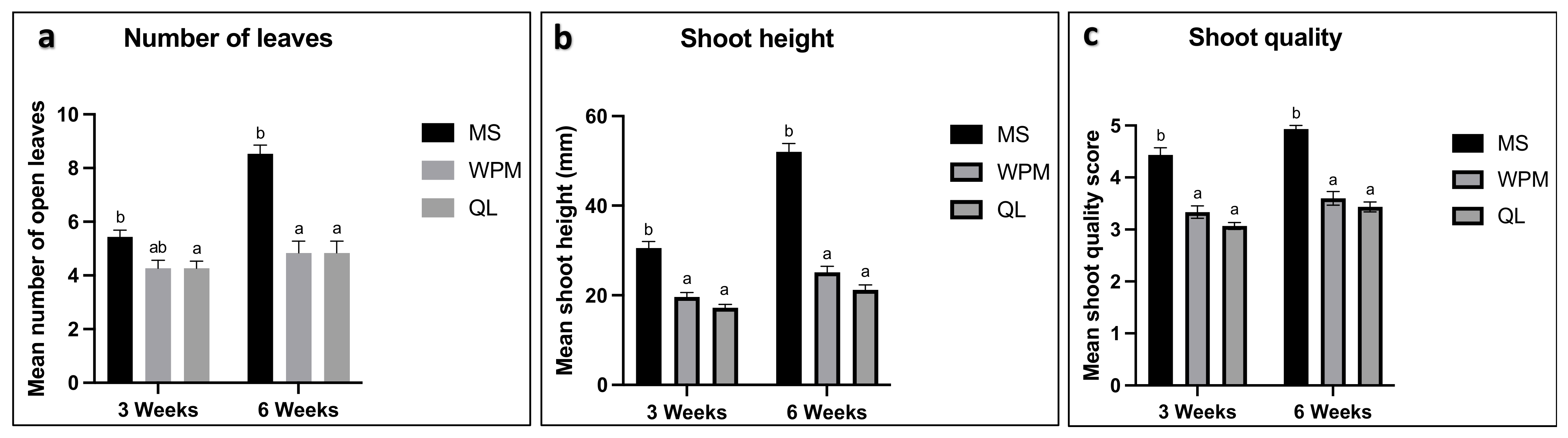
3.2. Basal Medium Selection
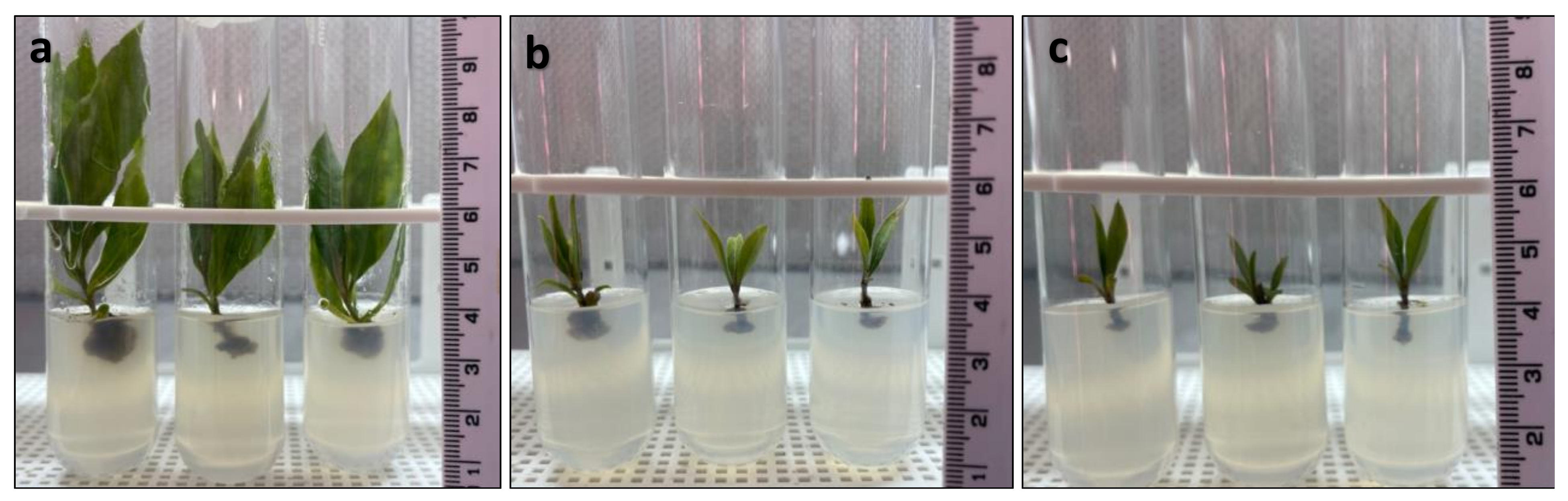
3.3. Meristem Induction and Multiplication
3.4. Rooting
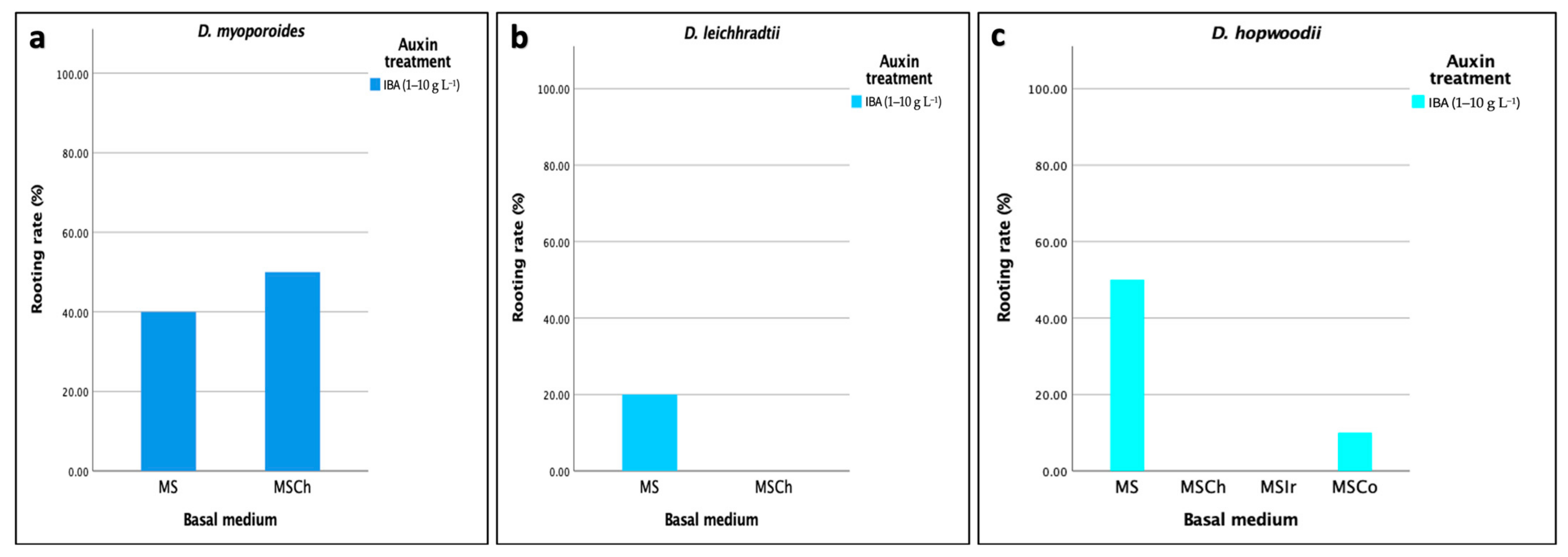
3.4.1. Effect of Different Rooting Media on Agar Medium-Based Rooting
3.4.2. Effects of Different Rooting Substrates and IBA Concentrations on Substrate-Based Rooting
3.4.3. Rockwool as a Rooting Substrate for D. hopwoodii Rooting
3.4.4. Comparison of Agar Medium-Based Rooting and Substrate-Based Rooting
3.5. Acclimatisation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnard, C. The Duboisias of Australia. Econ. Bot. 1952, 6, 3–17. [Google Scholar] [CrossRef]
- Craven, L.A.; Lepschi, B.J.; Haegi, L.A.R. A New Australian Species of Duboisia R. BR.(Solanaceae). J. Adel. Bot. Gard. 1995, 16, 27–31. [Google Scholar]
- Foley, P. Duboisia myoporoides: The Medical Career of a Native Australian Plant. Hist. Rec. Aust. Sci. 2006, 17, 31–69. [Google Scholar] [CrossRef]
- Ratsch, A.; Steadman, K.J.; Bogossian, F. The Pituri Story: A Review of the Historical Literature Surrounding Traditional Australian Aboriginal Use of Nicotine in Central Australia. J. Ethnobiol. Ethnomed. 2010, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Besher, S.; Al-Ammouri, Y.; Murshed, R. Production of Tropan Alkaloids in the in Vitro and Callus Cultures of Hyoscyamus Aureus and Their Genetic Stability Assessment Using ISSR Markers. Physiol. Mol. Biol. Plants 2014, 20, 343–349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, L.; Ding, R.; Chai, Y.; Bonfill, M.; Moyano, E.; Oksman-Caldentey, K.-M.; Xu, T.; Pi, Y.; Wang, Z.; Zhang, H. Engineering Tropane Biosynthetic Pathway in Hyoscyamus niger Hairy Root Cultures. Proc. Natl. Acad. Sci. USA 2004, 101, 6786–6791. [Google Scholar] [CrossRef]
- Häkkinen, S.T.; Moyano, E.; Cusido, R.M.; Palazon, J.; Pinol, M.T.; Oksman-Caldentey, K.-M. Enhanced Secretion of Tropane Alkaloids in Nicotiana Tabacum Hairy Roots Expressing Heterologous Hyoscyamine-6β-Hydroxylase. J. Exp. Bot. 2005, 56, 2611–2618. [Google Scholar] [CrossRef]
- Palazón, J.; Navarro-Ocaña, A.; Hernandez-Vazquez, L.; Mirjalili, M.H. Application of Metabolic Engineering to the Production of Scopolamine. Molecules 2008, 13, 1722–1742. [Google Scholar] [CrossRef]
- Wang, X.; Chen, M.; Yang, C.; Liu, X.; Zhang, L.; Lan, X.; Tang, K.; Liao, Z. Enhancing the Scopolamine Production in Transgenic Plants of Atropa Belladonna by Overexpressing Pmt and H6h Genes. Physiol. Plant. 2011, 143, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.; Smolke, C.D. Biosynthesis of Medicinal Tropane Alkaloids in Yeast. Nature 2020, 585, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, A.B.; Otálvaro, A.Á.M.; Busto, V.D.; Talou, J.R.; Velásquez, L.M.E.; Giulietti, A.M. Scopolamine, Anisodamine and Hyoscyamine Production by Brugmansia Candida Hairy Root Cultures in Bioreactors. Process Biochem. 2010, 45, 1577–1581. [Google Scholar] [CrossRef]
- Loftus-Hills, K.; Kelenyi, G.P. A Preliminary Report on the Cultivation of Duboisia Spp. J. Counc. Sci. Ind. Res. Aust. 1946, 19, 359–375. [Google Scholar]
- Singh, A.; Singh, D.V.; Rao, M.R.; Shukla, Y.N.; Husain, A. Cultivation of Duboisia myoporoides R. Brown as Source of Tropane Alkaloids in India. Indian J. Pharm. Sci. 1985, 47, 120–121. [Google Scholar]
- Gerson, E.A.; Kelsey, R.G.; St Clair, J.B. Genetic Variation of Piperidine Alkaloids in Pinus Ponderosa: A Common Garden Study. Ann. Bot. 2009, 103, 447–457. [Google Scholar] [CrossRef]
- Luanratana, O. Micropropagation of Duboisia Species. In High-Tech and Micropropagation VI; Springer: Berlin/Heidelberg, Germany, 1997; pp. 313–331. [Google Scholar]
- Hiti-Bandaralage, J.; Hayward, A.; O’Brien, C.; Gleeson, M.; Nak, W.; Mitter, N. Advances in Avocado Propagation for the Sustainable Supply of Planting Materials. In Achieving Sustainable Cultivation of Tropical Fruits; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 215–238. [Google Scholar]
- Hiti-Bandaralage, J.C.; Hayward, A.; Mitter, N. Micropropagation of Avocado (Persea Americana Mill.). Am. J. Plant Sci. 2017, 8, 2898. [Google Scholar] [CrossRef]
- Hiti-Bandaralage, J. Micropropagation as an Alternative for Avocado Clonal Propagation; The University of Queensland: Brisbane, Australia, 2019. [Google Scholar]
- Grout, B.W. Meristem-Tip Culture for Propagation and Virus Elimination. Plant Cell Cult. Protoc. 1999, 115–125. [Google Scholar]
- Gupta, S.; Singh, A.; Yadav, K.; Pandey, N.; Kumar, S. Chapter 2—Micropropagation for Multiplication of Disease-Free and Genetically Uniform Sugarcane Plantlets. In Advances in Plant Tissue Culture; Chandra Rai, A., Kumar, A., Modi, A., Singh, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 31–49. ISBN 978-0-323-90795-8. [Google Scholar]
- Getnet, B. In Vitro Shoot Multiplication of Two Sugarcane (Saccharum Officinarum L.) Genotypes Using Shoot Apical Meristem. Adv. Life Sci. Technol. 2017, 53, 13. [Google Scholar]
- Khaskheli, A.J.; Khaskheli, M.I.; Khaskheli, M.A.; Shar, T.; Ahmad, W.; Lighari, U.A.; Khaskheli, M.A.; Khaskheli, A.A.; Makan, F.H. Proliferation, Multiplication and Improvement of Micro-Propagation System for Mass Clonal Production of Rose through Shoot Tip Culture. Am. J. Plant Sci. 2018, 9, 296–310. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.-J. Plant Propagation by Tissue Culture: Volume 1. The Background; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; Volume 1. [Google Scholar]
- Benmahioul, B.; Dorion, N.; Kaid-Harche, M.; Daguin, F. Micropropagation and Ex Vitro Rooting of Pistachio (Pistacia Vera L.). Plant Cell Tissue Organ Cult. 2012, 108, 353–358. [Google Scholar] [CrossRef]
- Stevens, M.E.; Pijut, P.M. Rapid in Vitro Shoot Multiplication of the Recalcitrant Species Juglans Nigra L. In Vitr. Cell. Dev. Biol. Plant 2018, 54, 309–317. [Google Scholar] [CrossRef]
- Walkey, D.G. Production of Apple Plantlets from Axillary-Bud Meristems. Canadian J. Plant Sci. 1972, 52, 1085–1087. [Google Scholar] [CrossRef]
- Shu, W.; Timon, B. Preliminary Study on the Methods of Getting Virus-Free Peach Plantlets in Vitro. In Proceedings of the III International Peach Symposium 374, Beijing, China, 6–10 September 1993; pp. 191–194. [Google Scholar]
- Lane, W.D. Regeneration of Pear Plants from Shoot Meristem-Tips. Plant Sci. Lett. 1979, 16, 337–342. [Google Scholar] [CrossRef]
- Xue, Y.; Hiti-Bandaralage, J.C.A.; Mitter, N. Micropropagation of Duboisia Species: A Review on Current Status. Agronomy 2023, 13, 797. [Google Scholar] [CrossRef]
- Kukreja, A.K.; Mathur, A.K. Tissue Culture Studies in Duboisia myoporoides; 1. Plant Regeneration and Clonal Propagation by Stem Node Cultures. Planta Med. 1985, 51, 93–96. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- McCown, B.H. Woody Plant Medium (WPM)—A Mineral Nutrient Formulation for Microculture for Woody Plant Species. Hort. Sci. 1981, 16, 453. [Google Scholar]
- Quoirin, M.; Lepoivre, P.H. Improved Media for in Vitro Culture of Prunus Sp. In Proceedings of the Symposium on Tissue Culture for Horticultural Purposes 78, Ghent, Belgium, 6–9 September 1977; pp. 437–442. [Google Scholar]
- Kitamura, Y. Duboisia Spp.: In Vitro Regeneration, and the Production of Tropane and Pyridine Alkaloids. In Medicinal and Aromatic Plants I; Bajaj, Y.P.S., Ed.; Biotechnology in Agriculture and Forestry; Springer: Berlin/Heidelberg, Germany, 1988; Volume 4, pp. 419–436. ISBN 978-3-642-73028-3. [Google Scholar]
- Lin, G.-L.D. Tissue Culture of a Duboisia Hybrid (D. leichhardtii × D. myoporoides) and the Production of Tropane Alkaloids. Ph.D. Thesis, School of Pharmacy, The University of Queensland, Brisbane, QLD, Australia, 1991. [Google Scholar]
- Bairu, M.W.; Aremu, A.O.; Van Staden, J. Somaclonal Variation in Plants: Causes and Detection Methods. Plant Growth Regul. 2011, 63, 147–173. [Google Scholar] [CrossRef]
- Kitamura, Y.; Miura, H.; Sugii, M. Alkaloid Composition and Atropine Esterase Activity in Callus and Differentiated Tissues of Duboisia myoporoides R. BR. Chem. Pharm. Bull. 1985, 33, 5445–5448. [Google Scholar] [CrossRef]
- Hashimoto, T.; Nakajima, K.; Ongena, G.; Yamada, Y. Two Tropinone Reductases with Distinct Stereospecificities from Cultured Roots of Hyoscyamus Niger 1. Plant Physiol. 1992, 100, 836–845. [Google Scholar] [CrossRef]
- Kaviani, B.; Deltalab, B.; Kulus, D.; Tymoszuk, A.; Bagheri, H.; Azarinejad, T. In Vitro Propagation of Pyracantha angustifolia (Franch.) C.K. Schneid. Horticulturae 2022, 8, 964. [Google Scholar] [CrossRef]
- Okello, D.; Yang, S.; Komakech, R.; Rahmat, E.; Chung, Y.; Gang, R.; Kim, Y.-G.; Omujal, F.; Kang, Y. An in Vitro Propagation of Aspilia Africana (Pers.) C. D. Adams, and Evaluation of Its Anatomy and Physiology of Acclimatized Plants. Front. Plant Sci. 2021, 12, 704896. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Trikka, F.A.; Tsoktouridis, G.; Krigas, N.; Sarropoulou, V.; Papanastasi, K.; Maloupa, E.; Makris, A.M. Μicropropagation and Cultivation of Salvia Sclarea for Essential Oil and Sclareol Production in Northern Greece. In Vitr. Cell. Dev. Biol.-Plant 2020, 56, 51–59. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Habashi, A.A.; Emadpour, M.; Kazemi, N. Recovery of Virus-Free Almond (Prunus Dulcis) Cultivars by Somatic Embryogenesis from Meristem Undergone Thermotherapy. Sci. Rep. 2022, 12, 14948. [Google Scholar] [CrossRef]
- Sharmin, S.A.; Kabir, A.H.; Mandal, A.; Sarker, K.K.; Alam, M.F. In Vitro Propagation of Eggplant through Meristem Culture. Agric. Conspec. Sci. 2008, 73, 149–155. [Google Scholar]
- Yakuwa, H.; Oka, S. Plant Regeneration through Meristem Culture from Vegetative Buds of Mulberry (Morus Bombycis Koidz.) Stored in Liquid Nitrogen. Ann. Bot. 1988, 62, 79–82. [Google Scholar] [CrossRef]
- Beck, S.L.; Bunlop, R.; Van Staden, J. Meristem Culture of Acacia Mearnsii. Plant Growth Regul. 2000, 32, 49–58. [Google Scholar] [CrossRef]
- Alam, I.; Sharmin, S.A.; Naher, K.; Alam, J.; Anisuzzaman, M.; Alam, M.F. Effect of Growth Regulators on Meristem Culture and Plantlet Establishment in Sweet Potato [’Ipomoea Batatas’(L.) Lam.]. Plant Omics 2010, 3, 35–39. [Google Scholar]
- Deepa, A.V.; Anju, M.; Dennis Thomas, T. The Applications of TDZ in Medicinal Plant Tissue Culture. In Thidiazuron: From Urea Derivative to Plant Growth Regulator; Springer: Singapore, 2018; pp. 297–316. [Google Scholar]
- Erland, L.A.; Shukla, M.R.; Glover, W.; Saxena, P.K. A Simple and Efficient Method for Analysis of Plant Growth Regulators: A New Tool in the Chest to Combat Recalcitrance in Plant Tissue Culture. Plant Cell Tissue Organ Cult. 2017, 131, 459–470. [Google Scholar] [CrossRef]
- Gaba, V.P. Plant Growth Regulators in Plant Tissue Culture and Development. In Plant Development and Biotechnology; CRC Press: Boca Raton, FL, USA, 2005; pp. 87–99. [Google Scholar]
- De Klerk, G.-J. Rooting of Microcuttings: Theory and Practice. In Vitr. Cell. Dev. Biol. Plant 2002, 38, 415–422. [Google Scholar] [CrossRef]
- Ainsley, P.J.; Collins, G.G.; Sedgley, M. In Vitro Rooting of Almond (Prunus Dulcis Mill.). In Vitr. Cell. Dev. Biol. Plant 2001, 37, 778–785. [Google Scholar] [CrossRef]
- Amerson, H.V.; Mott, R.L. Notes: Improved Rooting of Western White Pine Shoots From Tissue Cultures. For. Sci. 1982, 28, 822–825. [Google Scholar] [CrossRef]
- Quambusch, M.; Gruß, S.; Pscherer, T.; Winkelmann, T.; Bartsch, M. Improved in Vitro Rooting of Prunus Avium Microshoots Using a Dark Treatment and an Auxin Pulse. Sci. Hortic. 2017, 220, 52–56. [Google Scholar] [CrossRef]
- Dumas, E.; Monteuuis, O. In Vitro Rooting of Micropropagated Shoots from Juvenile and Mature Pinus Pinaster Explants: Influence of Activated Charcoal. Plant Cell Tiss. Organ. Cult. 1995, 40, 231–235. [Google Scholar] [CrossRef]
- Magyar-Tábori, K.; Dobránszky, J.; Jámbor-Benczúr, E.; Lazányi, J.; Szalai, J.; Ferenczy, A. Effects of Indole-3-Butyric Acid Levels and Activated Charcoal on Rooting of in Vitro Shoots of Apple Rootstocks. Int. J. Hortic. Sci. 2002, 8, 25–28. [Google Scholar] [CrossRef]
- Da Costa, C.; De Almeida, M.; Ruedell, C.; Schwambach, J.; Maraschin, F.; Fett-Neto, A. When Stress and Development Go Hand in Hand: Main Hormonal Controls of Adventitious Rooting in Cuttings. Front. Plant Sci. 2013, 4, 133. [Google Scholar] [CrossRef]
- Marín, M.L.; Marín, J.A. Excised Rootstock Roots Cultured in Vitro. Plant Cell Rep. 1998, 18, 350–355. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pruski, K.; Astatkie, T.; Nowak, J. Tissue Culture Propagation of Mongolian Cherry (Prunus Fruticosa) and Nanking Cherry (Prunus Tomentosa). Plant Cell Tiss. Organ. Cult. 2005, 82, 207–211. [Google Scholar] [CrossRef]
- Kim, M.-S.; Klopfenstein, N.B.; Cregg, B.M. In Vitro and Ex Vitro Rooting of Micropropagated Shoots Using Three Green Ash (Fraxinus Pennsylvanica) Clones. New For. 1998, 16, 43–57. [Google Scholar] [CrossRef]
- Oakes, A.D.; Pilkey, H.C.; Powell, W.A. Improving Ex Vitro Rooting and Acclimatization Techniques for Micropropagated American Chestnut1. J. Environ. Hortic. 2020, 38, 149–157. [Google Scholar] [CrossRef]
- Silvestri, C.; Sabbatini, G.; Marangelli, F.; Rugini, E.; Cristofori, V. Micropropagation and Ex Vitro Rooting of Wolfberry. HortScience 2018, 53, 1494–1499. [Google Scholar] [CrossRef]
- Jagiełło-Kubiec, K.; Nowakowska, K.; Ilczuk, A.; Łukaszewska, A.J. Optimizing Micropropagation Conditions for a Recalcitrant Ninebark (Physocarpus Opulifolius L. Maxim.) Cultivar. In Vitr. Cell. Dev. Biol.-Plant 2021, 57, 281–295. [Google Scholar] [CrossRef]
- Bonin, J. Coir and Peat: An Optimum Rooting Substrate for Propagation\copyright. In Proceedings of the 2014 Annual Meeting of the International Plant Propagators Society 1085, Bellefonte, PA, USA, 1 January 2014; pp. 95–98. [Google Scholar]
- Newell, C.; Growns, D.; McComb, J. The Influence of Medium Aeration on in Vitro Rooting of Australian Plant Microcuttings. Plant Cell Tissue Organ Cult. 2003, 75, 131–142. [Google Scholar] [CrossRef]
- Lin, X.; Bergmann, B.A.; Stomp, A.-M. Effect of Medium Physical Support, Shoot Length and Genotype on in Vitro Rooting and Plantlet Morphology of Sweetgum. J. Environ. Hortic. 1995, 13, 117–121. [Google Scholar] [CrossRef]
- Xiaohuan, Y.; Xiangyong, P.; Qing, L.; Kaichun, Z. Research on Tissue Culture Rooting of Prunus Avium L. J. Northwest Sci-Tech Univ. Agric. For. (Nat. Sci. Ed.) 2004, 32, 71–73. [Google Scholar]
- Gislerød, H.R. Physical Conditions of Propagation Media and Their Influence on the Rooting of Cuttings. Plant Soil 1982, 69, 445–456. [Google Scholar] [CrossRef]
- Chandra, S.; Bandopadhyay, R.; Kumar, V.; Chandra, R. Acclimatization of Tissue Cultured Plantlets: From Laboratory to Land. Biotechnol. Lett. 2010, 32, 1199–1205. [Google Scholar] [CrossRef]
- Hiti-Bandaralage, J.; Hayward, A.; Mitter, N. Structural Disparity of Avocado Rootstocks In Vitro for Rooting and Acclimation Success. Int. J. Plant Biol. 2022, 13, 426–442. [Google Scholar] [CrossRef]
- Gonçalves, J.C.; Diogo, G.; Amâncio, S. In Vitro Propagation of Chestnut (Castanea sativa×C. Crenata): Effects of Rooting Treatments on Plant Survival, Peroxidase Activity and Anatomical Changes during Adventitious Root Formation. Sci. Hortic. 1998, 72, 265–275. [Google Scholar] [CrossRef]
- Addae-Frimpomaah, F.; Amponsah, J.; Tengey, T.K. Regeneration of Three Sweet Potato (Ipomoea Batatas (L.)) Accessions in Ghana via, Meristem and Nodal Culture. Int. J. Plant Breed. Genet. 2014, 8, 121–138. [Google Scholar] [CrossRef]
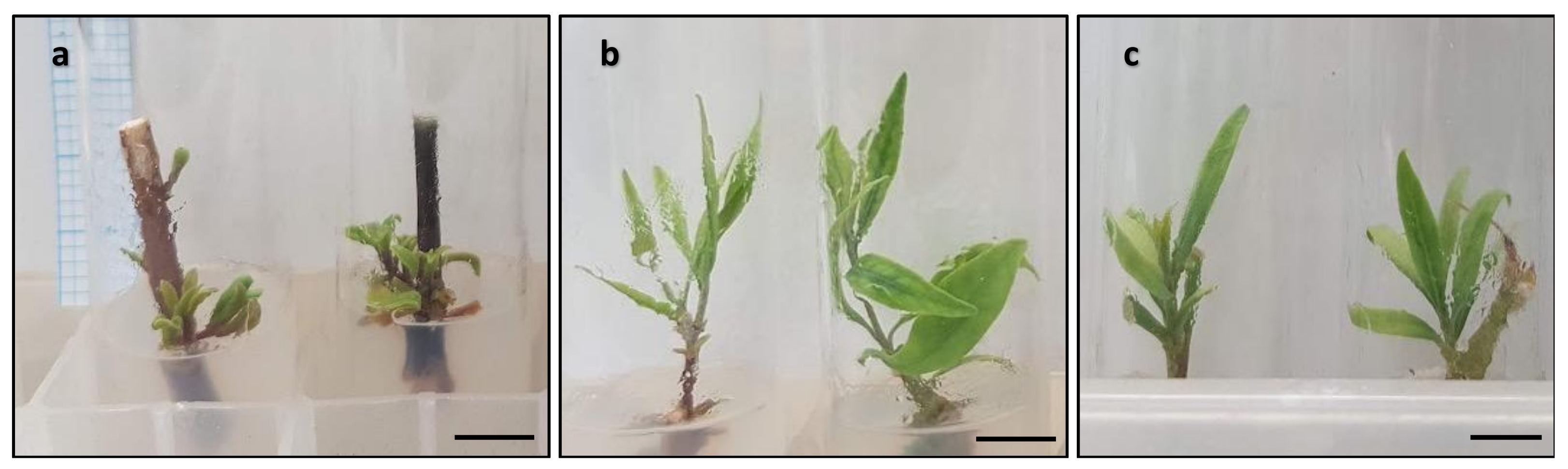

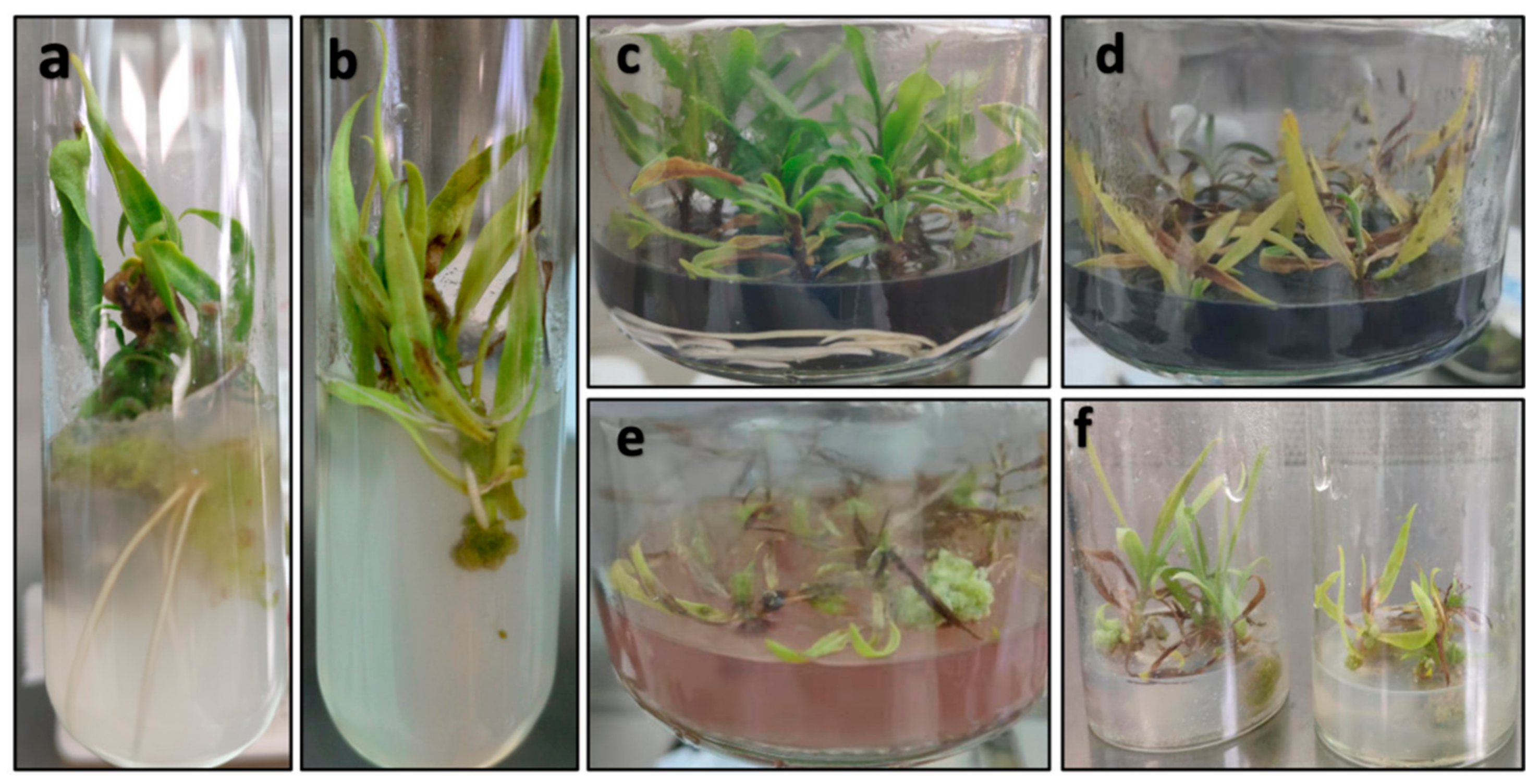



| Scores | Description of Morphological Features for Scoring |
|---|---|
| 1 | No bud breaking, dead shoot, vitrified shoot or callus overgrowth. |
| 2 | Unopened green, healthy buds or shoots. |
| 3 | Shoots with open leaves between 0–10 mm in length or leaves showing yellowing symptoms. |
| 4 | Shoots with at least one fully expanded green, healthy leaf larger than 10 mm and without leaf yellowing. |
| 5 | Shoots with more than three green, healthy leaves larger than 25 mm and without leaf yellowing. |
| Plant Species | Optimum Cytokinin Treatment | Multiplication Rate from F1 to F5 | Total Multiplication Rate |
|---|---|---|---|
| Duboisia myoporoides | BA (1–15 mg L−1) | 2 × 9.33 × 3.39 × 6.85 × 3.93 | 1702 |
| Duboisia leichhradtii | BA (1–15 mg L−1) | 3.6 × 3.4 × 5.1 × 3.44 × 4.2 | 901 |
| Duboisia hopwoodii | Kinetin (1–10 mg L−1) | 3.4 × 6.8 × 3.8 × 3.4 × 3.6 | 1075 |
| Plant Species | IBA Treatments | Rooting (%) | |
|---|---|---|---|
| Jiffy Cube Experiments | Foam Cube Experiments | ||
| Duboisia myoporoides | IBA (1–10 g L−1) | 100 a | 97.33 a |
| Duboisia leichhradtii | IBA (1–10 g L−1) | 100 b | 0 a |
| Duboisia hopwoodii | IBA (1–10 g L−1) | 11.67 b | 6 a |
| IBA (0.1–5 g L−1) | 44.33 c | - | |
| Plant Species | The Highest Rooting Rate Achieved (%) | |
|---|---|---|
| Agar Medium-Based Rooting | Substrate-Based Rooting | |
| Duboisia myoporoides | 50 | 100 |
| Duboisia leichhradtii | 20 | 100 |
| Duboisia hopwoodii | 50 | 70 |
| Plant Species | Acclimatisation Survival Rate (%) | |
|---|---|---|
| Acclimatisation in Seed Tray | Acclimatisation in Ziploc Bag | |
| Duboisia myoporoides | 100 b | 60 a |
| Duboisia leichhradtii | 100 | - |
| Duboisia hopwoodii | 80 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Hiti-Bandaralage, J.C.A.; Jambuthenne, D.T.; Zhao, Z.; Mitter, N. Micropropagation of Duboisia Species via Shoot Tip Meristem. Horticulturae 2023, 9, 1313. https://doi.org/10.3390/horticulturae9121313
Xue Y, Hiti-Bandaralage JCA, Jambuthenne DT, Zhao Z, Mitter N. Micropropagation of Duboisia Species via Shoot Tip Meristem. Horticulturae. 2023; 9(12):1313. https://doi.org/10.3390/horticulturae9121313
Chicago/Turabian StyleXue, Yuxin, Jayeni Chathurika Amarathunga Hiti-Bandaralage, Dilani Tharanga Jambuthenne, Zizhu Zhao, and Neena Mitter. 2023. "Micropropagation of Duboisia Species via Shoot Tip Meristem" Horticulturae 9, no. 12: 1313. https://doi.org/10.3390/horticulturae9121313
APA StyleXue, Y., Hiti-Bandaralage, J. C. A., Jambuthenne, D. T., Zhao, Z., & Mitter, N. (2023). Micropropagation of Duboisia Species via Shoot Tip Meristem. Horticulturae, 9(12), 1313. https://doi.org/10.3390/horticulturae9121313








