UV-B Radiation in the Acclimatization Mechanism of Psidium guajava in Sunlight
Abstract
:1. Introduction
2. Material and Methods
2.1. Growing Conditions
2.2. Fluorescence of Chlorophyll a
2.3. Gas Exchanges
2.4. Foliar Pigments
2.5. Hydrogen Peroxide, Lipid Peroxidation, and Total Phenolic Compounds
2.6. Carbohydrates
2.7. Statistical Analysis
3. Results and Discussion
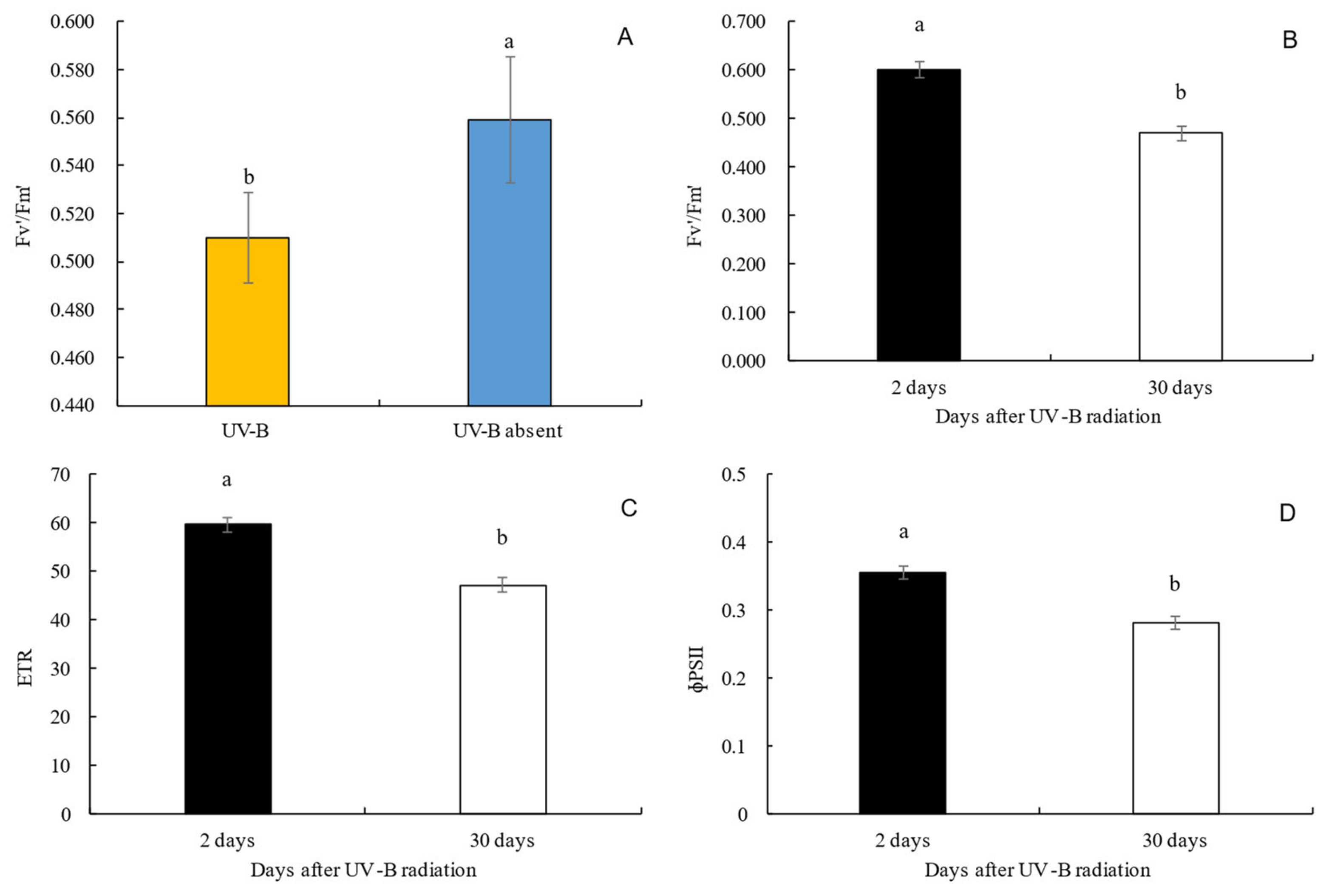
3.1. Fluorescence of Chlorophyll a
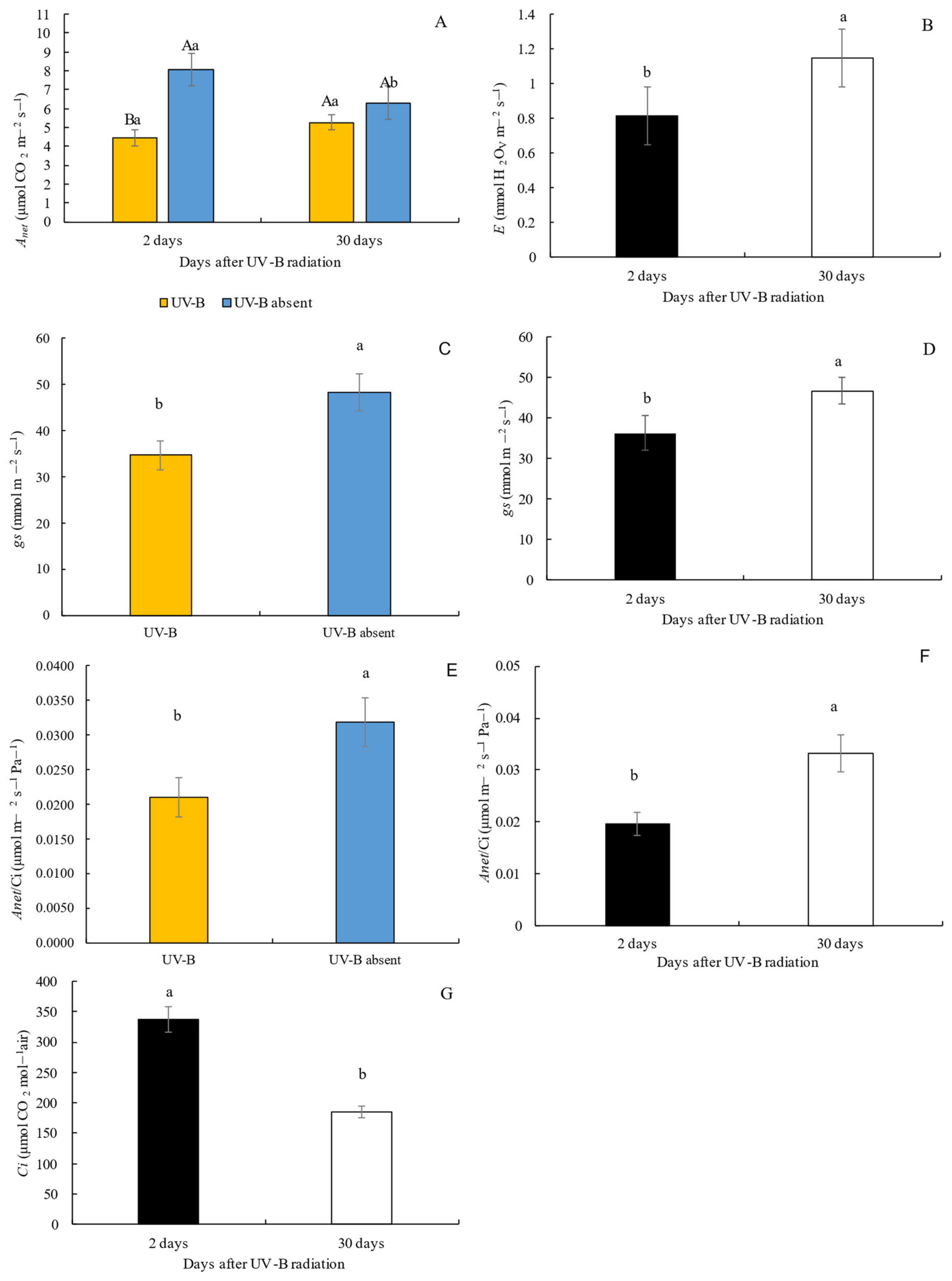
3.2. Gas Exchanges
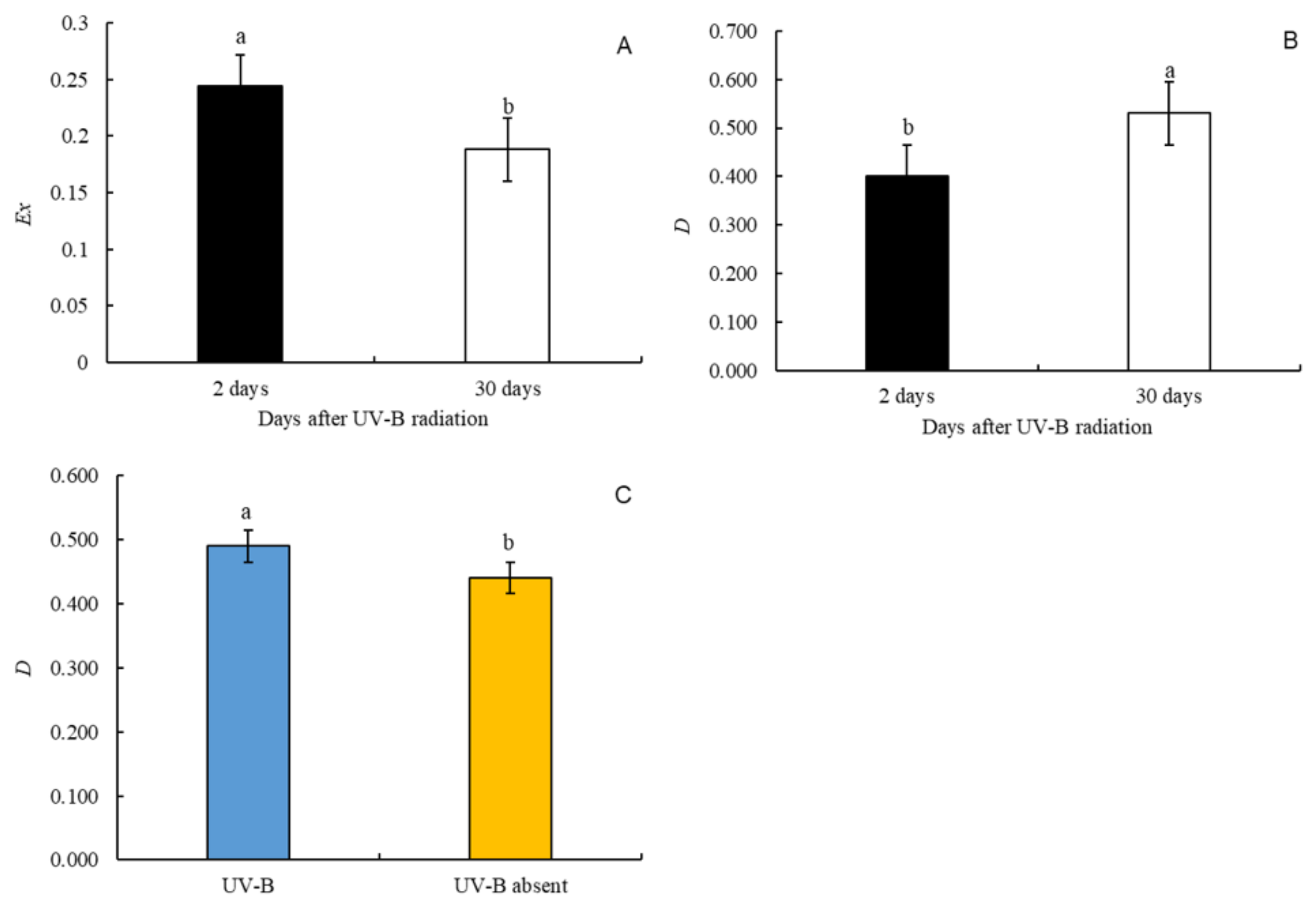
3.3. Foliar Pigments
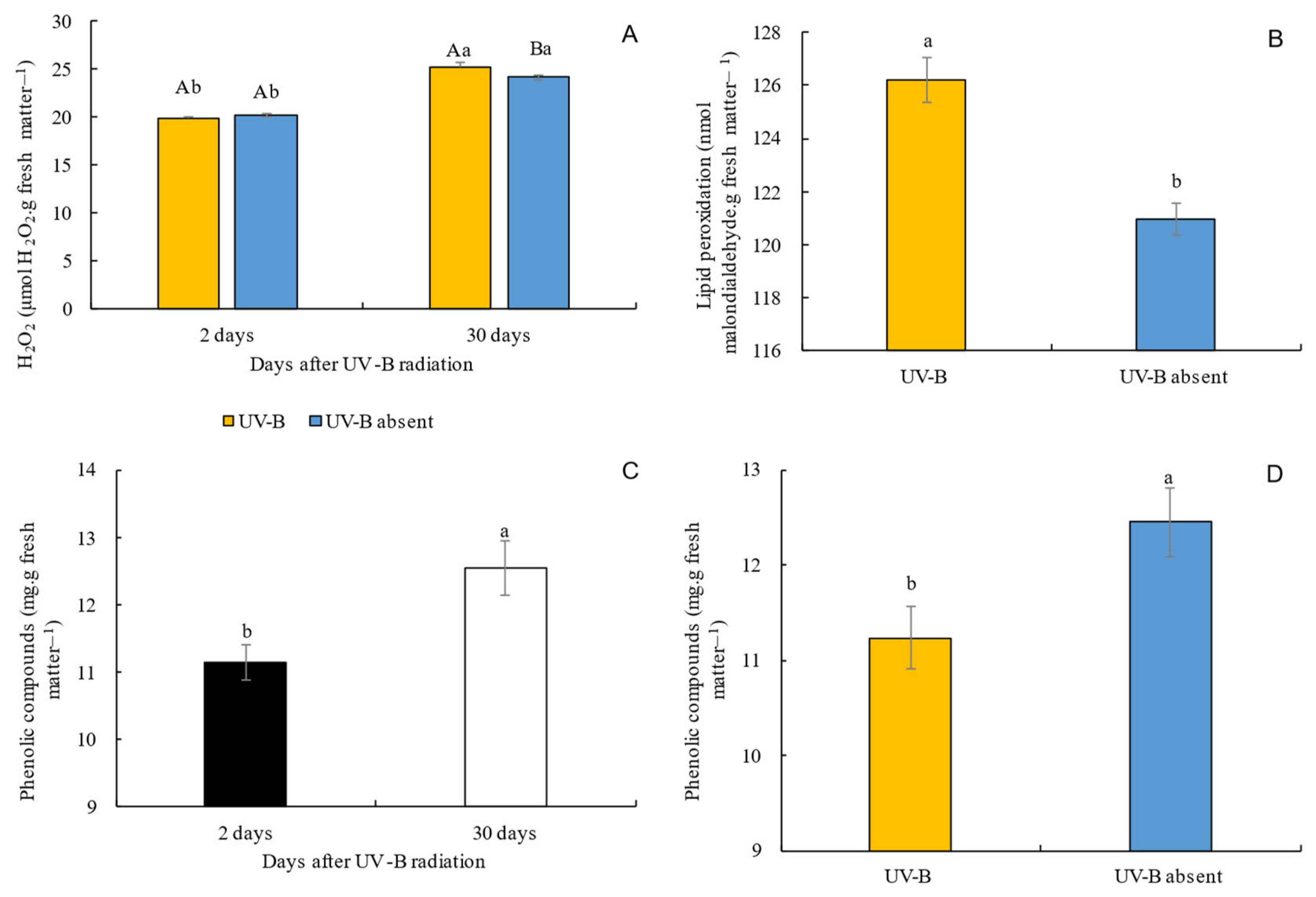
3.4. Hydrogen Peroxide, Lipid Peroxidation, and Total Phenolic Compounds
3.5. Carbohydrates
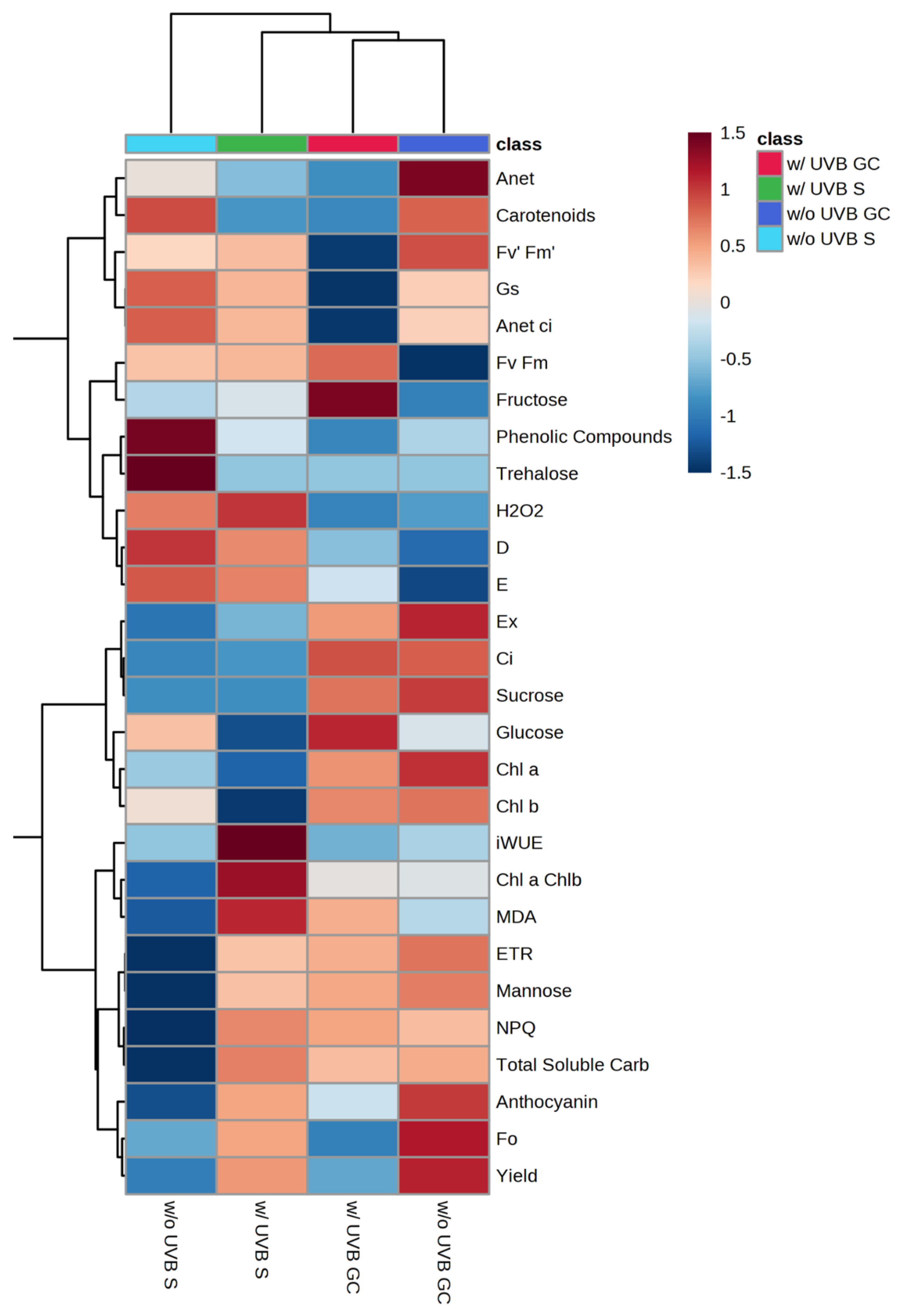
3.6. Heatmap
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hylander, A.M.; Longstreth, S.; Madronich, J.; Neale, S.; Neale, P.J.; Pandey, R.; Rhodes, K.K.; Robinson, L.E.; Robson, S.A.; Rose, M.; et al. Environmental Effects of Stratospheric Ozone Depletion, UV Radiation, and Environmental Effects of Stratospheric Ozone Depletion, UV Radiation, and Interactions with Climate Change: 2022 Assessment Report Interactions with Climate Change: 2022 Assessment Report Publication Details Citation Publication Details Citation; UNEP: Gigiri Nairobi, Kenya, 2023; ISBN 9789914733914. [Google Scholar]
- Larin, I.K. On the Influence of Global Warming on the Ozone Layer and UVB Radiation. Izv. Atmos. Ocean. Phys. 2021, 57, 110–115. [Google Scholar] [CrossRef]
- Mátai, A.; Nagy, D.; Hideg, É. UV-B Strengthens Antioxidant Responses to Drought in Nicotiana Benthamiana Leaves Not Only as Supplementary Irradiation but also as Pre-Treatment. Plant Physiol. Biochem. 2019, 134, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Faseela, P.; Puthur, J.T. The Imprints of the High Light and UV-B Stresses in Oryza sativa L. ‘Kanchana’ Seedlings Are Differentially Modulated. J. Photochem. Photobiol. B 2018, 178, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ahammed, G.J. Hormonal Regulation of Anthocyanin Biosynthesis for Improved Stress Tolerance in Plants. Plant Physiol. Biochem. 2023, 201, 107835. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Choi, M.G.; Kang, C.S.; Park, C.S.; Choi, S.B.; Park, Y.I. Overexpressing the Wheat Dihydroflavonol 4-Reductase Gene TaDFR Increases Anthocyanin Accumulation in an Arabidopsis Dfr Mutant. Genes Genom. 2016, 38, 333–340. [Google Scholar] [CrossRef]
- Mishra, P.; Prasad, S.M. Low Dose UV-B Radiation Induced Mild Oxidative Stress Impact on Physiological and Nutritional Competence of Spirulina (Arthrospira) Species. Plant Stress 2021, 2, 100039. [Google Scholar] [CrossRef]
- Romanatti, P.V.; Rocha, G.A.; Veroneze Júnior, V.; Santos Filho, P.R.; de Souza, T.C.; Pereira, F.J.; Polo, M. Limitation to Photosynthesis in Leaves of Eggplant under UVB According to Anatomical Changes and Alterations on the Antioxidant System. Sci. Hortic. 2019, 249, 449–454. [Google Scholar] [CrossRef]
- Nogués, S.; Allen, D.J.; Morison, J.I.L.; Baker, N.R. Characterization of Stomatal Closure Caused by Ultraviolet-B Radiation 1. Plant Physiol. 1999, 121, 489–496. [Google Scholar] [CrossRef]
- Liu, M.; Sun, Q.; Cao, K.; Xu, H.; Zhou, X. Acetylated Proteomics of UV-B Stress-Responsive in Photosystem II of Rhododendron chrysanthum. Cells 2023, 12, 478. [Google Scholar] [CrossRef]
- Yang, J.; Li, C.; Kong, D.; Guo, F.; Wei, H. Light-Mediated Signaling and Metabolic Changes Coordinate Stomatal Opening and Closure. Front. Plant Sci. 2020, 11, 601478. [Google Scholar] [CrossRef]
- Valledor, L.; Cañal, M.J.; Pascual, J.; Rodríguez, R.; Meijón, M. Early Induced Protein 1 (PrELIP1) and Other Photosynthetic, Stress and Epigenetic Regulation Genes Are Involved in Pinus Radiata D. Don UV-B Radiation Response. Physiol. Plant 2012, 146, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Cuzzuol, G.R.F.; Gama, V.N.; Zanetti, L.V.; Werner, E.T.; Pezzopane, J.E.M. UV-B Effects on Growth, Photosynthesis, Total Antioxidant Potential and Cell Wall Components of Shade-Tolerant and Sun-Tolerant Ecotypes of Paubrasilia Echinata. Flora Morphol. Distrib. Funct. Ecol. Plants 2020, 271, 151679. [Google Scholar] [CrossRef]
- Ruuhola, T.; Nybakken, L.; Randriamanana, T.; Lavola, A.; Julkunen-Tiitto, R. Effects of Long-Term UV-Exposure and Plant Sex on the Leaf Phenoloxidase Activities and Phenolic Concentrations of Salix myrsinifolia (Salisb.). Plant Physiol. Biochem. 2018, 126, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant Phenolics: Recent Advances on Their Biosynthesis, Genetics, Andecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, A.; Choudhary, K.K. Revisiting the Role of Phenylpropanoids in Plant Defense against UV-B Stress. Plant Stress 2023, 7, 100143. [Google Scholar] [CrossRef]
- Fasano, R.; Gonzalez, N.; Tosco, A.; Dal Piaz, F.; Docimo, T.; Serrano, R.; Grillo, S.; Leone, A.; Inzé, D. Role of Arabidopsis UV RESISTANCE LOCUS 8 in Plant Growth Reduction under Osmotic Stress and Low Levels of UV-B. Mol. Plant 2014, 7, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Eichholz, I.; Huyskens-Keil, S.; Keller, A.; Ulrich, D.; Kroh, L.W.; Rohn, S. UV-B-Induced Changes of Volatile Metabolites and Phenolic Compounds in Blueberries (Vaccinium corymbosum L.). Food Chem. 2011, 126, 60–64. [Google Scholar] [CrossRef]
- Rai, R.; Singh, S.; Yadav, S.; Chatterjee, A.; Rai, S.; Shankar, A.; Rai, L.C. Impact of UV-B Radiation on Photosynthesis and Productivity of Crop Molecular Evaluation of Abiotic Stress on Cyanobacterium Anabaena View Project. 2018. Available online: https://www.researchgate.net/publication/326572675_Impact_of_UV-B_Radiation_on_Photosynthesis_and_Productivity_of_Crop (accessed on 5 May 2023).
- Machado, F.; Dias, M.C.; de Pinho, P.G.; Araújo, A.M.; Pinto, D.; Silva, A.; Correia, C.; Moutinho-Pereira, J.; Santos, C. Photosynthetic Performance and Volatile Organic Compounds Profile in Eucalyptus globulus after UVB Radiation. Environ. Exp. Bot. 2017, 140, 141–149. [Google Scholar] [CrossRef]
- Kaur, S.; Tiwari, V.; Kumari, A.; Chaudhary, E.; Sharma, A.; Ali, U.; Garg, M. Protective and Defensive Role of Anthocyanins under Plant Abiotic and Biotic Stresses: An Emerging Application in Sustainable Agriculture. J. Biotechnol. 2023, 361, 12–29. [Google Scholar] [CrossRef]
- Doménech-Carbó, A.; Domínguez, I. In Situ Electrochemical Analysis of Anthocyanin Activation by ROS in Blueberries. Electrochem. Commun. 2023, 149, 107468. [Google Scholar] [CrossRef]
- Ai, T.N.; Naing, A.H.; Arun, M.; Jeon, S.M.; Kim, C.K. Expression of RsMYB1 in Petunia Enhances Anthocyanin Production in Vegetative and Floral Tissues. Sci. Hortic. 2017, 214, 58–65. [Google Scholar] [CrossRef]
- Patil, V.; Chauhan, A.K.; Singh, R.P. Optimization of the Spray-Drying Process for Developing Guava Powder Using Response Surface Methodology. Powder Technol. 2014, 253, 230–236. [Google Scholar] [CrossRef]
- Narváez-Cuenca, C.E.; Inampues-Charfuelan, M.L.; Hurtado-Benavides, A.M.; Parada-Alfonso, F.; Vincken, J.P. The Phenolic Compounds, Tocopherols, and Phytosterols in the Edible Oil of Guava (Psidium Guava) Seeds Obtained by Supercritical CO2 Extraction. J. Food Compos. Anal. 2020, 89, 103467. [Google Scholar] [CrossRef]
- Sandesh Kamath, B.; Vidhyavathi, R.; Sarada, R.; Ravishankar, G.A. Enhancement of Carotenoids by Mutation and Stress Induced Carotenogenic Genes in Haematococcus pluvialis Mutants. Bioresour. Technol. 2008, 99, 8667–8673. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.; Linatoc, A.C.; Bin Abu Bakar, M.F.; Takai, Z.I. Effect of Light Intensity on the Gas Exchange Characteristics and Total Pigment Content of Psidium Guajava. In Proceedings of the IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Bristol, UK, 2019; Volume 269. [Google Scholar]
- Abbasi, N.A.; Chaudhary, M.A.; Ali, M.I.; Hussain, A.; Ali, I.; Akhtar Abbasi, N.; Chaudhary, M.A.; Ali, M.I.; Hussain, A.; Ali, I. On Tree Fruit Bagging Influences Quality of Guava Harvested at Different Maturity Stages during Summer. Int. J. Agric. Biol. 2014, 16, 543–549. [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística—IBGE. Produção de Goiaba. Available online: http://https://www.ibge.gov.br/explica/producao-agropecuaria/goiaba/br (accessed on 20 August 2023).
- Vitti, K.A.; de Lima, L.M.; Filho, J.G.M. Agricultural and Economic Characterization of Guava Production in Brazil. Rev. Bras. Frutic. 2020, 42, e-447. [Google Scholar] [CrossRef]
- Gutiérrez, R.M.P.; Mitchell, S.; Solis, R.V. Psidium guajava: A Review of Its Traditional Uses, Phytochemistry and Pharmacology. J. Ethnopharmacol. 2008, 117, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Akter, R.; Hasan, M.K.; Kabir, K.H.; Darr, D.; Roshni, N.A. Agroforestry Systems and Their Impact on Livelihood Improvement of Tribal Farmers in a Tropical Moist Deciduous Forest in Bangladesh. Trees For. People 2022, 9, 100315. [Google Scholar] [CrossRef]
- Brenda Souza Chaves, V.; Rodrigues Gomes, F.; Acácio Barbosa, M.; Kamila Ferreira de Souza, L.; Laura Pereira Souza, A.; Danielle Pereira, L.; Marques Costa, M.; Fabíola Pereira da Silva, D.; Nunes da Silveira Neto, A. Revista Brasileira de Agropecuária Sustentável. Rev. Bras. Agropecu. Sustent. 2019, 9, 95–100. [Google Scholar]
- Otuoma, J.; Nyongesah, J.M.; Owino, J.; Onyango, A.A.; Okello, V.S. Ecological Manipulation of Psidium guajava to Facilitate Secondary Forest Succession in Tropical Forests. J. Ecol. Eng. 2020, 21, 210–221. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 1–32. [Google Scholar]
- Bilger, W.; Schreiber, U.; Bock, M. Determination of the Quantum Efficiency of Photosystem II and of Non-Photochemical Quenching of Chlorophyll Fluorescence in the Field; Springer: Berlin/Heidelberg, Germany, 1995; Volume 102. [Google Scholar]
- Kitajima, M.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta (BBA)-Bioenerg. 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Von Caemmerer, S.; Farquhar, G.D. Some Relationships between the Biochemistry of Photosynthesis and the Gas Exchange of Leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, Q.; Ma, K.; Chen, L. Temperature-Dependent Gas Exchange and Stomatal/Non-Stomatal Limitation to CO2 Assimilation of Quercus liaotungensis under Midday High Irradiance. Photosynthetica 2001, 39, 383–388. [Google Scholar] [CrossRef]
- Prado, C.H.B.A.; De Moraes, J.A.P.V. Photosynthetic Capacity and Specific Leaf Mass in Twenty Woody Species of Cerrado Vegetation under Field Conditions. Photosynthetica 1997, 33, 103–112. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. In Current Protocols in Food Analytical Chemistry; Wiley Online Library: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between Leaf Pigment Content and Spectral Reflectance across a Wide Range of Species, Leaf Structures and Developmental Stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The Effect of Drought and Ultraviolet Radiation on Growth and Stress Markers in Pea and Wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Devi, S.R.; Prasad, M.N. V Copper Toxicity in Ceratophyllum demersum L. (Coontail), a Free Floating Macrophyte: Response of Antioxidant Enzymes and Antioxidants. Plant Sci. 1998, 138, 157–165. [Google Scholar] [CrossRef]
- Bonoli, M.; Verardo, V.; Marconi, E.; Caboni, M.F. Antioxidant Phenols in Barley (Hordeum vulgare L.) Flour: Comparative Spectrophotometric Study among Extraction Methods of Free and Bound Phenolic Compounds. J. Agric. Food Chem. 2004, 52, 5195–5200. [Google Scholar] [CrossRef]
- Garcia, I.S.; Souza, A.; Barbedo, C.J.; Dietrich, S.M.C. Figueiredo-Ribeiro Endangered Leguminous Tree from the Brazilian Atlantic Forest. Braz. J. Biol. 2006, 66, 739–745. [Google Scholar] [CrossRef]
- Morris, D.L. Quantitative Determination of Carbohydrates with Dreywood’s Anthrone Reagent. Science (1979) 1948, 107, 254–255. [Google Scholar] [CrossRef]
- Suksom, W.; Wannachai, W.; Boonchiangma, S.; Chanthai, S.; Srijaranai, S. Ion Chromatographic Analysis of Monosaccharides and Disaccharides in Raw Sugar. Chromatographia 2015, 78, 873–879. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; De Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Tay, S.; He, J.; Yam, T.W. Photosynthetic Light Utilization Efficiency, Water Relations and Leaf Growth of C3 and CAM Tropical Orchids under Natural Conditions. Am. J. Plant Sci. 2015, 6, 2949–2959. [Google Scholar] [CrossRef]
- Takahashi, S.; Badger, M.R. Photoprotection in Plants: A New Light on Photosystem II Damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, R.; Canaccini, F.; Cortes, S.; Magnani, F.; Rapparini, F.; Zamboni, A.; Raddi, S. Role of Xanthophyll Cycle-Mediated Photoprotection in Arbutus Unedo Plants Exposed to Water Stress during the Mediterranean Summer. Photosynthetica 2008, 46, 378–386. [Google Scholar] [CrossRef]
- Ortiz, D.; Moreno, F.; Díez, M.C. Photosynthesis, Growth, and Survival in Seedlings of Four Tropical Fruit-Tree Species under Intense Radiation. Acta Amaz. 2021, 51, 1–9. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll Fluorescence Analysis: A Guide to Good Practice and Understanding Some New Applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Eppel, A.; Shaked, R.; Eshel, G.; Barak, S.; Rachmilevitch, S. Low Induction of Non-Photochemical Quenching and High Photochemical Efficiency in the Annual Desert Plant Anastatica Hierochuntica. Physiol. Plant 2014, 151, 544–558. [Google Scholar] [CrossRef]
- Yuan, L.B.; Chen, M.X.; Wang, L.N.; Sasidharan, R.; Voesenek, L.A.C.J.; Xiao, S. Multi-Stress Resilience in Plants Recovering from Submergence. Plant Biotechnol. J. 2023, 21, 466–481. [Google Scholar] [CrossRef]
- Zhong, Y.; Xu, J.; Zhao, X.; Qu, T.; Guan, C.; Hou, C.; Tang, X.; Wang, Y. Balancing Damage via Non-Photochemical Quenching, Phenolic Compounds and Photorespiration in Ulva prolifera Induced by Low-Dose and Short-Term UV-B Radiation. Int. J. Mol. Sci. 2022, 23, 2693. [Google Scholar] [CrossRef]
- Sisson, W.B.; Caldwell, M.M. Photosynthesis, Dark Respiration, and Growth of Rumex patientia L. Exposed to Ultraviolet Irradiance (288 to 315 Nanometers) Simulating a Reduced Atmospheric Ozone Column. Plant Physiol. 1976, 58, 563–568. [Google Scholar] [CrossRef]
- Shi, S.; Shi, R.; Li, T.; Zhou, D. UV-B Radiation Effects on the Alpine Plant Kobresia Humilis in a Qinghai-Tibet Alpine Meadow. Plants 2022, 11, 3102. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, A.; Mariotti, L.; Castagna, A.; Santin, M.; Trivellini, A.; Reyes, H.; Mensuali-Sodi, A.; Ranieri, A.; Quartacci, M.F. Hormone Profile Changes Occur in Roots and Leaves of Micro-Tom Tomato Plants When Exposing the Aerial Part to Low Doses of UV-B Radiation. Plant Physiol. Biochem. 2020, 148, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.G.; Seixas, D.P.; Barzotto, G.R.; Jorge, L.G.; Ducatti, K.R.; Ferreira, G.; Rodrigues, T.M.; da Silva, E.A.A.; Boaro, C.S.F. Roles of Calcium Signaling in Gene Expression and Photosynthetic Acclimatization of Solanum Lycopersicum Micro-Tom (MT) after Mechanical Damage. Int. J. Mol. Sci. 2022, 23, 13571. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lal, N.K.; Lin, Z.J.D.; Ma, S.; Liu, J.; Castro, B.; Toruño, T.; Dinesh-Kumar, S.P.; Coaker, G. Regulation of Reactive Oxygen Species during Plant Immunity through Phosphorylation and Ubiquitination of RBOHD. Nat. Commun. 2020, 11, 1838. [Google Scholar] [CrossRef] [PubMed]
- Barzotto, G.R.; Cardoso, C.P.; Jorge, L.G.; Campos, F.G.; Boaro, C.S.F. Hydrogen Peroxide Signal Photosynthetic Acclimation of Solanum lycopersicum L. Cv Micro-Tom under Water Deficit. Sci. Rep. 2023, 13, 13059. [Google Scholar] [CrossRef]
- Nazir, F.; Fariduddin, Q.; Khan, T.A. Hydrogen Peroxide as a Signalling Molecule in Plants and Its Crosstalk with Other Plant Growth Regulators under Heavy Metal Stress. Chemosphere 2020, 252, 126486. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ma, Y.; Weng, Y.; Yang, R.; Gu, Z.; Wang, P. Effects of UV-B Radiation on Phenolic Accumulation, Antioxidant Activity and Physiological Changes in Wheat (Triticum aestivum L.) Seedlings. Food Biosci. 2019, 30, 100409. [Google Scholar] [CrossRef]
- Živanović, B.; Komić, S.M.; Tosti, T.; Vidović, M.; Prokić, L.; Jovanović, S.V. Leaf Soluble Sugars and Free Amino Acids as Important Components of Abscisic Acid—Mediated Drought Response in Tomato. Plants 2020, 9, 1147. [Google Scholar] [CrossRef]
- Penna, S. Building Stress Tolerance through Over-Producing Trehalose in Transgenic Plants. Trends Plant Sci. 2003, 8, 355–357. [Google Scholar] [CrossRef]
- Wu, N.; Li, Z.; Wu, F.; Tang, M. Comparative Photochemistry Activity and Antioxidant Responses in Male and Female Populus cathayana Cuttings Inoculated with Arbuscular Mycorrhizal Fungi under Salt. Sci. Rep. 2016, 6, 37663. [Google Scholar] [CrossRef]
- Schmitz, J.; Heinrichs, L.; Scossa, F.; Fernie, A.R.; Oelze, M.L.; Dietz, K.J.; Rothbart, M.; Grimm, B.; Flügge, U.I.; Häusler, R.E. The Essential Role of Sugar Metabolism in the Acclimation Response of Arabidopsis thaliana to High Light Intensities. J. Exp. Bot. 2014, 65, 1619–1636. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, F.G.; Dantas, M.O.; Santos, J.P.M.; Froes, S.S.; Gama, J.P.S.; Boaro, C.S.F. UV-B Radiation in the Acclimatization Mechanism of Psidium guajava in Sunlight. Horticulturae 2023, 9, 1291. https://doi.org/10.3390/horticulturae9121291
Campos FG, Dantas MO, Santos JPM, Froes SS, Gama JPS, Boaro CSF. UV-B Radiation in the Acclimatization Mechanism of Psidium guajava in Sunlight. Horticulturae. 2023; 9(12):1291. https://doi.org/10.3390/horticulturae9121291
Chicago/Turabian StyleCampos, Felipe G., Mariana O. Dantas, João P. M. Santos, Sophia S. Froes, João P. S. Gama, and Carmen S. F. Boaro. 2023. "UV-B Radiation in the Acclimatization Mechanism of Psidium guajava in Sunlight" Horticulturae 9, no. 12: 1291. https://doi.org/10.3390/horticulturae9121291
APA StyleCampos, F. G., Dantas, M. O., Santos, J. P. M., Froes, S. S., Gama, J. P. S., & Boaro, C. S. F. (2023). UV-B Radiation in the Acclimatization Mechanism of Psidium guajava in Sunlight. Horticulturae, 9(12), 1291. https://doi.org/10.3390/horticulturae9121291




