Abstract
Orostachys spp., considered economically important succulent plants for both the medicinal and ornamental plant industries, are known to exhibit slow growth in their offsets during the long, cold winter months. Due to the slow growth, this study investigated the application of gibberellic acid (GA3) and determined the optimal GA3 concentration (control, 200 mg·L−1, 400 mg·L−1, and 600 mg·L−1) for promoting the propagation of three Orostachys species (i.e., O. fimbriata, O. japonica, and O. minuta), as well as its impact on their growth and development. According to our study findings, O. fimbrata and O. minuta influenced by GA3 exhibited higher survival rates (7–38%) and offset growth rates (3–87%) compared to the control. Similarly, its application resulted in significant shoot and root development, along with increased moisture content for the majority of the species. The results of this research demonstrate the potential and practical applications of using GA3 to increase the propagation and growth of Orostachys spp. during cold conditions for year-round propagation of these succulent plants, which could have ecological and horticultural significance for related species as well as other vegetatively propagated crops.
1. Introduction
A member of the Crassulaceae family, the genus Orostachys is composed of 12 recognized perennial succulent species that are abundantly grown in Korea, Japan, and Russia and operate via Crassulacean acid metabolism (CAM), which allows them to easily adapt to drought, cold, and stressful environments [1,2].
Several species of the Orostachys genus are economically important as high-valued ornamental potted plants (O. ‘Nungyu bawisol’) [3], ground covers (O. japonica) [4], landscape flower beds (O. japonica, O. minuta, and O. chongsunensis) [5], and green roofing plants (O. fimbrata) due to their small and exquisite leaves and formation of beautiful offsets [6]. Aside from its horticultural purpose, this genus has recently risen in popularity as a source of food and medicine [2]. Thus, many studies have engaged in characterizing and developing purification methods of Orostachys species as a rich source of flavonoids (i.e., epicatechin gallate, quercetin, and kaempferol) [7,8], anti-inflammatory novel polysaccharide, anti-tumor, antioxidant, and antifebrile components (i.e., 4-α-Glcp-(1 and 4,6)-β-Glcp-(1)) [9,10,11].
Because of their medicinal benefits, there is a large demand, especially in China, for Orostachys products and raw materials for their production, which are derived from different wild species [12]. However, the wild resources of these species are scarce and have already been endangered; hence, there is a need for improved propagation techniques to meet its demand as well as maintain progenies for conservation [13].
Succulents, such as Orostachys species, are commonly propagated through axillary buds attached to the leaves, stems, and offsets. These buds are attached to the stems, grow outward, detach, and should grow separately, but they are highly dormant [14]. They have a natural dormancy period wherein these species slow down their growth and are triggered by environmental factors that usually occur during winter and thus also affect plant propagules for propagation [14,15,16]. In order to establish a large-scale and less sophisticated solution to this barrier in propagation, the use of exogenous hormones is commonly applied to the propagules prior to planting [17].
Among the key plant growth regulators, gibberellins (GAs) have emerged as pivotal hormones with remarkable effects on plant propagation, as they are integral in the development of seeds and fruits and the primary hormone controlling plant height, shoot, and root elongation [18]. In recent years, the application of exogenous gibberellins has garnered significant attention as a promising strategy to modulate plant physiology such as those with problems in lagged growth and triggering branching. The application of GA is more prominently used to increase growth rates in various crops in in vivo and in vitro conditions [19,20,21]. The main mechanism by which GA expedites growth is by targeting the intercalary meristem as well as affecting gene transcription for specific steps in the biosynthetic pathways to mediate environmental factors such as temperature and photoperiod [22].
Studies have also reported that the exogenous application of GA has also encouraged growth in vegetative propagules of several economically important crops such as Allium sativum cloves [23], Rhododendron species flower buds [24], detached apple shoots [25], and strawberry petioles [26], among others. Lang et al. [27] explained that lagged or slower growth rates are affected by seasonal changes that are indicative of temperature extremes during winter and chilling and/or photoperiodic responses during autumn. As a complex process necessary for plant survival, propagation, and development, dormancy regulation in vegetative buds is highly associated with the action of specific hormones [28]. In plant models for vegetative propagation, among other plant hormones, GA is known to promote growth through the upregulation of D-type cyclins and cyclin-dependent kinase as well as A- and B-type cyclins [29], which are involved in G1-S and G2-M and are paramount to the growth and progression of bud spurge overcoming inhibiting environmental factors experienced by plant propagules being propagated in colder months [30,31].
However, the majority of the studies on Orostachys species propagation with the application of exogenous plant growth regulators have used in vitro methods such as tissue culture [13,32,33]. Several plant nurseries do not have sophisticated facilities to propagate using controlled sterile conditions grown in artificial media, and using exogenous hormones is the most suitable way to improve propagation efficiency.
Hence, in this study, the use of the exogenous application of GA3 at different concentrations on the offsets of three economically important Orostachys species is investigated to determine its effect on their propagation, growth, and development.
2. Materials and Methods
2.1. Plant Materials
Three Orostachys species (Figure 1), namely, O. fimbriata, O. japonica, and O. minuta, were utilized in this study and were brought to the Experiment Greenhouse of the Department of Environmental Horticulture, Sahmyook University, South Korea.
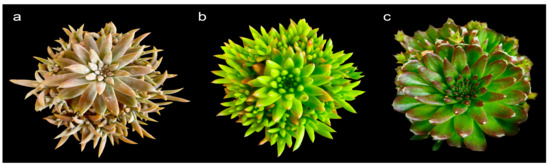
Figure 1.
Orostachys species used in this study: (a) O. fimbriata; (b) O. japonica; and (c) O. minuta.
2.2. Experimental Design and Treatment Applications
To determine the optimized concentration for breaking the dormancy of Orostachys propagules, we used gibberellic acid (GA3; CAS No. 77-06-5, Sigma Aldrich, St. Louis, USA) at different concentrations. The following concentrations were used as treatments: control (no exogenous GA3 application), 200 mg·L−1, 400 mg·L−1, and 600 mg·L−1. Due to the limited supply of these wild species, each treatment was replicated three times with 12 plants per replication treatment.
Mother plants with 1 cm-sized offsets were detached (Figure 2) and collected during the autumn season, which is considered the onset of lagged growth for the plant propagules. The offsets were planted in trays (28.0 × 52.0 × 4.5 cm; L × W × H) having 72 individual cells (45 cc per cell) that were filled with horticultural substrates (Hanareumsagto, Shinsung Mineral, Goesan-gun, Republic of Korea) as a potting medium. Exogenous application of GA3 was carried out using the drenching method with 10 mL solution of their respective treatment concentrations at planting.
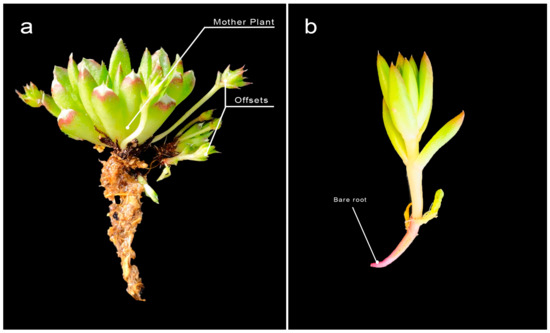
Figure 2.
Plant propagules of an Orostachys species showing: (a) the mother plant with several attached offsets growing at its basal area (O. minuta); and (b) the detached offset as bare root, which was used as propagule (O. japonica).
2.3. Data Gathered
The survival rate was calculated by counting the number of successfully developed offsets over the initial number of planted propagules and is presented as percentages, while the offset growth rate was measured by calculating the percentage increase over time. Other plant growth and development parameters were also gathered, such as shoot length and width, stem diameter, root length, number of offsets, offset length, leaf length and width, and ground cover.
Fresh weight was measured by removing the soil attached to the plant, rinsing the plant with water, and then allowing it to air-dry naturally in a sealed space for 24 h before measurement. Dry weight was measured after hot-air drying the samples in a dry oven (HK-DO135F, Hankook S&I, Republic of Korea) at 85 °C for 24 h. To determine the moisture content (MC), it was finally calculated using this formula: % MC = [(A−B)/A] * 100, where A is the fresh weight and B is the dry weight.
2.4. Care and Management
The offsets were placed inside the greenhouse with controlled conditions with a recorded temperature of 20.4 °C ± 2.1 and relative humidity of 45.8% ± 13.8 throughout the duration of the study. Overhead watering was carried out three times a week for water management.
2.5. Statistical Analysis
Numerical data obtained from Orostachys species were subjected to analysis of variance (ANOVA). Significant differences between means were analyzed using Duncan’s multiple range test at a 5% significance. All statistical analyses were performed using SAS 9.4 (SAS Institute, USA).
3. Results
3.1. Survival Rate and Growth
The exogenous application of gibberellic acid (GA3) had a significant impact on the survival rate (Figure 3) and offset growth rate (Figure 4) of Orostachys species. In O. fimbriata, control plants had the lowest survival rate of 66%, while propagules treated with GA3 had significantly higher survival rates with the use of 400 mg·L−1 (88%), 600 mg·L−1 (83%), and 200 mg·L−1 (72%) (Figure 3A). Among the species, only O. japonica showed no significant differences after the application of GA3, which had a 100% survival rate three months after treatment (MAT) (Figure 3B). On the other hand, O. minuta treated with GA3, regardless of the concentration, had the highest survival rates (94%) compared to the control (66%) (Figure 3C).
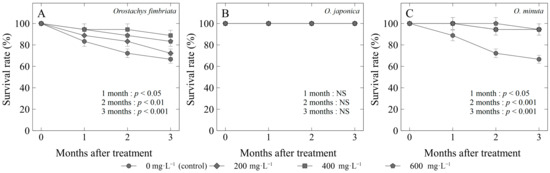
Figure 3.
Survival rate (%) of three Orostachys species: (A) O. fimbriata; (B) O. japonica; and (C) O. minuta offsets treated with different concentrations of gibberellic acid (GA3). Non-significant (NS) or significant at p < 0.05, 0.01, or 0.001.
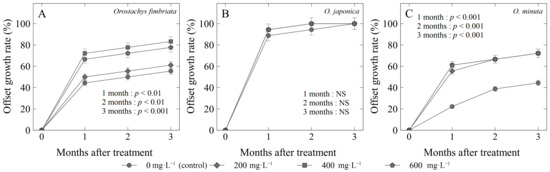
Figure 4.
Offset growth rate (%) of three Orostachys species: (A) O. fimbriata; (B) O. japonica; and (C) O. minuta offsets treated with different concentrations of gibberellic acid (GA3). Non-significant (NS) or significant at p < 0.01 or 0.001.
The offset growth rate of Orostachys species was determined for three consecutive months (Figure 4). For O. fimbriata, the use of 400 mg·L−1 (83%) on the offsets gave the highest growth rate, while the control gained the lowest value of 55% (Figure 4A). Similar to the survival rate, O. japonica with applied GA3 did not have any significant differences with those of the control from the first up to the third month (Figure 4B). However, it may be noted that the earliest and highest offset growth rate at 1 month (94%) and 2 months (100%) after treatment was taken from those treated with GA3 compared with the control (88% at 1 month, 94% at 2 months). The application of gibberellic acid gave the significantly highest offset growth in O. minuta species for the three consecutive months, with 72% (200 and 400 mg·L−1) to 83% (600 mg·L−1), while the control lagged at 44%.
3.2. Plant Growth Parameters
Aside from the survival rate and growth rate of Orostachys species offsets, the application of GA3 also significantly affected the growth and development parameters of these succulents (Table 1).

Table 1.
Plant growth parameters of Orostachys species in response to gibberellic acid (GA3) concentrations.
The use of GA3 significantly affected the shoot height in all Orostachys species, wherein all concentrations significantly differ from those of the control. The use of 600 mg·L−1 had the highest value for O. fimbriata (5.31 cm) and O. japonica (6.67 cm), which did not significantly differ from other concentrations. However, among O. minuta, the application of 600 mg·L−1 GA3 (5.15 cm) differed significantly from those of 200–400 mg·L−1 (3.42–3.83 cm) and of the control (1.88 cm).
Likewise, the shoot width of O. fimbriata and O. minuta treated with GA3 at different concentrations was significantly wider than that of the control. Similarly, the stem diameter of O. fimbrata and O. minuta was significantly affected by treatment application, wherein the use of 600 mg·L−1 gave the widest diameter, which was different from the other concentrations.
In terms of root growth, O. fimbriata and O. minuta were not significantly affected by GA3 application. On the other hand, control plants of O. japonica species had the longest root length of 6.36 cm compared to those treated with gibberellic acid.
For O. minuta, the use of 400 mg·L−1 GA3 produced the highest number of offsets with 20.5, compared to those of 200, 600 mg·L−1 (16.8, 13.6 offsets, respectively), and the control (8.7 offsets). In the case of O. japonica, the offset length was found to be highest in GA3 treatments (3.23–3.73 cm) regardless of the concentration. Nevertheless, for O. minuta, the use of the highest concentration (600 mg·L−1) resulted in the highest offset length of 2.42 cm, which significantly differed from other treatments.
The application of 600 mg·L−1 gave the significantly highest leaf length and width in O. fimbriata (3.87 cm, 0.60 cm) and O. minuta (3.62 cm, 0.53 cm), which significantly differed from those of other treatment concentations and the control. The results of the shoot and root growth of Orostachys species are evident in the representative samples shown in Figure 5.
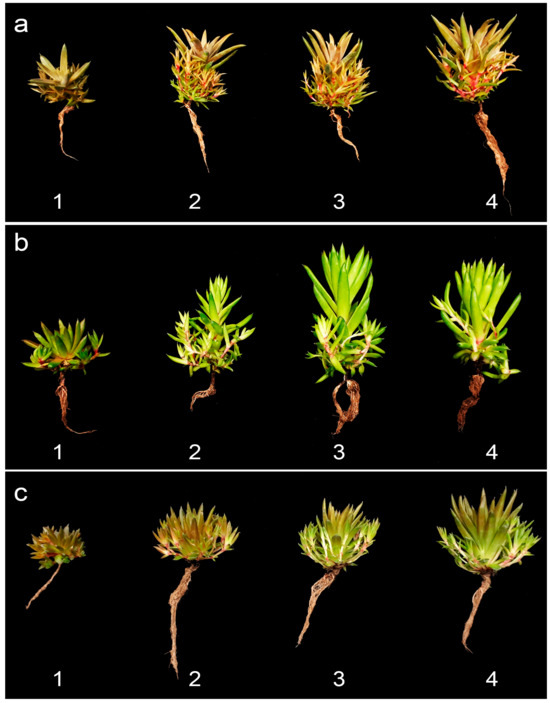
Figure 5.
Comparison of growth and development of Orostachys species: (a) O. fimbrata; (b) O. japonica; and (c) O. minuta of the (1) control and those treated with gibberellic acid (GA3) at different concentrations: (2) 200 mg·L−1; (3) 400 mg·L−1; and (4) 600 mg·L−1.
With regards to the ground cover, the application of 600 mg·L−1 resulted in the greatest ground cover in O. fimbrata (38.0 cm2) and O. minuta (27.7 cm2). However, in O. japonica, the ground cover showed no significant differences by treatment.
3.3. Moisture Content
Determining the moisture content is deemed crucial and provides a good overview of the overall growth and development performance of offsets concerning their nutrient uptake, water regulation, and plant vigor. The fresh and dry weight of Orostachys species were gathered to further determine their moisture content (Table 2). The results of the analysis revealed that the moisture content and dry and fresh weight of O. fimbriata and O. minuta were significantly affected by gibberellic acid application, except for the root moisture content. However, for O. japonica, no significant differences between the treatments were recognized.

Table 2.
Fresh and dry weight and moisture content of Orostachys species in response to gibberellic acid (GA3) concentrations.
The results showed that the use of 600 mg·L−1 GA3 exhibited a significantly higher fresh and dry weight of roots and shoots of O. fimbrata offsets compared to other concentrations and the control. Interestingly, the moisture content was observed to be higher in those treated with 200 mg·L−1 (94.9%), which did not significantly differ from those of the control (94.3%) and 400 mg·L−1 (93.3%).
Similar results were found to those of O. minuta offsets, wherein the 600 mg·L−1 treatment resulted in the heaviest shoot (7.23 g) and root (0.09 g) fresh weight. The heaviest shoot dry weight was found in those treated with 600 mg·L−1, which significantly differed from all other treatments; however, for the root dry weight, the application of both 200 mg·L−1 and 600 mg·L−1 gave the highest root dry weight of 0.02 g. The highest shoot content was finally found in those treated with 400 and 200 mg·L−1, with 94.5% and 94.0%, respectively.
4. Discussion
The dormant axillary buds of three Orostachys spp. were treated with various concentrations of gibberellic acid to determine its efficacy in inducing growth and development for better plant propagation methods of these economically valuable succulent plants. The use of GA in plant propagation, especially among horticulturally valued plants, has been a common practice, as it has impactful results at low concentrations and is easily handled by nursery propagators [18,34]. However, it was always recommended that trials on its concentration should be performed, as these exogenous hormones have varying effects on different plant species, wherein it could lead to inhibitory effects such as dwarfism in some cases [35]. Hence, this study was deemed necessary.
The results revealed that the use of GA3 had a significantly higher survival rate in O. fimbrata and O. minuta species compared to the controls. Despite the common use of gibberellins for shoot development, it has also been reported to promote tissue callus development [36] and increase cell division and elongation [37], which are important mechanisms for plant propagule survival, especially of those that are vegetatively propagated such as suckers, offsets, runners, and plant cuttings [18]. Callus formation, the development of unorganized cell masses, has profound importance in the propagation of horticultural plants, as it prevents further infection and water loss [38,39,40] and is often suggested to be highly pluripotent or associated with the regeneration of new organs and tissues [41]. Efferth [42] reported that callus formation may be driven by the application of plant hormones, including gibberellins.
On the other hand, O. japonica showed a 100% survival rate regardless of the treatment. This result has also been found in some plants wherein GA3 did not affect plant regeneration, such as those in the study of Tamaki et al. [43] on Graptopetalum paraguayense succulents. According to Kato [44], gibberellins are sometimes unable to stimulate cells in this regard, which would still warrant further investigation of its mechanism.
The offset growth rate of the majority of the Orostachys species was also significantly affected by the exogenous application of gibberellins, regardless of the concentration, which significantly differed from those of the controls. Several crops have been reported to show positive results in enhancing the growth of dormant propagules of asexually propagated crops such as Acer and Prunus hardwood cuttings [45], Gladiolus corms [46], Picrorhiza kurroa rooting runner cuttings [47], garlic bulbs [48], globe artichoke [49], and even seed-bearing plants such as Panax notoginseng [50], Vigna mungo [51], and papaya [52], which are usually perpetuated using mucilage, cold conditions, low light intensities, etc. The use of GA3 on plant propagules causes an alteration of the hormone balance, thus inducing hydrolytic enzyme formation, which regulates the mobilization of reserved food resources [18,46,53]. It has also been shown that GA-promoted growth may mediate these food reserves through the accumulation of soluble sugars, starch, and cell wall polysaccharides [54]. Aside from the mobilization of food, GA3 has also been associated with antagonistic interactions with abscisic acid, which induces dormancy and lagged growth; however, the exogenous application of GAs counters it [27]. Likewise, the study of Beauvieux et al. [55] reported that when GA3 is applied, it could indirectly promote vegetative development through the stimulation of oxidative stress responses, which was found to increase the production of proteins involved in oxidative reduction responses by 40% in Japanese apricots [56]. These mechanisms coherently work to enhance the survival rate and give a higher offset growth rate in O. fimbrata and O. japonica.
In terms of shoot growth parameters, the use of GA3, especially at the highest concentration, resulted in the tallest and widest plants with long stem lengths. Our results are similar to the numerous studies that have been conducted showing gibberellin as an effective plant growth enhancer of shoot development and plant production in general, especially for horticulture and viticulture [57]. The most prominent effect of gibberellins is their role in internode elongation [58]. Due to their first discovery in rice, stem-promoting gibberellins have been studied and applied in other crops to induce bolting and leaf growth [59]. GA’s action targets the intercalary meristem, where cell division and elongation activities are increased [22,60].
Similarly, the root growth parameters were also found to be higher in those treated with GA3 than those of the control, but the differences between treatments were only significant in two species, i.e., O. japonica and O. minuta. Although several papers suggest that GA plays a minimal and less remarkable role, sometimes even detrimental, in root functions and development [60,61,62], studies by Bidadi et al. [63] suggest that there just needs to be a proper balance between the production of bioactive GAs in the root to promote root growth. Likewise, Binenbaum et al. [64] emphasized that GA movement is deemed necessary at multiple stages of development in all plant organs, such as the local accumulation of bioactive GA in the root elongation zone, because it is directly correlated with cellular growth. Other vegetatively propagated plants have also reported root growth with the application of GA, such as Pennisetum purpureum [65], Lemna minor [66], and sugar beets [67], which had similar results to those of Orostachys species.
Gibberellin has been found to support leaf expansion [68,69], which would explain the leaf length, width, and ground coverage of Orostachys species. The use of GA has been prominent in ground cover crops [70,71,72] and other ornamentals [73,74,75] to increase leaf growth, thus increasing ground coverage. In the study of Nelissen et al. [76], this leaf expansion was suggested to be caused when there is maximum GA in the division zone (DZ). When altering the GA levels, it would also have a definitive effect on the DZ size, resulting in proportional growth or a change in organ growth rates.
The moisture content of Orostachys species treated with GA3 had only significant differences in O. fimbriata and O. minuta. The results suggest that the majority of GA-treated offsets had an increased moisture content than those of untreated offsets. GA, being a key plant hormone, has a vital role in regulating signal pathways that help plants adapt to several abiotic stress conditions and promote plant growth, including water uptake [18,77]. The accompanying leaf expansion and shoot growth prompted by the application of GA as a signaling hormone that promoted water uptake sheds light on the increased moisture content of treated Orostachys plants. Studies of several vegetatively propagated plants have shown increased rates of shoot and propagation rates when treated with GA3 [78,79,80].
5. Conclusions
Offsets of Orostachys species, particularly O. fimbrata and O. minuta, induced with gibberellic acid (GA3) had significantly higher and earlier offset growth rates. Through the evaluation of their offset growth and development, significant differences in shoot and root growth, including moisture content, were observed, which would allow faster and more efficient vegetative propagation rates for this economically important succulent throughout the year, even during the cold winter months where slow growth is prevalent.
Author Contributions
Conceptualization, J.H.L., E.J.S. and S.Y.N.; methodology, J.H.L. and S.Y.N.; formal analysis, J.H.L. and S.Y.N.; investigation, J.H.L. and E.J.S.; writing—original draft preparation, J.H.L., E.J.S. and S.Y.N.; writing—review and editing, J.H.L. and S.Y.N.; project administration, S.Y.N.; All authors have read and agreed to the published version of the manuscript.
Funding
This paper was supported by the Sahmyook University Research Fund in 2023.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, J.H.; Kim, H.; Lee, C. Analysis of genetic relationships among Korean native Orostachys species using RAPD. Korean J. Hortic. Sci. Technol. 2001, 19, 159–162. [Google Scholar]
- An, J.; Moon, J.C.; Jang, C.S. Markers for distinguishing Orostachys species by SYBR green-based real-time PCR and verification of their application in commercial O. japonica food products. Appl. Biol. Chem. 2018, 61, 499–508. [Google Scholar] [CrossRef]
- Chon, Y.S.; Lee, S.W.; Jeong, K.J.; Ha, S.Y.; Bae, J.H.; Yun, J.G. Growth and quality affected by light intensity, potting media and fertilization level in potted Orostachys ‘Nungyu bawisol’. J. Bio-Environ. Control 2011, 20, 357–364. [Google Scholar]
- Ryu, J.H.; Lee, H.B.; Kim, C.M.; Jung, H.H.; Kim, K.S. Cold tolerance of ground cover plants for use as green roofs and walls. Korean J. Hortic. Sci. Technol. 2014, 32, 590–599. [Google Scholar] [CrossRef][Green Version]
- Jung, J.H.; Park, N.B. Morphological characteristics and material comparison of native Orostachys species (Orostachys japonica (Maxim.) A. Berger, Orostachys minuta (Kom.) A. Berger, Orostachys chongsunensis Y.N. Lee). J. Pract. Agric. Fish Res. 2019, 21, 5–13. [Google Scholar]
- Chen, J.Y. Development characteristics and cultivation techniques of Orostachys fimbriata. South China Agric. 2011, 5, 15–18. [Google Scholar]
- Lee, J.; Son, H.; Lee, K.H.; Kim, S.; Myagmar, G.; Kim, S.Y.; Chun, Y.; Yoo, H.Y. Identification and characterization of major flavonoids in extracts from an unexplored medicinal herb Orostachys fimbriata. Horticulturae 2022, 8, 1092. [Google Scholar] [CrossRef]
- Shin, D.Y.; Lee, W.S.; Jung, J.H.; Hong, S.H.; Park, C.; Kim, H.J.; Kim, G.-Y.; Hwang, H.J.; Kim, G.S.; Jung, J.-M.; et al. Flavonoids from Orostachys japonicus A. Berger inhibit the invasion of LnCaP prostate carcinoma cells by inactivating akt and modulating tight junctions. Int. J. Mol. Sci. 2013, 14, 18407–18420. [Google Scholar] [CrossRef]
- Hu, D.; Su, F.; Yang, G.; Wang, J.; Zhang, Y. Purification, structural characterization, and anti-inflammatory effects of a novel polysaccharide isolated from Orostachys fimbriata. Molecules 2021, 26, 7116. [Google Scholar] [CrossRef]
- Hur, S.; Jang, E.; Lee, J.-H. Beneficial actions of Orostachys japonica and its compounds against tumors via MAPK signaling pathways. Nutrients 2021, 13, 555. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, J.S.; Lee, H.-S.; Lim, Y.-M.; So, J.-H.; Hahn, D.; Ha, Y.S.; Nam, J.-O. Bioconverted Orostachys japonicas extracts suppress angiogenic activity of Ms-1 endothelial cells. Int. J. Mol. Sci. 2017, 18, 2615. [Google Scholar] [CrossRef]
- Piao, X.C.; Zhang, W.B.; Jiang, J.; Jin, Y.H.; Park, P.J.; Kim, S.E.; Lian, M.L. Cell suspension culture of Orostachys cartilaginous in bioreactor systems for bioactive compound production and evaluation of their antioxidant properties. Acta Physiol. Plant. 2017, 39, 70. [Google Scholar] [CrossRef]
- Li, Q.W.; Hou, D.M.; Liang, M.Q.; Liu, R.N. Construction of rapid propagation system for Orostachys fimbriatus. Acta Agricult. Zhejiangensis 2014, 26, 84–88. [Google Scholar]
- Gorelick, R. Why vegetative propagation of leaf cuttings is possible in succulent and semi-succulent plants. Haseltonia 2015, 20, 51–57. [Google Scholar] [CrossRef]
- Succulents Australia. Available online: https://www.succulents-australia-sales.com/blogs/blog/orostachys-iwarenge-chinese-dunce-cap-succulent-care (accessed on 25 May 2023).
- Soppe, W.J.; Bentsink, L. Dormancy in plants. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 1–7. [Google Scholar]
- Cabahug, R.A.M.; Nam, S.Y.; Lim, K.B.; Jeon, J.K.; Hwang, Y.J. Propagation techniques for ornamental succulents. Flower Res. J. 2018, 26, 90–101. [Google Scholar] [CrossRef]
- Hartmann, T.; Kester, D.E.; Davies, T.F., Jr.; Geneve, R.L. Hartmann & Kester’s Plant Propagation Principles and Practices, 8th ed.; Pearson Education Ltd.: England, UK, 2014; pp. 20–398. [Google Scholar]
- Ogawa, M.; Hanada, A.; Yamauchi, Y.; Kuwahara, A.; Kamiya, Y.; Yamaguchi, S. Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 2003, 15, 1591–1604. [Google Scholar] [CrossRef] [PubMed]
- Kucera, B.; Cohn, M.A.; Leubner-Metzger, G. Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res. 2005, 15, 281–307. [Google Scholar] [CrossRef]
- Finkelstein, R.; Reeves, W.; Ariizumi, T.; Steber, C. Molecular aspects of seed dormancy. Annu. Rev. Plant Biol. 2008, 59, 387–415. [Google Scholar] [CrossRef]
- Taiz, L.; Moller, I.M.; Murphy, A.; Zeiger, E. Germination and establishment. In Plant Physiology and Development, 7th ed.; Sinauer Associates: England, UK, 2022; pp. 431–580. [Google Scholar]
- Rahman, M.H.; Haque, M.S.; Karim, M.A.; Ahmed, M. Effects of gibberellic acid (GA3) on breaking dormancy in garlic (Allium sativum L.). Int. J. Agric. Biol. 2005, 8, 63–65. [Google Scholar]
- Chang, Y.S.; Sung, F.H. Effects of gibberellic acid and dormancy-breaking chemicals on flower development of Rhododendron pulchrum Sweet and R. scabrum Don. Sci. Hortic. 2000, 83, 331–337. [Google Scholar] [CrossRef]
- Pavia, E.; Robitaille, H. Breaking bud rest on detached apple shoots: Interaction of gibberellic acid with some rest-breaking chemicals. HortScience 1978, 13, 57–58. [Google Scholar] [CrossRef]
- Guttridge, C.G. Interaction of photoperiod, chilling and exogenous gibberellic acid on growth of strawberry petioles. Ann. Bot. 1970, 34, 349–364. [Google Scholar] [CrossRef]
- Lang, G.A.; Early, J.D.; Martin, G.C.; Darnell, R.L. Endo-, para, and ecodormancy: Physiological terminology and classification for dormancy research. HortScience 1987, 22, 371–377. [Google Scholar] [CrossRef]
- Horvath, D.P.; Anderson, J.V.L.; Chao, W.S.; Foley, M.E. Knowing when to grow: Signals regulating bud dormancy. Trends Plant Sci. 2003, 8, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, C.; Ramirez-Parra, E.; Castellano, M.M.; del Pozo, J.C. G(1) to S transition: More than a cell cycle engine switch. Curr. Opin. Plant Biol. 2002, 5, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Sauter, M. Differential expression of a CAK (CDC2-activating kinase)-like protein kinase, cyclins and CDC2 genes from rice during the cell cycle and in response to gibberellin. Plant J. 1997, 11, 181–190. [Google Scholar] [CrossRef]
- Horvath, D.P.; Chao, W.S.; Anderson, J.V. Molecular analysis of signals controlling dormancy and growth in underground adventitious buds of leafy spurge. Plant Physiol. 2002, 128, 1439–1446. [Google Scholar] [CrossRef]
- Kang, Y.M.; Moon, B.C.; Choi, M.S. Production of useful compounds and application of propagation in Korean medicinal plants. Plant Med. 2014, 80, 33. [Google Scholar] [CrossRef]
- Kim, W.J.; Jung, H.Y.; Min, J.Y.; Park, D.J.; Kim, Y.D.; Kang, Y.M.; Choi, M.S. Effects of growth regulators on shoot regeneration and polysaccharide production of Orostachys japonicus Berger. Korean J. Med. Crop Sci. 2004, 12, 391–396. [Google Scholar]
- Lee, I.J. Practical application of plant growth regulator on horticultural crops. J. Hort. Sci. 2003, 10, 211–217. [Google Scholar]
- Brian, P.W. Effects of gibberellins on plant growth and development. Biol. Rev. 1959, 34, 37–77. [Google Scholar] [CrossRef]
- Lance, B.; Reid, D.M.; Thorpe, T.A. Endogenous gibberellins and growth of tobacco callus cultures. Physiol. Plant. 1976, 36, 287–292. [Google Scholar] [CrossRef]
- Guttridge, C.; Thompson, P. Effect of gibberellic acid on length and number of epidermal cells in petioles of strawberry. Nature 1959, 183, 197–198. [Google Scholar] [CrossRef] [PubMed]
- Cline, M.N.; Neely, D. The histology and histochemistry of wound-healing process in Geranium cuttings. J. Am. Soc. Hortic. Sci. 1983, 108, 496–502. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Sugimoto, K.; Iwase, A. Plant callus: Mechanisms of induction and repression. Plant Cell 2023, 25, 3159–3173. [Google Scholar] [CrossRef]
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of plant secondary metabolites: A historical perspective. Plant Sci. 2001, 161, 839–851. [Google Scholar] [CrossRef]
- Stobbe, H.; Schmitt, U.; Eckstein, D.; Dujesiefken, D. Developmental stages and fine structure of surface callus formed after debarking of living lime trees (Tilia sp.). Ann. Bot. 2002, 89, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Tamaki, T.; Kubo, S.; Shimomura, K.; Umehara, M. Effects of gibberellin and abscisic acid on asexual reproduction from Graptopetalum paraguayense leaves. J. Plant Growth Regul. 2020, 39, 1373–1380. [Google Scholar] [CrossRef]
- Kato, J. Studies on the physiological effect of gibberellin (i): On the differential activity between gibberellin and auxin. Mem. Coll. Sci. Univ. Kyoto Ser. B 1953, 20, 189–193. [Google Scholar]
- Larson, P.R. Gibberellic acid-induced growth of dormant hardwood cuttings. For. Sci. 1960, 6, 232–239. [Google Scholar]
- Sarkar, M.A.H.; Hossain, M.I.; Uddin, A.F.M.J.; Uddin, M.A.N.; Sarkar, M.D. Vegetative, floral and yield attributes of gladiolus in response to gibberellic acid and corm size. Sci. Agric. 2014, 7, 142–146. [Google Scholar]
- Chandra, B.; Palni, L.M.S.; Nandi, S.K. Propagation and conservation of Picrorhiza kurrooa Royle ex Benth.: An endangered himalayan medicinal herb of high commercial value. Biodivers Conserv. 2006, 15, 2325–2338. [Google Scholar] [CrossRef]
- Desta, B.; Tena, N.; Amare, G. Regulation of garlic bulb dormancy. Asian J. Res. Rev. Agric. 2022, 4, 1–5. [Google Scholar]
- Soliman, A.G.M.; Alkharpotly, A.A.; Gabal, A.A.A.; Abido, A.I.A. The performance of globe artichoke plants as affected by propagation methods and spraying with gibberellic acid. J. Adv. Agric. Res. 2019, 24, 1–33. [Google Scholar]
- Ge, N.; Jia, J.S.; Yang, L.; Huang, R.M.; Wang, Q.Y.; Chen, C.; Meng, Z.G.; Li, L.G.; Chen, J.W. Exogenous gibberellic acid shortening after-ripening process and promoting seed germination in a medicinal plant Panax notoginseng. BMC Plant Biol. 2023, 23, 67. [Google Scholar] [CrossRef] [PubMed]
- Nedunchezhiyan, V.; Palanivel, M.; Akhila Jabeen, P.A.; Thangavel, P.; Ramakrishnan, B.; Velusamy, M.; Muthusamy, S.; Edm, I.A. Effects of gibberellic acid on seed dormancy of black gram (Vigna mungo L.). J. App. Biol. Biotech. 2023, 11, 256–259. [Google Scholar] [CrossRef]
- Salomão, A.N.; Mundim, R.C. Germination of papaya seed in response to desiccation, exposure to subzero temperatures, and gibberellic acid. HortScience 2000, 35, 904–906. [Google Scholar] [CrossRef]
- Bhalla, R.; Kumar, A. Response of plant bio-regulators on dormancy breaking in gladiolus. J. Ornam. Hortic. 2008, 11, 1–8. [Google Scholar]
- Miyamoto, K.; Ueda, J.; Kamisaka, S. Gibberellin-enhanced sugar accumulation in growing subhooks of etiolated Pisum sativum seedings. Effects of gibberellic acid, indoleacetic acid and cycloheximide on invertase activity, sugar accumulation and growth. Physiol. Plant. 1993, 88, 301–306. [Google Scholar] [CrossRef]
- Beauvieux, R.; Wenden, B.; Dirlewanger, E. Bud dormancy in perennial fruit tree species: A pivotal role for oxidative cues. Front. Plant Sci. 2018, 9, 657. [Google Scholar] [CrossRef]
- Zhuang, W.; Gao, Z.; Wang, L.; Zhong, W.; Ni, Z.; Zhang, Z. Comparative proteomic and transcriptomic approaches to address the active role of GA4 in Japanese apricot flower bud dormancy release. J. Exp. Bot. 2013, 64, 4953–4966. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, W. Chemical regulators of gibberellin status and their application in plant production. Ann. Plant Rev. 2016, 49, 359–403. [Google Scholar]
- Gupta, R.; Chakrabarty, S.K. Gibberellic acid in plant. Plant Signal. Behav. 2013, 8, 9. [Google Scholar] [CrossRef]
- Sun, T. Gibberellin signal transduction in stem elongation and leaf growth. In Plant Hormones; Davies, P.J., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 308–328. [Google Scholar]
- Taiz, L.; Zeiger, E. Gibberellins: Regulators of plant height. In Plant Physiology, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 2002; pp. 461–492. [Google Scholar]
- Mehouachi, J.; Tadeo, F.R.; Zaragoza, S.; Primo-Millo, E.; Talon, M. Effects of gibberellic acid and paclobutrazol on growth and carbohydrate accumulation in shoots and roots of citrus rootstock seedlings. J. Hortic. Sci. 1996, 71, 747–754. [Google Scholar] [CrossRef]
- Lustosa Sobrinho, R.; Zoz, T.; Finato, T.; Oliveira, C.E.d.S.; Neto, S.S.d.O.; Zoz, A.; Alaraidh, I.A.; Okla, M.K.; Alwasel, Y.A.; Beemster, G.; et al. Jatropha curcas L. as a plant model for studies on vegetative propagation of native forest plants. Plants 2022, 11, 2457. [Google Scholar] [CrossRef] [PubMed]
- Bidadi, H.; Yamaguchi, S.; Asahina, M.; Satoh, S. Effects of shoot-applied gibberellin/gibberellin-biosynthesis inhibitors on root growth and expression of gibberellin biosynthesis genes in Arabidopsis thaliana. Plant Root 2010, 4, 4–11. [Google Scholar] [CrossRef]
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin localization and transport in plants. Review 2018, 23, 410–421. [Google Scholar] [CrossRef]
- Ishii, Y.; Yamano, A.; Idota, S. Effects of short-day and gibberellic acid treatments on summer vegetative propagation of napier grass (Pennisetum purpureum Schumach). Int. J. Agron. 2016, 2016, 9606914. [Google Scholar] [CrossRef]
- Inada, S.; Shimmen, T. Regulation of elongation growth by gibberellin in root segments of Lemna minor. Plant Cell Physiol. 2000, 41, 932–939. [Google Scholar] [CrossRef]
- Schwabe, W.W. A simple technique for vegetative propagation of sugar beet. Ann. Appl. Biol. 1980, 94, 269–272. [Google Scholar] [CrossRef]
- Li, J.; Sima, W.; Ouyang, B.; Wang, T.; Ziaf, K.; Luo, Z.; Liu, L.; Li, H.; Chen, M.; Huang, Y.; et al. Tomato SIDREB gene restricts leaf expansion and internode elongation by downregulating key genes for gibberellin biosynthesis. J. Exp. Bot. 2012, 63, 6407–6420. [Google Scholar] [CrossRef] [PubMed]
- Sprangers, K.; Thys, S.; Dusschoten, D.V.; Beemster, G.T.S. Giberrellin enhances the anisotopy of cell expansion in the growth zone of the maize leaf. Front. Plant Sci. 2020, 11, 1163. [Google Scholar] [CrossRef]
- Leben, C.; Alder, E.F.; Chichuk, A. Influence of gibberellic acid on the growth of Kentucky bluegrass. Agron. J. 1959, 51, 116–117. [Google Scholar] [CrossRef]
- Caldiz, D.O.; Clúa, A.; Beltrano, J.; Tenenbaum, S.D. Ground cover, photosynthetic rate and tuber yield of potato (Solanum tuberosum L.) crops from seed tubers with different physiological age modified by foliar applications of plant growth regulators. Potato Res. 1998, 41, 175–185. [Google Scholar] [CrossRef]
- Bishnoi, N.R.; Krishnamoorthy, H.N. Effect of waterlogging and gibberellic acid on leaf gas exchange in peanut (Arachis hypogaea L.). J. Plant Physiol. 1992, 139, 503–505. [Google Scholar] [CrossRef]
- Budiarto, R.; Mubarok, S.; Nanda, M.A.; Nabiyyu, M.; Jaya, M.H.I.S. The increase in kaffir lime leaf production due to gibberellin is diminished by pruning. Horticulturae 2023, 9, 1018. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, S.; Chen, F.; Teng, N.; Fang, W.; Guan, Z. Anatomical structure and gravitropic response of the creeping shoots of ground-cover chrysanthemum ‘Yuhuajinhua’. Plant Growth Regul. 2008, 56, 141–150. [Google Scholar] [CrossRef]
- Douglas, G.C.; Rutledge, C.B.; Casey, A.D.; Richardson, D.H.S. Micropropagation of floribunda, ground cover and miniature roses. Plant Cell Tissue Organ Cult. 1989, 19, 55–64. [Google Scholar] [CrossRef]
- Nelissen, H.; Rymenm, B.; Jikumaru, Y.; Demuynck, K.; Lijsebettens, M.V.; Kamiya, Y.; Inze, D.; Beemster, G.T.S. A local maximum in gibberellin levels regulates maize leaf growth by spatial control of cell division. Curr. Biol. 2012, 22, 1183–1187. [Google Scholar] [CrossRef]
- Zhu, G.; An, L.; Jia, X.; Chen, X.; Zhou, G.; Mclaughlin, N. Effects of gibberellic acid on water uptake and germination of sweet sorghum seeds under salinity stress. Chil. J. Agric. Res. 2019, 79, 415–424. [Google Scholar] [CrossRef]
- Wang, M.; Chen, J.; Zhang, X.; Li, S.; Zhang, T.; Li, W.; Lin, L. Gibberellin A3 induces polyaerial shoot formation and increases the propagation rate in Paris polyphylla rhizomes. Ind. Crops Prod. 2021, 167, 113511. [Google Scholar] [CrossRef]
- Hartmann, A.; Senning, M.; Hedden, P.; Sonnewald, U.; Sonnewald, S. Reactivation of meristem activity and sprout growth in potato tubers require both cytokinin and gibberellin. Plant Physiol. 2011, 155, 776–796. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Shiraiwa, N.; Itai, A.; Honda, I. Involvement of gibberellins in the regulation of tillering in Welsh Onion (Allium fistulosum L.). Hortic. J. 2015, 84, 334–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).