Dynamics of Bioactive Compounds under the Influence of Yellow, Blue, and Violet Light Filters on Hippophae rhamnoides L. (Sea Buckthorn) Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Reagents
2.2. The HPLC-DAD-ESI+ Phenolic Compounds and Vitamin C
2.2.1. HPLC Analysis
2.2.2. Phenolic Compounds
2.2.3. Vitamin C Assay
2.3. EPR Measurement of Antioxidant Activity
2.4. Statistical Analysis
3. Results
3.1. Phenolic Compounds and Vitamin C Content
3.2. EPR Investigations of the Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- El-Sohaimy, S.A.; Shehata, M.G.; Mathur, A.; Darwish, A.G.; Abd El-Aziz, N.M.; Gauba, P.; Upadhyay, P. Nutritional Evaluation of Sea Buckthorn “Hippophae rhamnoides” Berries and the Pharmaceutical Potential of the Fermented Juice. Fermentation 2022, 8, 391. [Google Scholar] [CrossRef]
- Wang, H.H.; Sun, X.; Hua, S.Z.; Teng, X.P. Historical record and current situation of pharmaceutical R & D about sea buckthorn in China. Glob. Sea Buckthorn Res. Dev. 2012, 10, 25–28. [Google Scholar]
- Wang, Z.; Zhao, F.; Wei, P.; Chai, X.; Hou, G.; Meng, Q. Phytochemistry, health benefits, and food applications of sea buckthorn (Hippophae rhamnoides L.): A comprehensive review. Front. Nutr. 2022, 9, 1036295. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Important therapeutic uses of sea buckthorn (Hippophae): A review. J. Biol. Sci. 2004, 4, 687–693. [Google Scholar]
- Zhong, M.; Zhao, S.; Xie, J.; Wang, Y. Molecular and Cellular Mechanisms of the Anti-oxidative Activity of Seabuckthorn (Hippophae rhamnoides L.). In Seabuckthorn Genome; Springer: Berlin/Heidelberg, Germany, 2022; pp. 301–313. [Google Scholar]
- Stobdan, T.; Chaurasia, O.P.; Korekar, G.; Mundra, S.; Ali, Z.; Yadav, A.; Singh, S.B. Attributes of Seabuckthorn (Hippophae rhamnoides L.) to Meet Nutritional Requirements in High Altitudes. Def. Sci. J. 2010, 60, 226–230. [Google Scholar] [CrossRef]
- Pop, R.M.; Weesepoel, Y.; Socaciu, C.; Pintea, A.; Vincken, J.-P.; Gruppen, H. Carotenoid composition of berries and leaves from six Romanian sea buckthorn (Hippophae rhamnoides L.) varieties. Food Chem. 2014, 147, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Suo, Y.; Chen, D.; Tong, L. Protection against vascular endothelial dysfunction by polyphenols in sea buckthorn berries in rats with hyperlipidemia. BioScience Trends 2016, 10, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yang, W.; Kallio, H.; Yang, B. Health promoting properties and sensory characteristics of phytochemicals in berries and leaves of sea buckthorn (Hippophaë rhamnoides). Crit. Rev. Food Sci. Nutr. 2022, 62, 3798–3816. [Google Scholar] [CrossRef]
- Krejcarová, J.; Straková, E.; Suchý, P.; Herzig, I.; Karásková, K. Sea buckthorn (Hippophae rhamnoides L.) as a potential source of nutraceutics and its therapeutic possibilities—A review. Acta Vet. Brno 2015, 84, 257–268. [Google Scholar] [CrossRef]
- Masoodi, K.Z.; Wani, W.; Dar, Z.A.; Mansoor, S.; Anam-ul-Haq, S.; Farooq, I.; Hussain, K.; Wani, S.A.; Nehvi, F.A.; Ahmed, N. Sea buckthorn (Hippophae rhamnoides L.) inhibits cellular proliferation, wound healing and decreases expression of prostate specific antigen in prostate cancer cells in vitro. J. Funct. Foods 2020, 73, 104102. [Google Scholar] [CrossRef]
- Wani, T.A.; Wani, S.M.; Ahmad, M.; Ahmad, M.; Gani, A.; Masoodi, F.A. Bioactive profile, health benefits and safety evaluation of sea buckthorn (Hippophae rhamnoides L.): A review. Cogent Food Agric. 2016, 2, 1128519. [Google Scholar] [CrossRef]
- Ganju, L.; Padwad, Y.; Singh, R.; Karan, D.; Chanda, S.; Chopra, M.K.; Bhatnagar, P.; Kashyap, R.; Sawhney, R.C. Anti-inflammatory activity of Seabuckthorn (Hippophae rhamnoides) leaves. Int. Immunopharmacol. 2005, 5, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Suryakumar, G.; Gupta, A. Medicinal and therapeutic potential of Sea buckthorn (Hippophae rhamnoides L.). J. Ethnopharmacol. 2011, 138, 268–278. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zhang, G.; Zhang, J.; Zeng, Y.; Liu, J. Integrated analysis of multiomic data reveals the role of the antioxidant network in the quality of sea buckthorn berry. FASEB J. 2017, 31, 1929–1938. [Google Scholar] [CrossRef]
- Fankhauser, C.; Chory, J. Light control of plant development. Annu. Rev. Cell Dev. Biol. 1997, 13, 203–229. [Google Scholar] [CrossRef] [PubMed]
- Gendreau, E.; Traas, J.; Desnos, T.; Grandjean, O.; Caboche, M.; Hofte, H. Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol. 1997, 114, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Clipsham, M.; Kang, D.; Safina, A.; Valgardson, D. Effect of different light wavelengths on the overall growth of Arabidopsis thaliana seedlings. Expedition 2014, 4, 1. [Google Scholar]
- Cornea-Cipcigan, M.; Pamfil, D.; Sisea, C.R.; Mărgăoan, R. Gibberellic Acid Can Improve Seed Germination and Ornamental Quality of Selected Cyclamen Species Grown Under Short and Long Days. Agronomy 2020, 10, 516. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Kim, S.A.; Choi, S.-J.; Yun, H.K. Comparison of accumulation of stilbene compounds and stilbene related gene expression in two grape berries irradiated with different light sources. Hortic. Environ. Biotechnol. 2015, 56, 36–43. [Google Scholar] [CrossRef]
- Dhakal, R.; Baek, K.-H. Short period irradiation of single blue wavelength light extends the storage period of mature green tomatoes. Postharvest Biol. Technol. 2014, 90, 73–77. [Google Scholar] [CrossRef]
- Samuolienė, G.; Sirtautas, R.; Brazaitytė, A.; Duchovskis, P. LED lighting and seasonality effects antioxidant properties of baby leaf lettuce. Food Chem. 2012, 134, 1494–1499. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Maruo, T.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Ito, Y.; Ichimura, T.; Shinohara, Y. Effects of supplemental lighting with light-emitting diodes (LEDs) on tomato yield and quality of single-truss tomato plants grown at high planting density. Environ. Control. Biol. 2012, 50, 63–74. [Google Scholar] [CrossRef]
- Ménard, C.; Dorais, M.; Hovi, T.; Gosselin, A. Developmental and physiological responses of tomato and cucumber to additional blue light. In Proceedings of the V International Symposium on Artificial Lighting in Horticulture, Lillehammer, Norway, 30 June 2005; pp. 291–296. [Google Scholar]
- Brown, C.S.; Schuerger, A.C.; Sager, J.C. Growth and photomorphogenesis of pepper plants under red light-emitting diodes with supplemental blue or far-red lighting. J. Am. Soc. Hortic. Sci. 1995, 120, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Kokalj, D.; Hribar, J.; Cigić, B.; Zlatić, E.; Demšar, L.; Sinkovič, L.; Šircelj, H.; Bizjak, G.; Vidrih, R. Influence of Yellow Light-Emitting Diodes at 590 nm on Storage of Apple, Tomato and Bell Pepper Fruit. Food Technol. Biotechnol. 2016, 54, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, K.; Hashimoto, T.; Yoshida, S.; Sungwon, P.; Fukuda, S. Short photoirradiation induces flavonoid synthesis and increases its production in postharvest vegetables. J. Agric. Food Chem. 2012, 60, 4359–4368. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-L.; Xu, Q.; Li, F.; Feng, Y.; Qin, F.; Fang, W. Applications of xerophytophysiology in plant production—LED blue light as a stimulus improved the tomato crop. Sci. Hortic. 2012, 148, 190–196. [Google Scholar] [CrossRef]
- Kim, B.S.; Lee, H.O.; Kim, J.Y.; Kwon, K.H.; Cha, H.S.; Kim, J.H. An effect of light emitting diode (LED) irradiation treatment on the amplification of functional components of immature strawberry. Hortic. Environ. Biotechnol. 2011, 52, 35–39. [Google Scholar] [CrossRef]
- Chonhenchob, V.; Kamhangwong, D.; Kruenate, J.; Khongrat, K.; Tangchantra, N.; Wichai, U.; Singh, S.P. Preharvest bagging with wavelength-selective materials enhances development and quality of mango (Mangifera indica L.) cv. Nam Dok Mai# 4. J. Sci. Food Agric. 2011, 91, 664–671. [Google Scholar]
- Diaconeasa, Z.; Florica, R.; Rugină, D.; Lucian, C.; Socaciu, C. Hplc/pda–esi/ms identification of phenolic acids, flavonol glycosides and antioxidant potential in blueberry, blackberry, raspberries and cranberries. J. Food Nutr. Res. 2014, 2, 781–785. [Google Scholar] [CrossRef]
- Felföldi, Z.; Ranga, F.; Roman, I.A.; Sestras, A.F.; Vodnar, D.C.; Prohens, J.; Sestras, R.E. Analysis of physico-chemical and organoleptic fruit parameters relevant for tomato quality. Agronomy 2022, 12, 1232. [Google Scholar] [CrossRef]
- Csillag, I.; Damian, G. EPR Study of organically-grown versus greenhaouse strawberries. Stud. Univ. Babes-Bolyai Phys. 2016, 61, 1. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K. R Cluster Package: Cluster Analysis Basics and Extensions. R Package, Version 2.1. 0; 2019. Available online: https://CRAN.R-project.org/package=cluster (accessed on 13 October 2023).
- Wickham, H.; Chang, W. ggplot2: An Implementation of the Grammar of Graphics. R Package Version 0.7. 2008. 3. Available online: http://CRAN.R-project.org/package=ggplot2 (accessed on 13 October 2023).
- Ullah, M.A.; Tungmunnithum, D.; Garros, L.; Hano, C.; Abbasi, B.H. Monochromatic lights-induced trends in antioxidant and antidiabetic polyphenol accumulation in in vitro callus cultures of Lepidium sativum L. J. Photochem. Photobiol. B Biol. 2019, 196, 111505. [Google Scholar] [CrossRef] [PubMed]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Nowicka, P. Triterpenoids, phenolic compounds, macro-and microelements in anatomical parts of sea buckthorn (Hippophaë rhamnoides L.) berries, branches and leaves. J. Food Compos. Anal. 2021, 103, 104107. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, J.; Zou, H.; Zhang, L.; Li, S.; Wang, Y. The combination of blue and red LED light improves growth and phenolic acid contents in Salvia miltiorrhiza Bunge. Ind. Crops Prod. 2020, 158, 112959. [Google Scholar] [CrossRef]
- Taulavuori, K.; Pyysalo, A.; Taulavuori, E.; Julkunen-Tiitto, R. Responses of phenolic acid and flavonoid synthesis to blue and blue-violet light depends on plant species. Environ. Exp. Bot. 2018, 150, 183–187. [Google Scholar] [CrossRef]
- Kubica, P.; Szopa, A.; Prokopiuk, B.; Komsta, Ł.; Pawłowska, B.; Ekiert, H. The influence of light quality on the production of bioactive metabolites—Verbascoside, isoverbascoside and phenolic acids and the content of photosynthetic pigments in biomass of Verbena officinalis L. cultured in vitro. J. Photochem. Photobiol. B Biol. 2020, 203, 111768. [Google Scholar] [CrossRef]
- Arakawa, O. Effect of ultraviolet light on anthocyanin synthesis in light-colored sweet cherry, cv. Sato Nishiki. J. Jpn. Soc. Hortic. Sci. 1993, 62, 543–546. [Google Scholar] [CrossRef]
- Kataoka, I.; Beppu, K.; Sugiyama, A.; Taira, S. Enhancement of coloration of “Satohnishiki” sweet cherry fruit by postharvest irradiation with ultraviolet rays. Environ. Control Biol. 1996, 34, 313–319. [Google Scholar] [CrossRef][Green Version]
- Klimek-Szczykutowicz, M.; Prokopiuk, B.; Dziurka, K.; Pawłowska, B.; Ekiert, H.; Szopa, A. The influence of different wavelengths of LED light on the production of glucosinolates and phenolic compounds and the antioxidant potential in in vitro cultures of Nasturtium officinale (watercress). Plant Cell Tissue Organ Cult. PCTOC 2022, 149, 113–122. [Google Scholar] [CrossRef]
- Ye, S.; Shao, Q.; Xu, M.; Li, S.; Wu, M.; Tan, X.; Su, L. Effects of light quality on morphology, enzyme activities, and bioactive compound contents in Anoectochilus roxburghii. Front. Plant Sci. 2017, 8, 857. [Google Scholar] [CrossRef] [PubMed]
- Muneer, S.; Kim, E.J.; Park, J.S.; Lee, J.H. Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.). Int. J. Mol. Sci. 2014, 15, 4657–4670. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Van Iersel, M.W. Photosynthetic physiology of blue, green, and red light: Light intensity effects and underlying mechanisms. Front. Plant Sci. 2021, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, G.; Gundogdu, M.; Karlović, K.; Židovec, V.; Vokurka, A.; Ercişli, S. Main agro-morphological and biochemical berry characteristics of wild-grown sea buckthorn (Hippophae rhamnoides L. ssp. caucasica Rousi) genotypes in Turkey. Sustainability 2021, 13, 1198. [Google Scholar] [CrossRef]
- Zielińska, A.; Nowak, I. Abundance of active ingredients in sea-buckthorn oil. Lipids Health Dis. 2017, 16, 1–11. [Google Scholar] [CrossRef]
- Pop, D.F.; Mitre, V.; Balcău, S.L.; Gocan, T.M. Correlation Between the Amount of Soluble Substance and Vitamin C in ten Varieties of Strawberries Under the Influence of Mulch and Fertilizer. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Hortic. 2013, 70, 259–260. [Google Scholar]
- Marta, G.T.; Ileana, A. Correlations between the soluble dry matter and the content of vitamin C in the carrot roots. J. Hortic. For. Biotechnol. 2014, 18, 45–48. [Google Scholar]
- Gocan, T.-M.; Andreica, I.; Poșta, G.; Rozsa, M.; Rozsa, S. The effects of the industrial processing of the tomato paste and tomato juice on the C vitamin content. Ser. Hortic. 2017, 60, 89–92. [Google Scholar]
- Mao, P.; Duan, F.; Zheng, Y.; Yang, Q. Blue and UV-A light wavelengths positively affected accumulation profiles of healthy compounds in pak-choi. J. Sci. Food Agric. 2021, 101, 1676–1684. [Google Scholar] [CrossRef]
- Ganganelli, I.; Molina Agostini, M.C.; Galatro, A.; Gergoff Grozeff, G.E. Specific wavelength LED light pulses modify vitamin C and organic acids content in raspberry and blackberry fruit during postharvest. J. Hortic. Sci. Biotechnol. 2023, 98, 649–661. [Google Scholar] [CrossRef]
- Cruz-Rus, E.; Amaya, I.; Sanchez-Sevilla, J.F.; Botella, M.A.; Valpuesta, V. Regulation of L-ascorbic acid content in strawberry fruits. J. Exp. Bot. 2011, 62, 4191–4201. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Rus, E.; Botella, M.A.; Valpuesta, V.; Gomez-Jimenez, M.C. Analysis of genes involved in L-ascorbic acid biosynthesis during growth and ripening of grape berries. J. Plant Physiol. 2010, 167, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Y.; Pan, D.-L.; Jia, Z.-H.; Wang, T.; Wang, G.; Guo, Z.-R. Chlorophyll, carotenoid and vitamin C metabolism regulation in Actinidia chinensis ‘Hongyang’outer pericarp during fruit development. PLoS ONE 2018, 13, e0194835. [Google Scholar]
- Cimpoiu, C.; Miclaus, V.; Damian, G.; Tarsiche, I.; Nastasia, P.O.P. Influence of intermittent heating during maceration on the antioxidant capacity of some grape seeds and skins. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 41–43. [Google Scholar]
- Nicolescu, A.; Babotă, M.; Zhang, L.; Bunea, C.I.; Gavrilaș, L.; Vodnar, D.C.; Mocan, A.; Crișan, G.; Rocchetti, G. Optimized ultrasound-assisted enzymatic extraction of phenolic compounds from Rosa canina L. pseudo-fruits (Rosehip) and their biological activity. Antioxidants 2022, 11, 1123. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Sea buckthorn as a source of important bioactive compounds in cardiovascular diseases. Food Chem. Toxicol. 2016, 97, 199–204. [Google Scholar] [CrossRef]
- Jia, Y.; Peng, C.; Gao, M.; Yang, K.; Wang, A.; Pan, Y. Optimization of the luminescence efficiency and moisture stability of a red phosphor KRb3Ge2F12: Mn4+ for indoor plant growth LED applications. Ceram. Int. 2022, 48, 4208–4215. [Google Scholar] [CrossRef]
- Meng, Y.; Chen, P.; Fan, H.; Lu, Z.; Zhong, X.; Lu, J.; Ou, Y.; Zhou, L. Mn4+-doped La (CaZr) 0.5 O3 phosphors for plant grow LEDs: Ab initio site occupy and photoluminescence properties. Mater. Res. Bull. 2022, 147, 111610. [Google Scholar] [CrossRef]
- Lauria, G.; Piccolo, E.L.; Ceccanti, C.; Guidi, L.; Bernardi, R.; Araniti, F.; Cotrozzi, L.; Pellegrini, E.; Moriconi, M.; Giordani, T. Supplemental red LED light promotes plant productivity, “photomodulate” fruit quality and increases Botrytis cinerea tolerance in strawberry. Postharvest Biol. Technol. 2023, 198, 112253. [Google Scholar] [CrossRef]
- Yap, E.S.P.; Uthairatanakij, A.; Laohakunjit, N.; Jitareerat, P.; Vaswani, A.; Magana, A.A.; Morre, J.; Maier, C.S. Plant growth and metabolic changes in ‘Super Hot’chili fruit (Capsicum annuum) exposed to supplemental LED lights. Plant Sci. 2021, 305, 110826. [Google Scholar] [CrossRef]
- Lin, K.-H.; Huang, M.-Y.; Hsu, M.-H. Morphological and physiological response in green and purple basil plants (Ocimum basilicum) under different proportions of red, green, and blue LED lightings. Sci. Hortic. 2021, 275, 109677. [Google Scholar] [CrossRef]
- Williams, C.A.; Goldstone, F.; Greenham, J. Flavonoids, cinnamic acids and coumarins from the different tissues and medicinal preparations of Taraxacum officinale. Phytochemistry 1996, 42, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, M. Separation of quercetin, sexangularetin, kaempferol and isorhamnetin for simultaneous HPLC determination of flavonoid aglycones in inflorescences, leaves and fruits of three Sorbus species. J. Pharm. Biomed. Anal. 2008, 48, 629–635. [Google Scholar] [CrossRef] [PubMed]

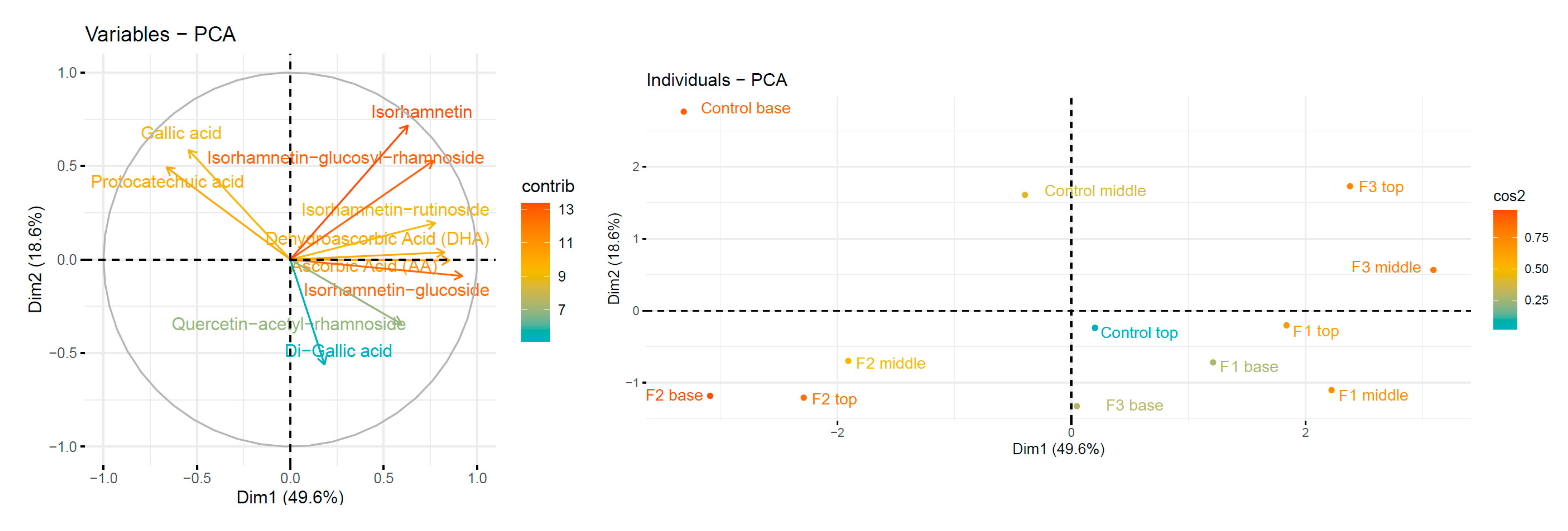
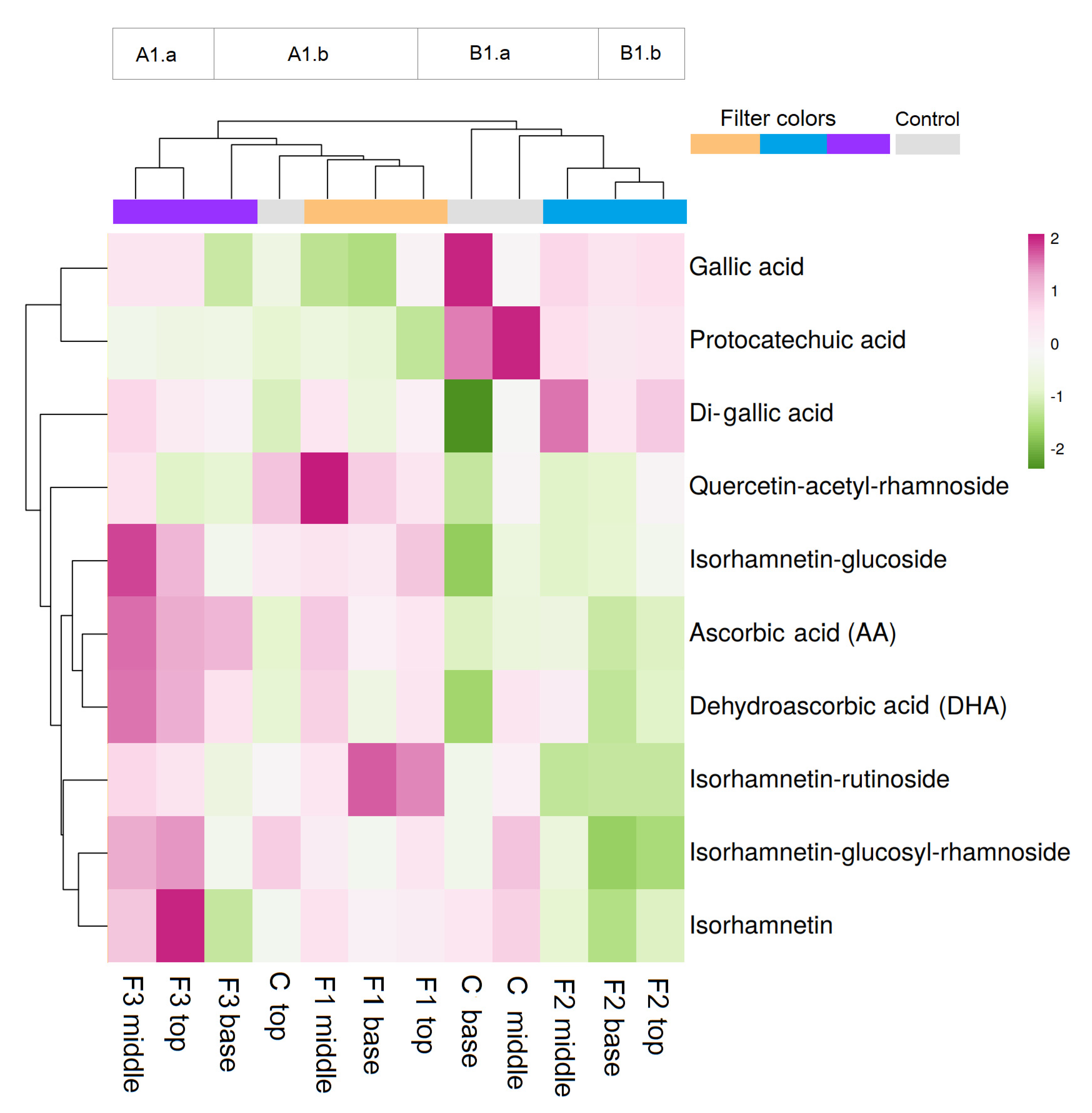
| Compound | Control Base | Control Middle | Control Top | F1 Base | F1 Middle | F1 Top | F2 Base | F2 Middle | F2 Top | F3 Base | F3 Middle | F3 Top |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Di-Gallic acid | 1.604 ± 0.12bA | 3.159 ± 0.13eA | 2.588 ± 0.32eA | 2.834 ± 0.21fB * | 3.578 ± 0.34eB * | 3.368 ± 0.38eB * | 3.576 ± 0.24dC* | 4.419 ± 0.51eC * | 3.900 ± 0.32eC * | 3.330 ± 0.21dBC * | 3.776 ± 0.09fB * | 3.476± 0.11eB * |
| Gallic acid | 0.538 ± 0.03aC | 0.254 ± 0.01aB | 0.193 ± 0.01aA | 0.067± 0.02aA * | 0.088± 0.021aA * | 0.269± 0.012aB * | 0.328± 0.03aB * | 0.358 ± 0.04aC * | 0.347 ± 0.05abC * | 0.104 ± 0.01aA * | 0.321 ± 0.03aBC | 0.319 ± 0.04aC * |
| Protocatechuic acid | 1.945 ± 0.03cC | 2.157 ± 0.11dC | 0.922 ± 0.02cdAB | 0.965 ± 0.05bcdA * | 1.01 ± 0.07bcA * | 0.724 ± 0.08abA | 1.411 ± 0.11cB * | 1.529 ± 0.09cdB * | 1.464 ± 0.06dC * | 1.068 ± 0.09bA * | 1.102 ± 0.11bdA * | 1.051 ± 0.09bcB |
| Quercetin-acetyl-rhamnoside | 0.243 ± 0.03aA | 0.454 ± 0.04abB | 0.623 ± 0.021bcC | 0.603 ± 0.03bB * | 0.823 ± 0.02bC * | 0.529 ± 0.04aBC | 0.306 ± 0.03aA | 0.293 ± 0.08aA * | 0.456 ± 0.06abB * | 0.319 ± 0.04aA | 0.556 ± 0.03abB * | 0.293 ± 0.05aA * |
| Isorhamnetin-glucosyl-rhamnoside | 2.064 ± 0.3cB | 2.825 ± 0.5eC | 2.759 ± 0.6eBC | 2.118 ± 0.8eB | 2.398 ± 0.7dB * | 2.518 ± 0.43dB | 1.33 ± 0.32cA * | 1.923 ± 0.4dA * | 1.421 ± 0.2dA * | 2.094 ± 0.11cB | 2.986 ± 0.43eC | 3.083 ± 0.28eC |
| Isorhamnetin-glucoside | 0.546 ± 0.08aA | 0.803 ± 0.09bA | 1.015 ± 0.17cdC | 1.015 ± 0.11cdB * | 1.056 ± 0.09bcB * | 1.156 ± 0.07bcC * | 0.754 ± 0.05abB * | 0.733 ± 0.08abA | 0.865 ± 0.05bcA * | 0.867 ± 0.09bB * | 1.376 ± 0.04dC* | 1.200 ± 0.09cC * |
| Isorhamnetin-rutinoside | 0.407 ± 0.05aA | 0.477 ± 0.08abB | 0.453 ± 0.05abB | 0.721 ± 0.07bcB * | 0.522 ± 0.07abB | 0.685 ± 0.08abC * | 0.278 ± 0.02aA | 0.273 ± 0.02aA * | 0.276 ± 0.03aA * | 0.379 ± 0.05aA | 0.566 ± 0.05abcB | 0.533 ± 0.06abB |
| Isorhamnetin | 1.443 ± 0.3bB | 1.568 ± 0.6cB | 1.224 ± 0.5dAB | 1.33 ± 0.12dB | 1.478 ± 0.17cB | 1.365 ± 0.2cC | 0.852 ± 0.12bA * | 1.049 ± 0.13bcA * | 1.002 ± 0.16cdA | 0.921 ± 0.11bA * | 1.611 ± 0.45dB | 1.977 ± 0.28dC * |
| Total phenolic content | 30.264 | 31.220 | 29.159 | 33.326 | ||||||||
| Ascorbic acid (AA) | 0.185 ± 0.01aA | 0.191 ± 0.01aA | 0.186 ± 0.05aA | 0.206 ± 0.07aB * | 0.221 ± 0.05aB * | 0.211 ± 0.04aB * | 0.180 ± 0.06aA | 0.192 ± 0.08aA | 0.185 ± 0.02aA | 0.225 ± 0.02aC * | 0.237 ± 0.03aB * | 0.228 ± 0.01aC * |
| Dehydroascorbic acid (DHA) | 0.253 ± 0.03bA | 0.278 ± 0.03bAB | 0.263 ± 0.06bA | 0.266 ± 0.04bB * | 0.281 ± 0.01bB | 0.278 ± 0.08bB * | 0.256 ± 0.09bA | 0.274 ± 0.04bA | 0.261 ± 0.04bA | 0.279 ± 0.02bC * | 0.292 ± 0.03bC * | 0.287 ± 0.05bC * |
| Total vitamin C | 1.356 | 1.463 | 1.350 | 1.550 |
| Filters | C1 (Control) [a.u] | F1 (Yellow Filter) [a.u] | F2 (Blue Filter) [a.u] | F3 (Violet Filter) [a.u] |
|---|---|---|---|---|
| The Amplitude of the EPR Signal, Measured from Figure 1 | ||||
| Base | 0.25 | 0.55 | 0.27 | 0.25 |
| Middle | 1.38 | 0.93 | 1.37 | 1.21 |
| Top | 0.15 | 0.63 | 1.17 | 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldovan, I.; Pop, V.C.; Borsai, O.; Lukacs, L.; Ranga, F.; Culea, E.; Damian, G.; Cornea-Cipcigan, M.; Margaoan, R. Dynamics of Bioactive Compounds under the Influence of Yellow, Blue, and Violet Light Filters on Hippophae rhamnoides L. (Sea Buckthorn) Fruits. Horticulturae 2023, 9, 1312. https://doi.org/10.3390/horticulturae9121312
Moldovan I, Pop VC, Borsai O, Lukacs L, Ranga F, Culea E, Damian G, Cornea-Cipcigan M, Margaoan R. Dynamics of Bioactive Compounds under the Influence of Yellow, Blue, and Violet Light Filters on Hippophae rhamnoides L. (Sea Buckthorn) Fruits. Horticulturae. 2023; 9(12):1312. https://doi.org/10.3390/horticulturae9121312
Chicago/Turabian StyleMoldovan, Ioana, Viorel Cornel Pop, Orsolya Borsai, Lehel Lukacs, Florica Ranga, Eugen Culea, Grigore Damian, Mihaiela Cornea-Cipcigan, and Rodica Margaoan. 2023. "Dynamics of Bioactive Compounds under the Influence of Yellow, Blue, and Violet Light Filters on Hippophae rhamnoides L. (Sea Buckthorn) Fruits" Horticulturae 9, no. 12: 1312. https://doi.org/10.3390/horticulturae9121312
APA StyleMoldovan, I., Pop, V. C., Borsai, O., Lukacs, L., Ranga, F., Culea, E., Damian, G., Cornea-Cipcigan, M., & Margaoan, R. (2023). Dynamics of Bioactive Compounds under the Influence of Yellow, Blue, and Violet Light Filters on Hippophae rhamnoides L. (Sea Buckthorn) Fruits. Horticulturae, 9(12), 1312. https://doi.org/10.3390/horticulturae9121312








