Effects of Exogenous 24-Epibrassinolide Leaves Spraying Application on Chlorophyll Accumulation and Gene Expression Profiles of Chlorophyll Metabolism in Celery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Exogenous 24-EBL Treatments
2.3. Extraction and Determination of Chlorophyll Content
2.4. Total RNA Extraction and cDNA Preparation
2.5. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
2.6. Data Analysis
3. Results
3.1. Effects of Exogenous 24-EBL on the Leaf Color of Celery
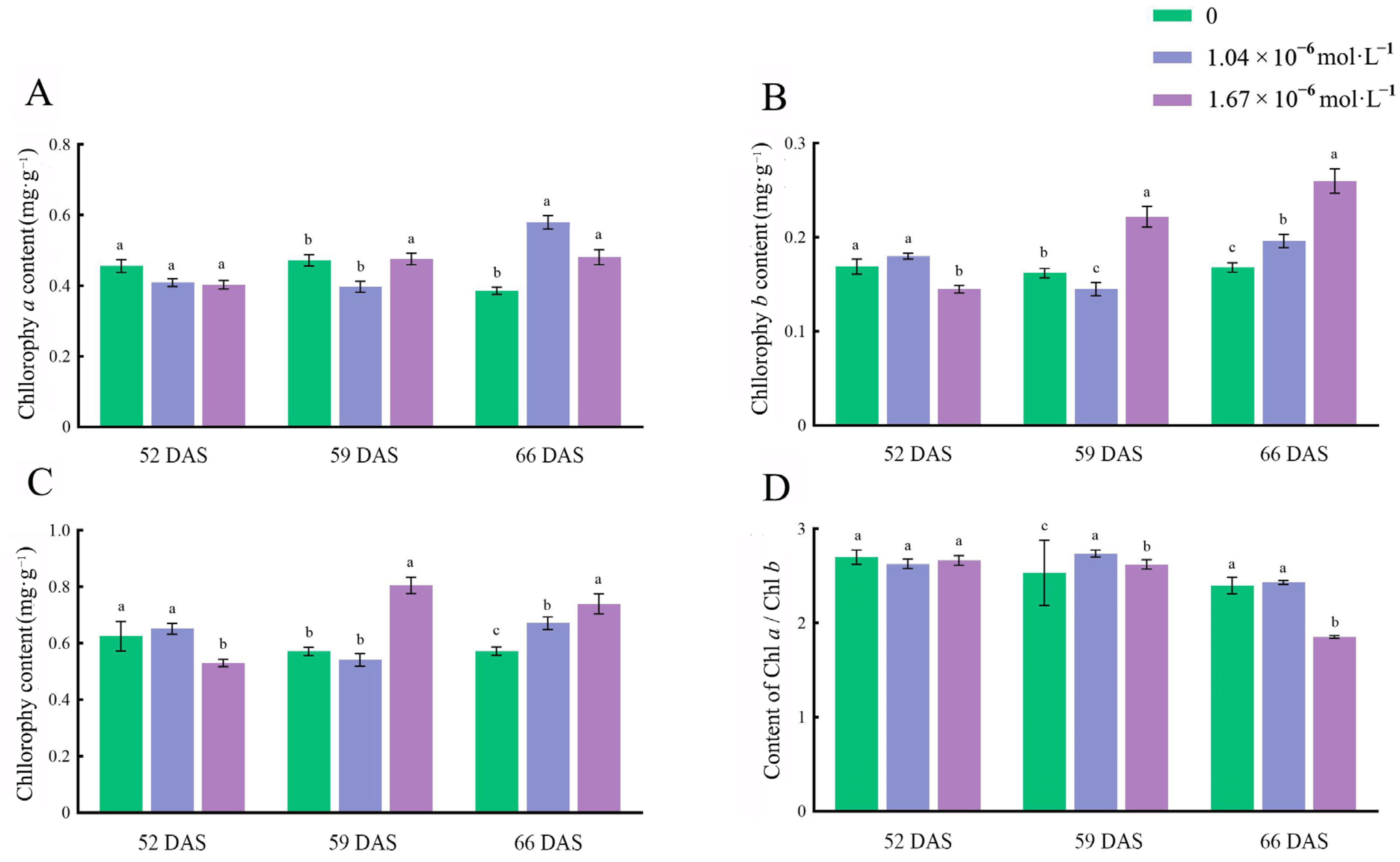
3.2. Effects of Exogenous 24-EBL on Chlorophyll Content and Proportion in Leaf Blades of Celery
3.3. Effects of Exogenous 24-EBL on Chlorophyll Content and Proportion in Petioles of Celery
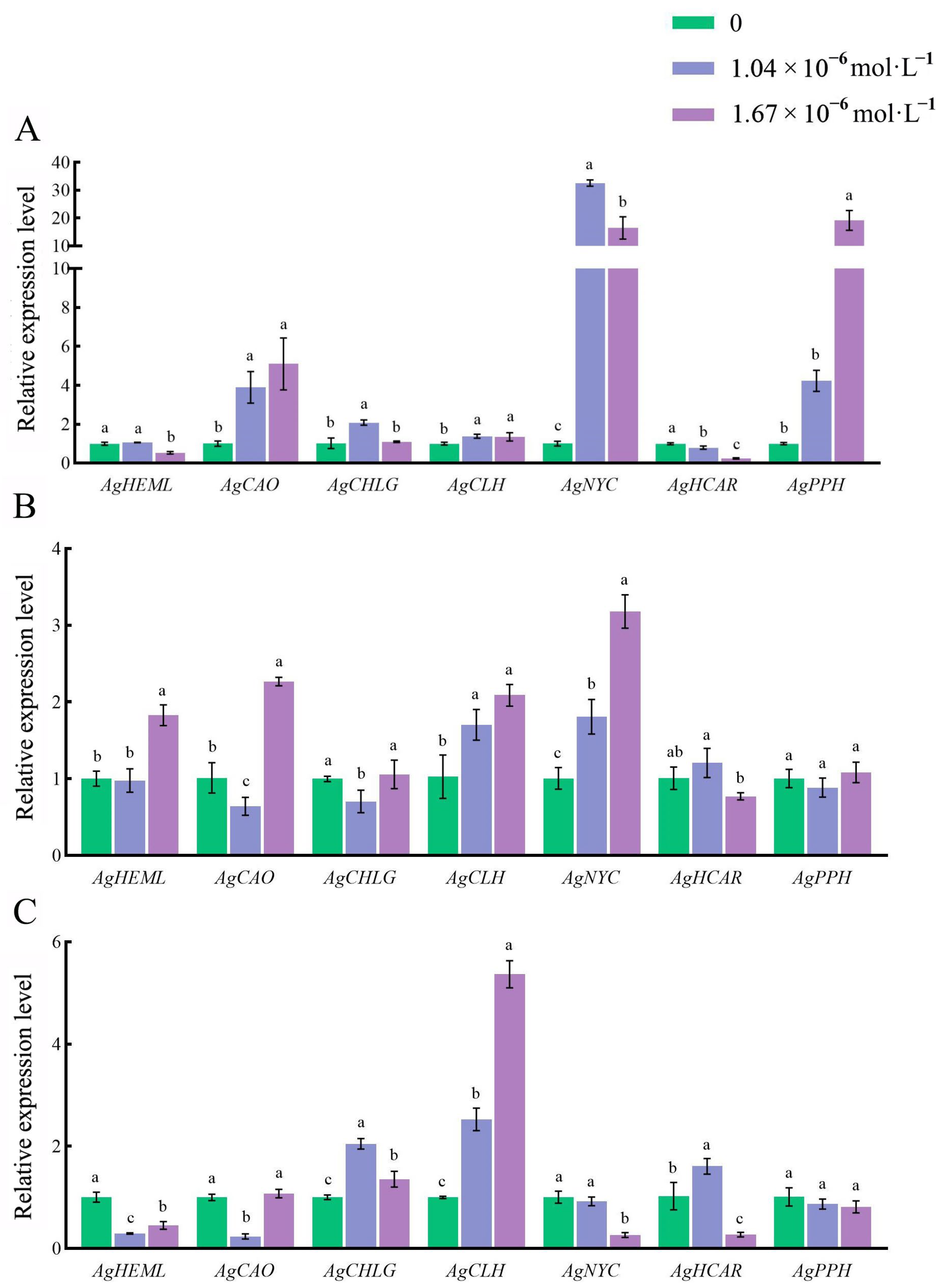
3.4. Effects of Exogenous 24-EBL on Expression of Genes Related to Chlorophyll Metabolism in Leaf Blades of Celery
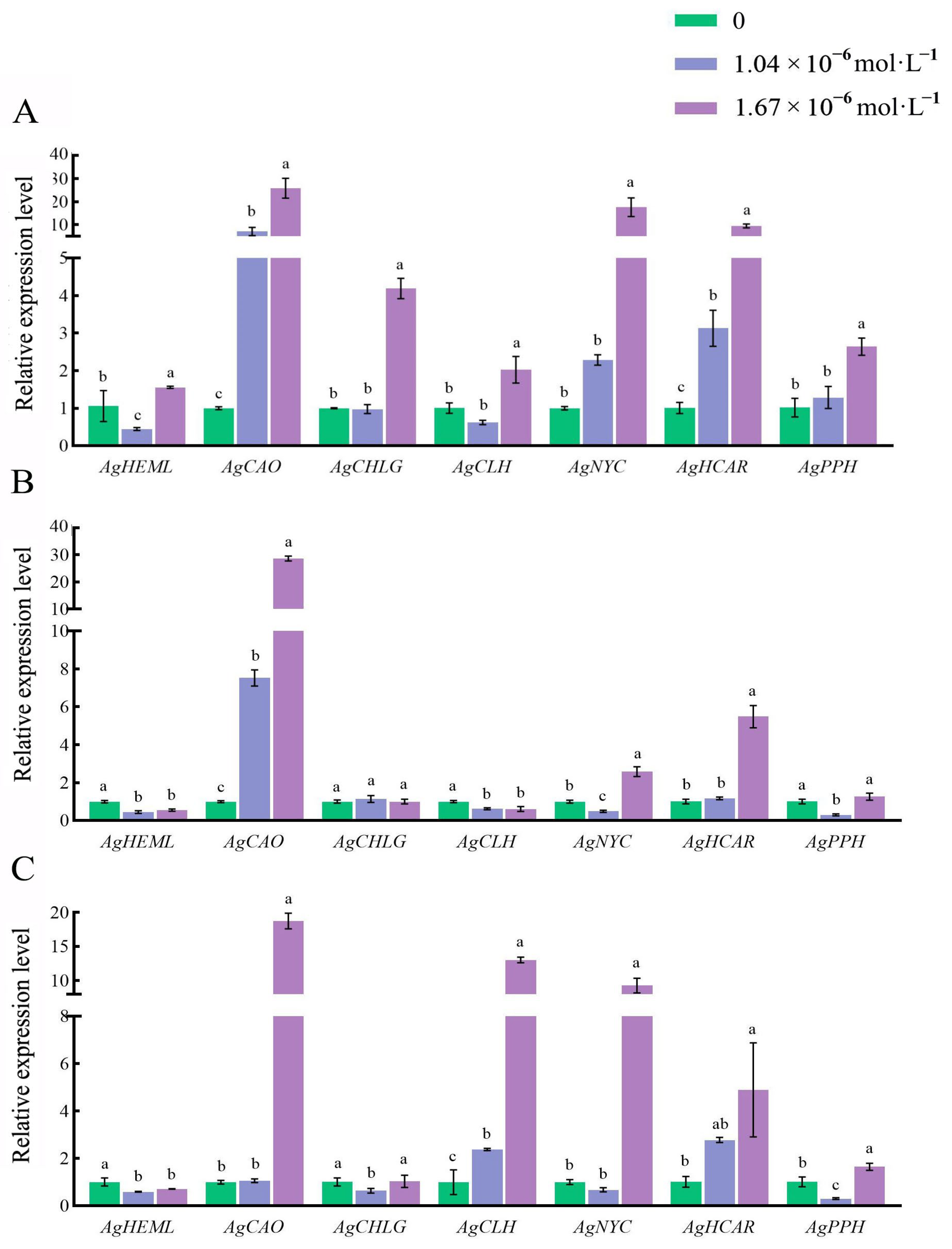
3.5. Effects of Exogenous 24-EBL on the Expression of Genes Related to Chlorophyll Metabolism in Celery Petioles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.Y.; Hou, X.L.; Wang, F.; Tan, G.F.; Xu, Z.S.; Xiong, A.S. Advances in the research of celery, an important Apiaceae vegetable crop. Crit. Rev. Biotechnol. 2018, 38, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Y.; Chen, Q.; Yang, Y.; Duan, Y.W.; Yang, Y.P. Correction: Exchanges of economic plants along the land silk road. BMC Plant Biol. 2023, 23, 71. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Luo, Q.; Li, T.; Meng, P.H.; Pu, Y.T.; Liu, J.X.; Zhang, J.; Liu, H.; Tan, G.F.; Xiong, A.S. Origin, evolution, breeding, and omics of Apiaceae: A family of vegetables and medicinal plants. Hortic. Res. 2022, 9, uhac076. [Google Scholar] [CrossRef]
- Song, X.M.; Li, N.; Zhang, Y.C.; Liang, Y.; Zhou, R.; Yu, T.; Shen, S.Q.; Feng, S.Y.; Zhang, Y.; Li, X.Q.; et al. Transcriptomics and genomics analysis uncover the differentially expressed chlorophyll and carotenoid-related genes in celery. Int. J. Mol. Sci. 2022, 23, 8986. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.H.; Gou, S.Z.; Sun, B.Y.; Lv, W.L.; Li, Y.W.; Peng, H.; Xiao, H.; Yang, G.; Wang, Y.J. Modeled dosage-response relationship on the net photosynthetic rate for the sensitivity to acid rain of 21 plant species. Bull. Environ. Contam. Toxicol. 2012, 89, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Nadezhda, A.; Golubkina, V.; Kharchenko, A. Yield, growth, quality, biochemical characteristics and elemental composition of plant parts of celery leafy. Stalk and root types grown in the northern hemisphere. Plants 2020, 9, 484. [Google Scholar]
- Liu, M.Y.; Ma, W.; Su, X.J.; Zhang, X.M.; Lu, Y.; Zhang, S.W.; Yan, J.H.; Feng, D.L.; Ma, L.S.; Taylor, A.; et al. Mutation in a chlorophyll-binding motif of Brassica ferrochelatase enhances both heme and chlorophyll biosynthesis. Cell Rep. 2022, 41, 111758. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Chen, Y.; Guo, Y.Y.; Ma, Y.L.; Yang, M.; Fu, R.Q.; Sun, Y.P. Elevated CO2 delayed yellowing by maintaining chlorophyll biosynthesis and inhibiting chlorophyll degradation and carotenoid accumulation of postharvest broccoli. Postharvest Biol. Technol. 2022, 194, 112089. [Google Scholar] [CrossRef]
- Wang, L.D.; Shang, L.; Wu, X.Y.; Hao, H.Q.; Jing, H.C. Genomic architecture of leaf senescence in sorghum (Sorghum bicolor). Theor. Appl. Genet. 2023, 136, 328. [Google Scholar] [CrossRef]
- Cai, W.Q.; Zhang, D.M.; Zhang, X.; Chen, Q.R.; Liu, Y.; Lin, L.; Xiang, L.L.; Yang, Y.J.; Xu, L.; Yu, X.Y.; et al. Leaf color change and photosystem function evaluation under heat treatment revealed the stress resistance variation between Loropetalum chinense and L. chinense var. rubrum. PeerJ. 2023, 11, e14834. [Google Scholar] [CrossRef]
- Ramirez-Olvera, S.M.; Trejo-Tellez, L.L.; Perez-Sato, J.A.; Gómez-Merino, F.C. Silicon stimulates initial growth and chlorophyll a/b ratio in rice seedlings, and alters the concentrations of Ca, B, and Zn in plant tissues. J. Plant Nutr. 2019, 42, 1928–1940. [Google Scholar] [CrossRef]
- Morley, P.; Jump, A.; West, M.D.; Donoghue, D. Spectral response of chlorophyll content during leaf senescence in European beech trees. Environ. Res. Commun. 2020, 2, aba7a0. [Google Scholar] [CrossRef]
- Singh, A.; Dwivedi, P.; Kumar, V.; Kumar, P.D. Brassinosteroids and their analogs: Feedback in plants under in vitro condition. S. Afr. J. Bot. 2021, 143, 256–265. [Google Scholar] [CrossRef]
- Wachsman, M.B.; Ramirez, G.A.; Talarico, L.B.; Galagovsky, L.R.; Coto, C.E. Antiviral activity of natural and synthetic brassinosteroids. Curr. Med. Chem. Anti-Infect. Agents 2004, 3, 163–179. [Google Scholar] [CrossRef]
- Kumari, S.; Thakur, A.; Singh, N.; Chandel, J.S.; Singh, N.; Rana, J.S.N. Influence of drought stress and brassinosteroid on growth and physio-biochemical characteristics of apple plants. Indian J. Hortic. 2020, 77, 88–93. [Google Scholar] [CrossRef]
- Lima, J.V.; Lobato, A.K.S. Brassinosteroids improve photosystem II efficiency, gas exchange, antioxidant enzymes and growth of cowpea plants exposed to water deficit. Physiol. Mol. Biol. Plants 2017, 23, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Zafari, M.; Ebadi, A.; Jahanbakhsh, S.; Sedghi, M. Safflower (Carthamus tinctorius) biochemical properties, yield and oil content affected by 24-epibrassinosteroid and genotype under drought stress. J. Agric. Food Chem. 2020, 68, 6040–6047. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Ma, J.; Huang, W.L.; Di, H.M.; Xia, X.; Ma, W.; Ma, J.; Yang, J.; Li, X.M.; Lian, H.S.; et al. Physiological and comparative transcriptome analysis reveals the mechanism by which exogenous 24-epibrassinolide application enhances drought resistance in potato (Solanum tuberosum L.). Antioxidants 2022, 11, 1701. [Google Scholar] [CrossRef]
- Hayat, S.; Alyemeni, M.N.; Hasan, S.A. Foliar spray of brassinosteroid enhances yield and quality of Solanum lycopersicum under cadmium stress. Saudi J. Biol. Sci. 2012, 19, 325–335. [Google Scholar] [CrossRef]
- Kolomeichuk, L.V.; Efimova, M.V.; Zlobin, I.E.; Kreslavski, V.D.; Murgan, O.K.; Kovtun, I.S.; Khripach, V.A.; Kuznetsov, V.V.; Allakhverdiev, S.I. 24-epibrassinolide alleviates the toxic effects of NaCl on photosynthetic processes in potato plants. Photosynth. Res. 2020, 146, 151–163. [Google Scholar] [CrossRef]
- Yonca, S.A. The effect of 24-epibrassinolide treatments at different concentrations on some growth parameters and crocin level in saffron (Crocus sativus L.). J. Int. Second. Metab. 2020, 7, 109–118. [Google Scholar]
- Liu, Y.H.; Sun, M.; Wang, H.; Liu, J.X.; Tan, G.F.; Yan, T.; Wang, Y.H.; Yan, Z.M.; Liu, H.; Tao, J.P.; et al. 24-epibrassinolide and 2,6-dichlorobenzonitrile promoted celery petioles and hypocotyl elongation by altering cellulose accumulation and cell length. Agronomy 2022, 12, 1670. [Google Scholar] [CrossRef]
- Li, M.Y.; Li, J.; Zhang, R.; Lin, Y.X.; Xiong, A.S.; Tan, G.F.; Lou, Y.; Zhang, Y.; Chen, Q.; Wang, Y.; et al. Combined analysisof the metabolome and transcriptome to explore heat stress responses andadaptation mechanisms in celery (Apium graveolens L.). Int. J. Mol. Sci. 2022, 23, 3367. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ma, H.Y.; Huang, Y.; Li, Y.; Wang, G.I.; Jiang, Q.; Wang, F.; Xiong, A.S. Comparative proteomic analysis provides novel insights into chlorophyll biosynthesis in celery under temperature stress. Physiol. Plant. 2017, 161, 468–485. [Google Scholar] [CrossRef] [PubMed]
- Gollan, J.P.; Grebe, S.; Roling, L.; Grimm, B.; Spetea, C.; Aro, E.M. Photosynthetic and transcriptome responses to fluctuating light in Arabidopsis thylakoid ion transport triple mutant. Plant Direct 2023, 7, e534. [Google Scholar] [CrossRef]
- Li, M.Y.; Feng, K.; Hou, X.L.; Jiang, Q.; Xu, Z.S.; Wang, G.L.; Liu, J.X.; Wang, F.; Xiong, A.S. The genome sequence of celery (Apium graveolens L.), an important leaf vegetable crop rich in apigenin in the Apiaceae family. Hortic. Res. 2020, 7, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Schäffer, A.A.; Aravind, L.; Madden, T.L.; Shavirin, S.; Spouge, J.L.; Wolf, Y.I.; Koonin, E.V.; Altschul, S.F. Improving the accuracy of PSI-BLAST protein database searches with composition-based statistics and other refinements. Nucleic Acids Res. 2001, 29, 2994–3005. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Mohammadreza, A.; Pari, Z.S. Foliar spray with 24-epibrassinolide enhanced strawberry fruit quality, phytochemical content, and postharvest life. J. Plant Growth Regul. 2019, 39, 920–929. [Google Scholar]
- Sridhara, S.; Ramesh, N.; Gopakkali, P.; Paramesh, V.; Tamam, N.; Abdelbacki, A.M.M.; Elansary, H.O.; El-Sabrout, A.M.; Abdelmohsen, S.A.M. Application of homobrassinolide enhances growth, yield and quality of tomato. Saudi J. Biol. Sci. 2021, 28, 4800–4806. [Google Scholar] [CrossRef]
- Santos, D.; Alves, L.; Batista; Lemos, B.; Lobato; Silva, A.K. 24-Epibrasinolide delays chlorophyll degradation and stimulates the photosynthetic machinery in magnesium-stressed soybean plants. J. Plant Growth Regul. 2021, 42, 183–198. [Google Scholar] [CrossRef]
- Li, N.; Guo, S.R.; Shu, S.; Sun, J. Effects of exogenous 24-epibrassinolde on leaf morphology and photosynthetic characteristics of tomato seedlings under low light stress. J. Appl. Ecol. 2015, 26, 847–852. [Google Scholar]
- Shu, S.; Tang, Y.Y.; Yuan, Y.H.; Sun, J.; Zhong, M.; Guo, S.R. The role of 24-epibrassinolide in the regulation of photosynthetic characteristics and nitrogen metabolism of tomato seedlings under a combined low temperature and weak light stress. Plant Physiol. Biochem. 2016, 107, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Hu, C.X.; Wang, Y.H. Effects of molybdenum on the intermediates of chlorophyll biosynthesis in winter wheat cultivars under low temperature. Agric. Sci. China 2006, 5, 670–677. [Google Scholar]
- Primka, E.J.; Smith, W.K. Synchrony in fall leaf drop: Chlorophyll degradation, color change, and abscission layer formation in three temperate deciduous tree species. Am. J. Bot. 2019, 106, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Hauenstein, M.; Hortensteiner, S.; Aubry, S. Side-chain modifications of phyllobilins may not be essential for chlorophyll degradation in Arabidopsis. Plant Direct 2022, 6, e441. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.X.; Li, Z.H.; Guo, H.W. New advances in the regulation of leaf senescence by classical and peptide hormones. Front. Recent Dev. Plant Sci. 2022, 13, 923136. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Cheng, S.C.; Cai, J.H.; Wei, B.D.; Zhou, X.; Zhou, Q.; Zhao, Y.B.; Ji, S.J. Chlorophyll degradation and carotenoid biosynthetic pathways: Gene expression and pigment content in broccoli during yellowing. Food Chem. 2019, 297, 124964. [Google Scholar] [CrossRef]
- Zhao, X.; Jia, T.; Hu, X. HCAR is a limitation factor for chlorophyll cycle and chlorophyll b degradation in chlorophyll-b-overproducing plants. Biomolecules 2020, 10, 10121639. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Kim, Y.S.; Yoo, S.C.; Hörtensteiner, S.; Paek, N.C. 7-Hydroxymethyl chlorophyll a reductase functions in metabolic channeling of chlorophyll breakdown intermediates during leaf senescence. Biochem. Biophys. Res. Commun. 2013, 430, 32–37. [Google Scholar] [CrossRef]
- Guan, J.; Fan, X.F.; Yue, Y.S.; Xu, L.X.; Teng, K.; Yin, S.X. Integrative transcriptome and chlorophyll fluorescence test analysis shed new light on the leaf senescence mechanism of Zoysia japonica. Agronomy 2023, 13, 623. [Google Scholar] [CrossRef]
- Teng, K.; Yve, Y.; Zhang, H.; Li, H.; Xu, L.X.; Han, C.; Fan, X.F.; Wu, J.Y. Functional characterization of the pheophytinase gene ZjPPH, from Zoysia japonica in regulating chlorophyll degradation and photosynthesis. Front. Recent Dev. Plant Sci. 2021, 12, 786570. [Google Scholar] [CrossRef]
- Peng, R.N.; Sun, W.Y.; Jin, X.X.; Yu, L.J.; Chen, C.; Yue, Z.H.; Dong, Y.I. Analysis of 2,4-epibrassinolide created an enhancement tolerance on Cd toxicity in Solanum nigrum L. Environ. Sci. Pollut. Res. 2020, 27, 16784–16797. [Google Scholar] [CrossRef]
- Khatypov, R.A.; Khmelnitskiy, A.Y.; Leonova, M.M.; Vasilieva, L.G.; Shuvalov, V.A. Primary light-energy conversion in tetrameric chlorophyll structure of photosystem II and bacterial reaction centers: I. A review. Photosynth. Res. 2008, 98, 81–93. [Google Scholar] [CrossRef]
- Çağ, S.; Gören-Sağlam, N.; Çıngıl-Barış, Ç.; Kaplan, E. The effect of different concentration of epibrassinolide on chlorophyll, protein and anthocyanin content and peroxidase activity in excised red cabbage (Brassica oleraceae L.) Cotyledons. Biotechnol. Biotechnol. Equip. 2007, 21, 422–425. [Google Scholar] [CrossRef]


| Period (Stage) | Treatment Concentration and Dosage of 24-EBL | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1.04 × 10−6 mol·L−1 | 1.67 × 10−6 mol·L−1 | |||||||
| 45 DAS (CK) | 100 mL | 100 mL | 100 mL | 100 mL | 100 mL | 100 mL | 100 mL | 100 mL | 100 mL |
| 52 DAS (The first stage) | √ | 100 mL | 100 mL | √ | 100 mL | 100 mL | √ | 100 mL | 100 mL |
| 59 DAS (The second stage) | √ | 100 mL | √ | 100 mL | √ | 100 mL | |||
| 66 DAS (The third stage) | √ | √ | √ | ||||||
| Gene Name | Sequences of Forward Primers (5′–3′) | Sequences of Reverse Primers (5′–3′) |
|---|---|---|
| AgTUB-B | TGGTGGCACTGGATCTGGTATGG | ACTTTCGGAGGAGGGAAGACTGAA |
| AgHEML | GTTGTCTTATGGCGGTGCTCAAG | GTGGATTCCTGCCGTCATTGC |
| AgCAO | TTGGTGAATGATAGGCTGTT | GGATGGTAAGGTTGGACTG |
| AgCHLG | CGCCTGACATAATTGTTCTTACACTCT | CACATCAATAGCACCAACACATATCCA |
| AgCHL | GCTTCATTGTCATTGCTCCTCAGTTAT | CCTTCAGATAGCCACTTGGTTATTGC |
| AgNYC | GGATGCGAGTAGTAGTAAAGGGAAATGGAA | TGGCGTCTGTAATTGATAACCGAGTC |
| AgHCAR | CAGTGGACAGGCATAGTGACAACAA | ACTTCCTCTGGCGTCCTTGCTAA |
| AgPPH | CAAGCAGAGGCATCACAA | CCGTATAATGGAGACAACAAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Wang, L.-X.; Li, M.-Y.; Tan, G.-F.; Zhang, E.-J.; Liu, P.-Z.; Liu, H.; Tao, J.-P.; Shu, S.; Zhou, J.-H.; et al. Effects of Exogenous 24-Epibrassinolide Leaves Spraying Application on Chlorophyll Accumulation and Gene Expression Profiles of Chlorophyll Metabolism in Celery. Horticulturae 2023, 9, 1279. https://doi.org/10.3390/horticulturae9121279
Chen C, Wang L-X, Li M-Y, Tan G-F, Zhang E-J, Liu P-Z, Liu H, Tao J-P, Shu S, Zhou J-H, et al. Effects of Exogenous 24-Epibrassinolide Leaves Spraying Application on Chlorophyll Accumulation and Gene Expression Profiles of Chlorophyll Metabolism in Celery. Horticulturae. 2023; 9(12):1279. https://doi.org/10.3390/horticulturae9121279
Chicago/Turabian StyleChen, Chen, Li-Xiang Wang, Meng-Yao Li, Guo-Fei Tan, Er-Jin Zhang, Pei-Zhuo Liu, Hui Liu, Jian-Ping Tao, Sheng Shu, Jian-Hua Zhou, and et al. 2023. "Effects of Exogenous 24-Epibrassinolide Leaves Spraying Application on Chlorophyll Accumulation and Gene Expression Profiles of Chlorophyll Metabolism in Celery" Horticulturae 9, no. 12: 1279. https://doi.org/10.3390/horticulturae9121279
APA StyleChen, C., Wang, L.-X., Li, M.-Y., Tan, G.-F., Zhang, E.-J., Liu, P.-Z., Liu, H., Tao, J.-P., Shu, S., Zhou, J.-H., & Xiong, A.-S. (2023). Effects of Exogenous 24-Epibrassinolide Leaves Spraying Application on Chlorophyll Accumulation and Gene Expression Profiles of Chlorophyll Metabolism in Celery. Horticulturae, 9(12), 1279. https://doi.org/10.3390/horticulturae9121279










