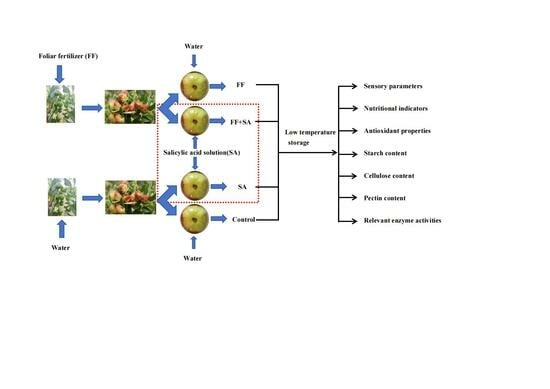
Preharvest Foliar Spraying Combined with Postharvest Salicylic Acid Treatment Regulates Panzao (Ziziphus jujuba Mill. cv. ‘Jingcang1’) Fruit Quality and Softening during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Storage Conditions
2.2. Firmness, Weight Loss Rate, Transverse and Longitudinal Diameter Loss Rates, and Color Differences
2.3. TSS, TA, SS, AsA, Total Phenols, Flavonoids, and SP Content
2.4. Determination of Antioxidant Properties
2.5. Determination of Starch and Cell Wall Material Content
2.5.1. Determination of Starch Content
2.5.2. Determination of Cellulose Content
2.5.3. Determination of Pectin Content
2.6. Determination of Relevant Enzyme Activities
2.6.1. Determination of Amylase Activity
2.6.2. Cellulase (Cx), Polygalacturonase (PG), and β-glucosidase (β-glu) Activities
2.7. Data Processing and Statistical Analysis
3. Results
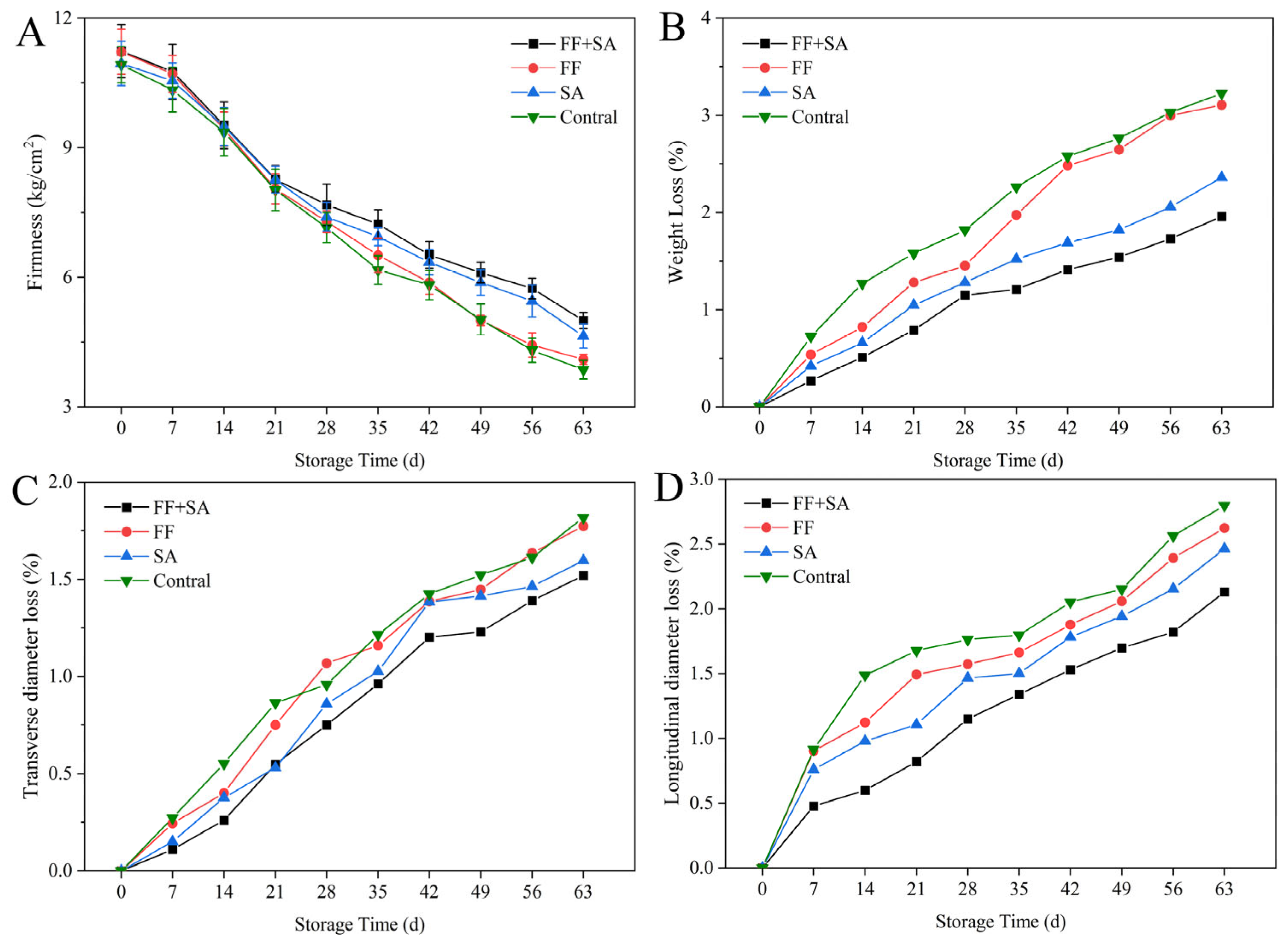
3.1. Firmness, Weight Loss Rate, Cross-Diameter Loss Rate, and Longitudinal Loss Rate
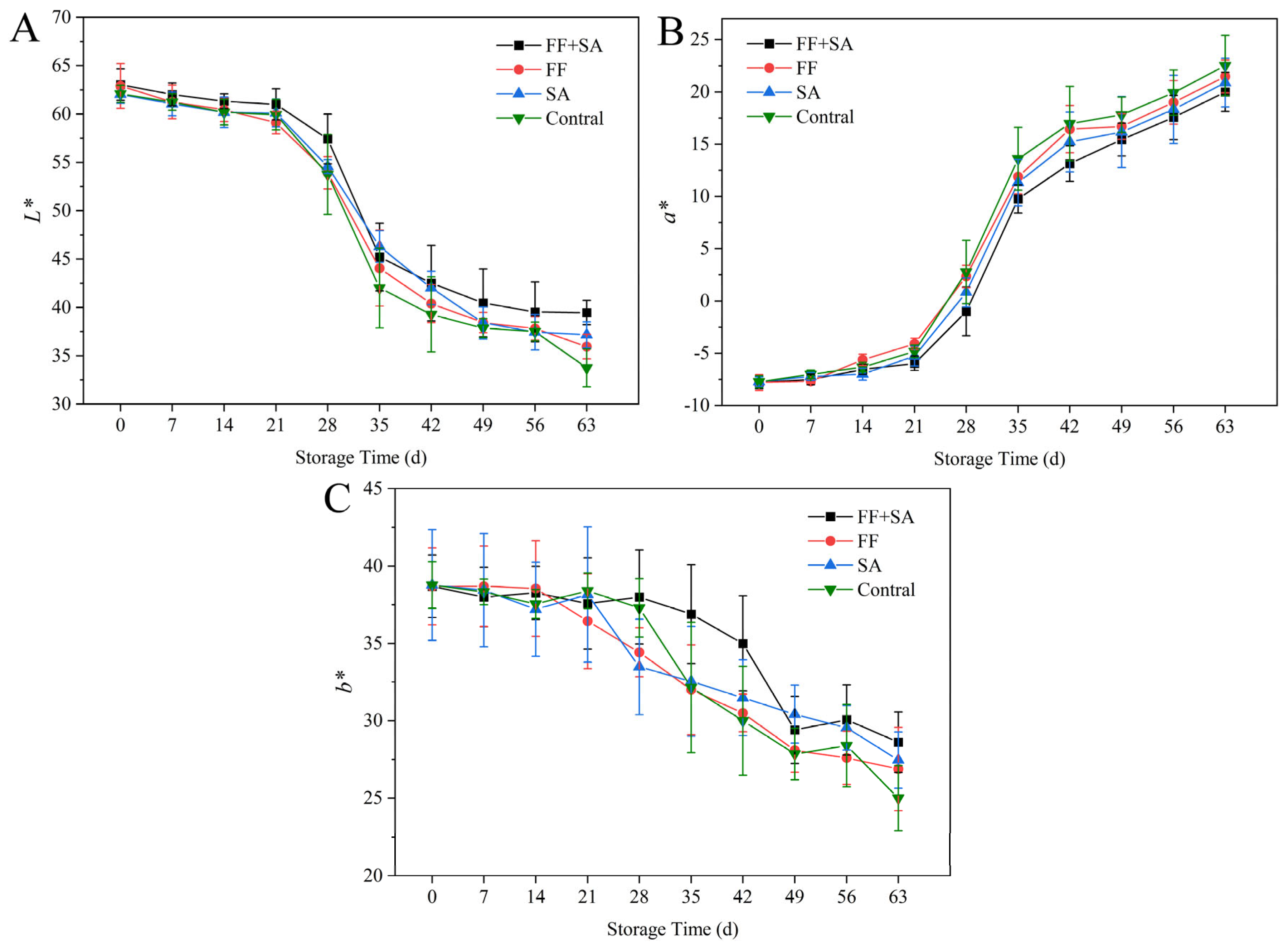
3.2. Fruit Chromatic Value
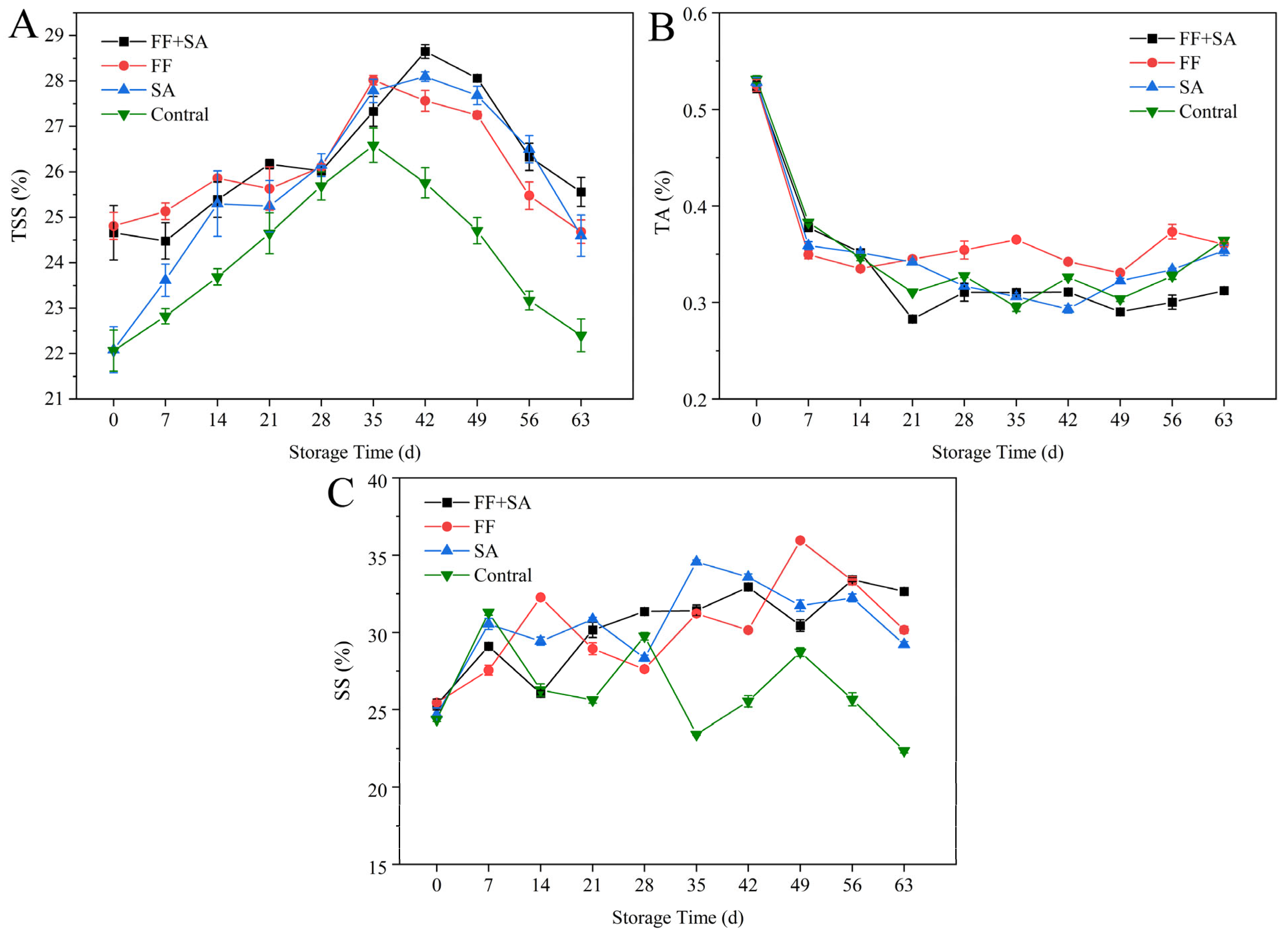
3.3. Total Soluble Solid, Titratable Acid, and Soluble Sugar
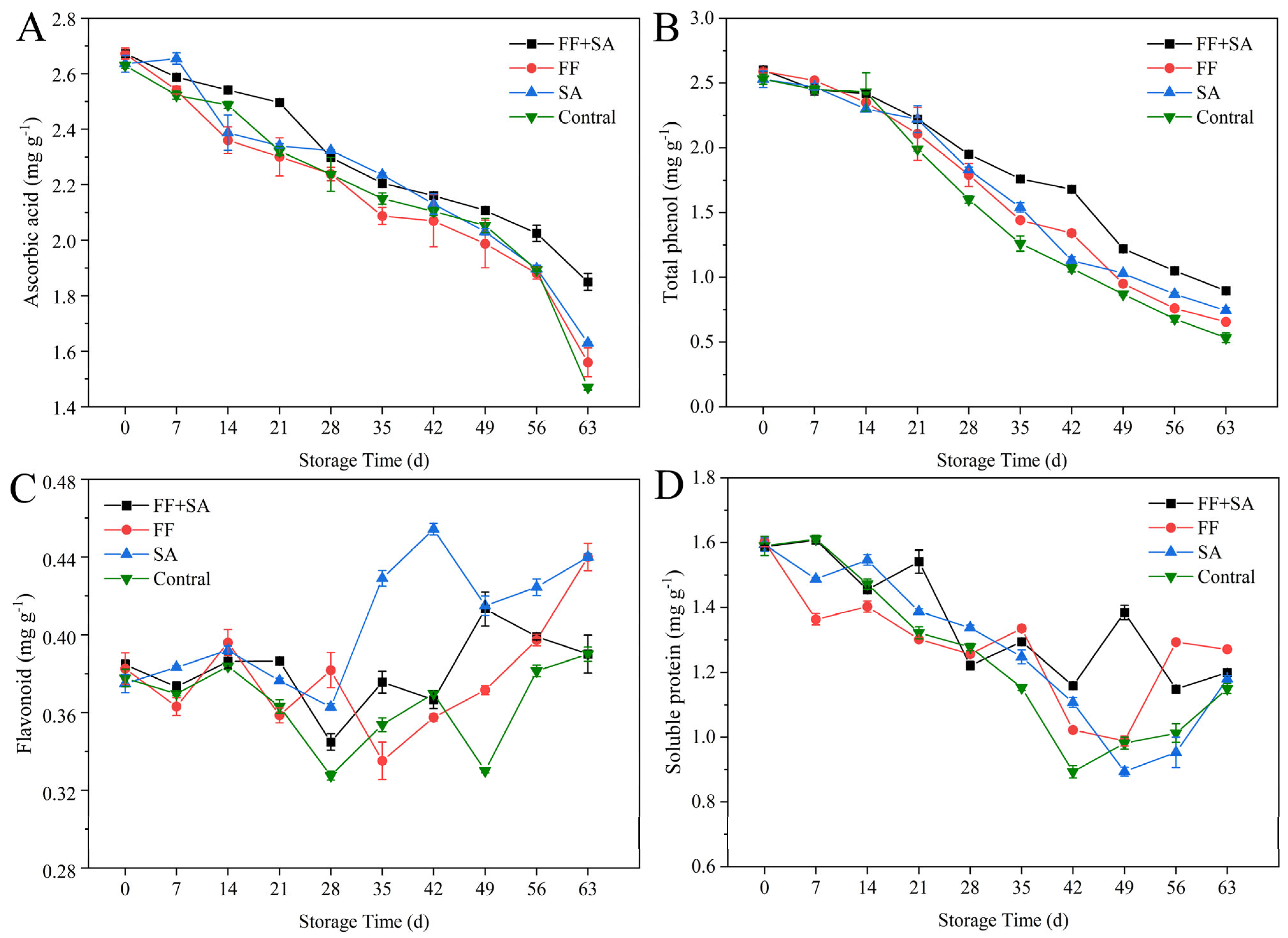
3.4. Ascorbic Acid, Total Phenol, Flavonoid, and Soluble Protein
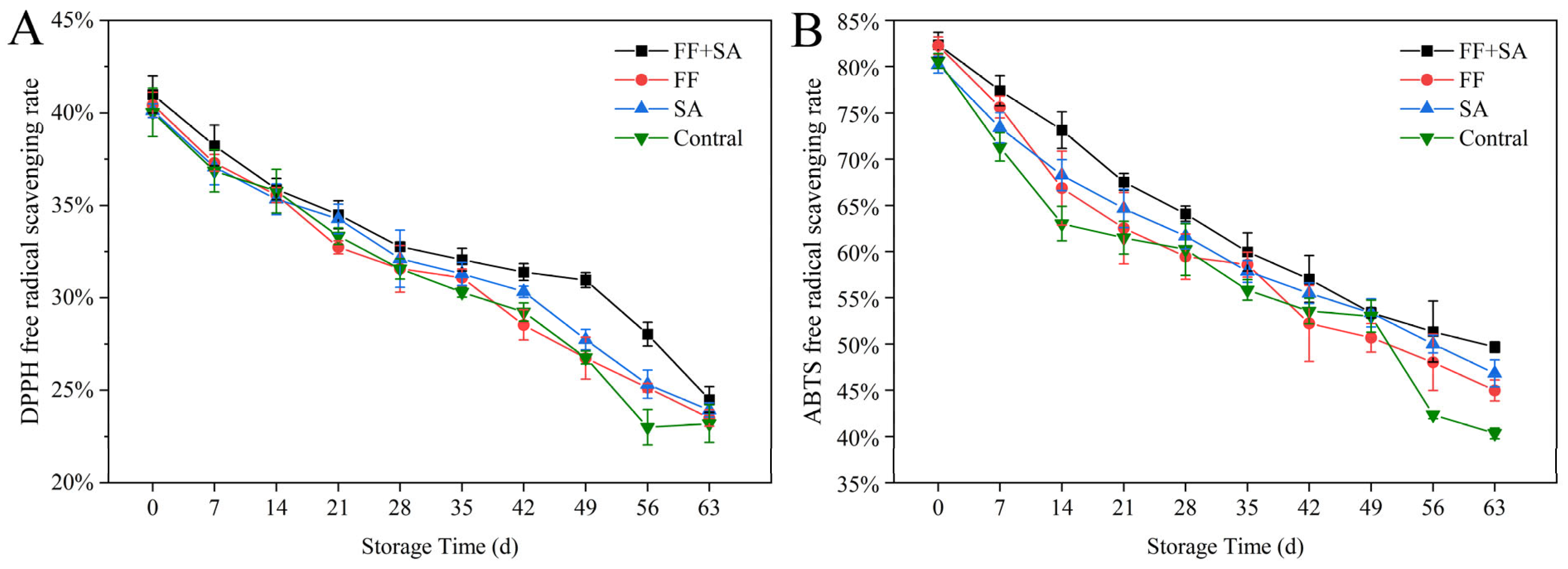
3.5. Antioxidant Activity
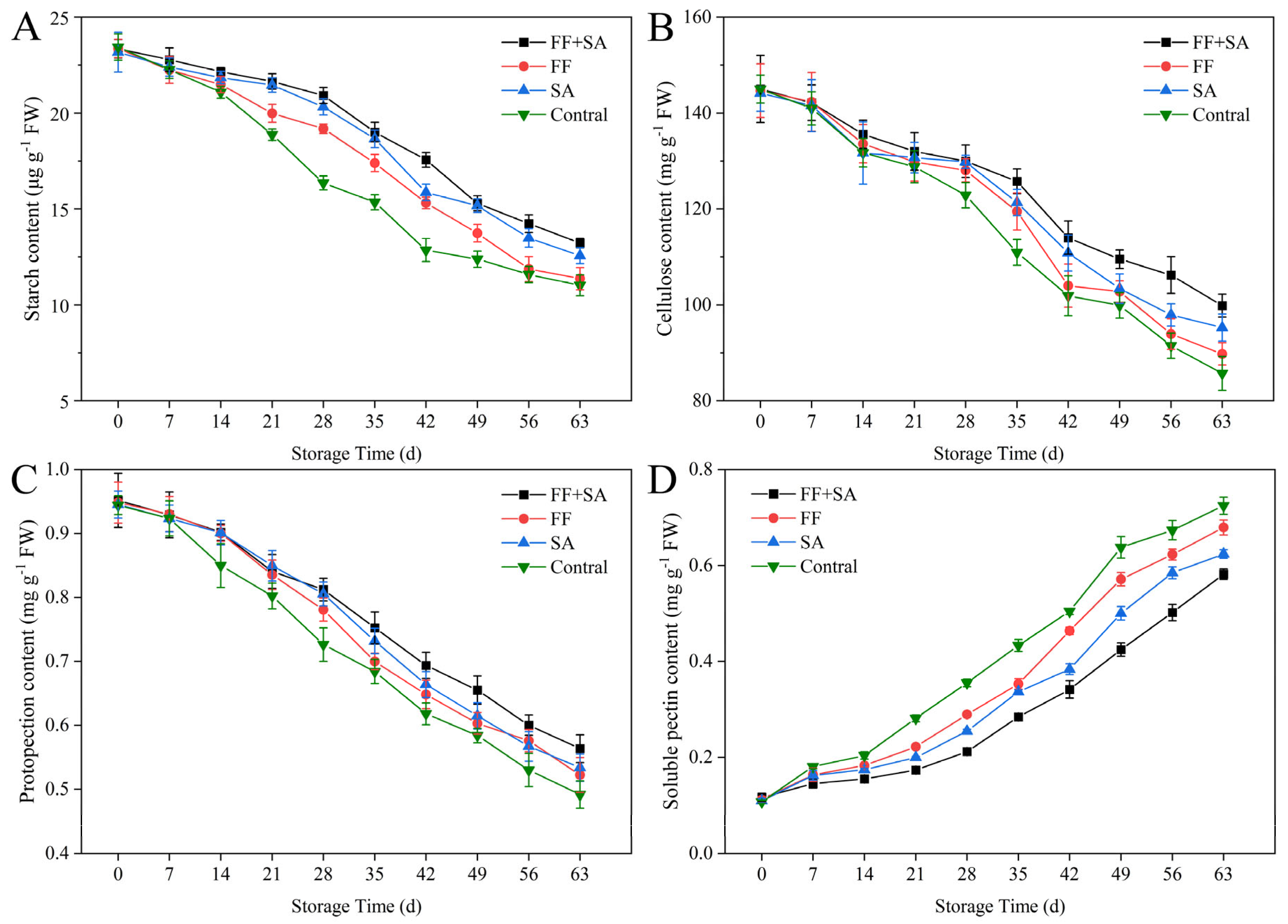
3.6. Starch and Cell Wall Material
3.7. Enzyme Activities
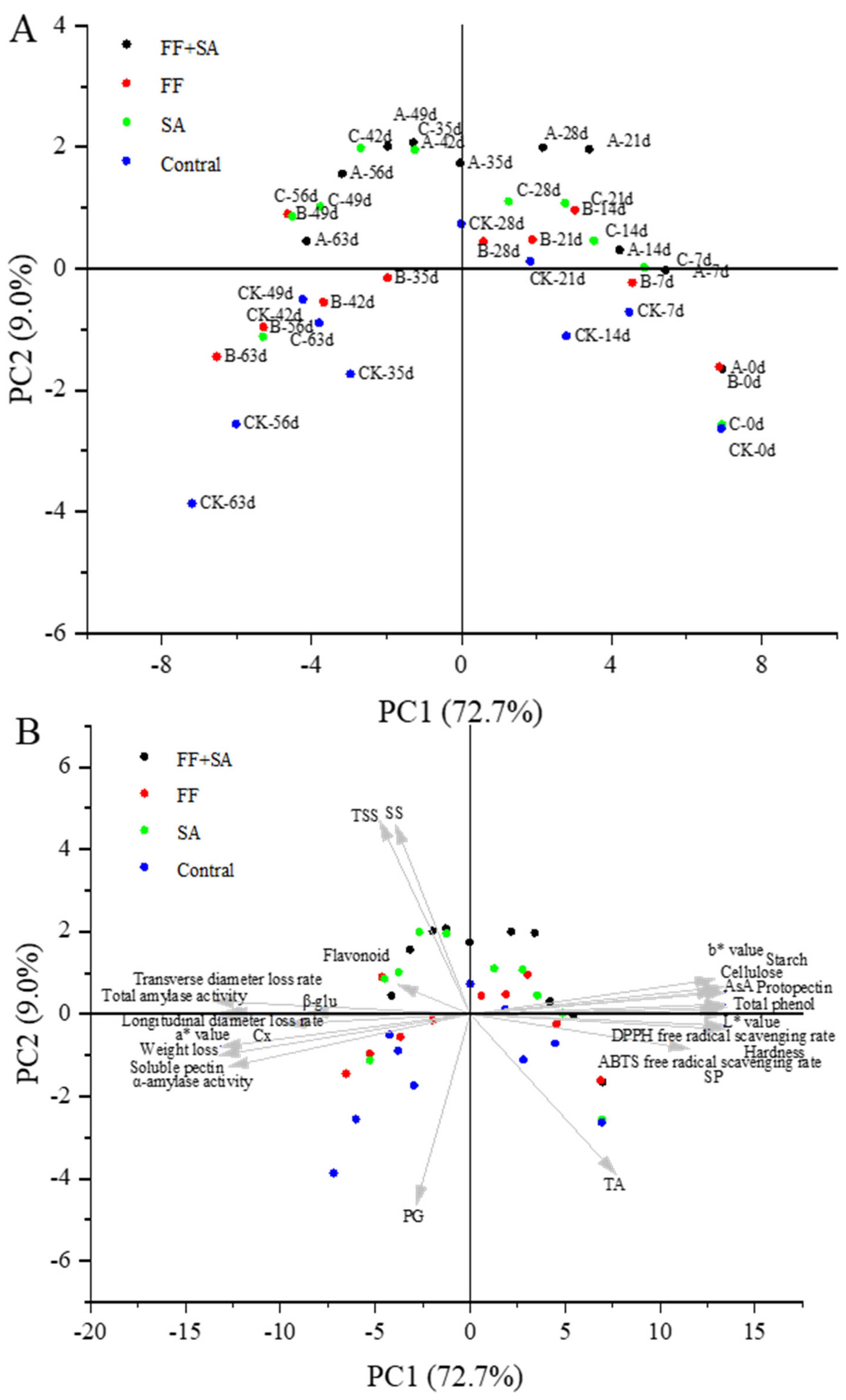
3.8. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Geng, Y.; Zhao, X.M.; Tan, Y.P.; Bao, L.W.; Xie, N.B.; Zhang, T.; Meng, Y.N. Effect of salicylic acid combined with microporous plastic bag on quality of Ziziphus jujube Mill. cv. ‘Jingcang1’ during storage. Sci. Technol. Food Ind. 2023, 44, 338–346. [Google Scholar]
- Zhang, W.L.; Pan, Y.G.; Jiang, Y.M.; Zhang, Z.K. Advances in gas fumigation technologies for postharvest fruit preservation. Crit. Rev. Food Sci. Nutr. 2023, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Reche, J.; García-Pastor, M.E.; Valero, D.; Hernández, F.; Almansa, M.S.; Legua, P.; Amorós, A. Effect of modified atmosphere packaging on the physiological and functional characteristics of Spanish jujube (Ziziphus jujuba Mill.) cv ‘Phoenix’ during cold storage. Sci. Hortic. 2019, 258, 108743. [Google Scholar] [CrossRef]
- Lu, X.G.; Meng, G.L.; Jin, W.G.; Gao, H. Effects of 1-MCP in combination with Ca application on aroma volatiles production and softening of ‘Fuji’ apple fruit. Sci. Hortic. 2018, 229, 91–98. [Google Scholar] [CrossRef]
- Nhi, T.T.Y.; Phat, D.T.; Truong, L.D.; Tri Nhut, P.; Long, H.B.; Quyen, T.N.; Giang, B.L. Antimicrobial activities of flavedo peel extract and its feasibility in the development of bio-based pectin coating film for fruit preservation. J. Food Saf. 2022, 42, e13013. [Google Scholar] [CrossRef]
- Khurshid, T.; McNeil, D.L.; Trought, M.C.T. Effect of foliar-applied gibberellins and soil-applied paclobutrazol on fruit quality at harvest and during storage of ‘Braebum’ apples growing under a high-density planting system. N. Z. J. Crop Hortic. Sci. 1997, 25, 59–65. [Google Scholar] [CrossRef]
- Yin, N.; Mu, L.; Liang, Y.L.; Hao, W.L.; Yin, H.F.; Zhu, S.M.; An, X.J. Effects of foliar selenium fertilizer on fruit yield, quality and selenium content of three varieties of Vitis vinifera. Yingyong Shengtai Xuebao 2020, 31, 953–958. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, N.; Misra, K.K.; Nirgude, V. Effect of foliar spray of calcium chloride and boric acid on shelf-life of guava. Indian J. Hortic. 2017, 74, 586–590. [Google Scholar] [CrossRef]
- Ali, I.; Abbasi, N.A.; Hafiz, I. Application of calcium chloride at different phenological stages alleviates chilling injury and delays climacteric ripening in peach fruit during low-temperature storage. Int. J. Fruit Sci. 2021, 21, 1040–1058. [Google Scholar] [CrossRef]
- Zydlik, Z.; Zydlik, P.; Kafkas, N.E.; Yesil, B.; Cieśliński, S. Foliar application of some macronutrients and micronutrients improves yield and fruit quality of Highbush Blueberry (Vaccinium corymbosum L.). Horticulturae 2022, 8, 664. [Google Scholar] [CrossRef]
- Prakash, R.; Jokhan, A.D.; Singh, R. Effects of foliar application of gibberellic acid, boric acid and sucrose on noni (M. citrifolia L.) fruit growth and quality. Sci. Hortic. 2022, 301, 111098. [Google Scholar] [CrossRef]
- Moale, C.; Ghiurea, M.; Sîrbu, C.E.; Somoghi, R.; Cioroianu, T.M.; Faraon, V.A.; Lupu, C.; Trică, B.; Constantinescu-Aruxandei, D.; Oancea, F. Effects of siliceous natural nanomaterials applied in combination with foliar fertilizers on physiology, yield and fruit quality of the apricot and peach trees. Plants 2021, 10, 2395. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic acid as a safe plant protector and growth regulator. Plant Pathol. J. 2020, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Sun, C.C.; Wang, Y.H.; Gong, H.S.; Zhang, A.D.; Yang, Y.Q.; Guo, F.J.; Cui, K.B.; Fan, X.G.; Li, X.L. The preharvest and postharvest application of salicylic acid and its derivatives on storage of fruit and vegetables: A review. Sci. Hortic. 2023, 312, 111858. [Google Scholar] [CrossRef]
- Geng, Y.; Zhao, X.M.; Tan, Y.P.; Wang, Y.H.; Lin, J.; Fan, D.Y. Effects of Different Foliar Fertilizer Treatments on the Growth and Storability of Ziziphus Jujube Mill. cv. ‘Jingcang1’. Soil Fertil. Sci. China 2023, 2, 186–194. [Google Scholar]
- Geng, Y.; Zhao, X.M.; Tan, Y.P.; Fan, D.Y.; Li, B.B.; Ma, S.J. Effects of harvest maturity on storage quality and antioxidative capacity of Ziziphus jujube Mill. cv. ‘Jingcang1’. Food Ferment. Ind. 2023, 49, 269–275. [Google Scholar] [CrossRef]
- Cao, J.K.; Jiang, W.B.; Zhao, Y.M. Guidance of Postharvest Physiological and Biochemical Experiment of Fruits and Vegetables; China Light Industry Press: Beijing, China, 2007; pp. 78–82,85–88. Available online: https://max.book118.com/html/2019/0824/5242233104002121.shtm (accessed on 1 February 2023).
- Liu, Y.N.; Wang, Y.; Bi, Y.; Li, S.E.; Jiang, H.; Zhu, Y.; Wang, B. Effect of preharvest acetylsalicylic acid treatments on ripening and softening of harvested muskmelon fruit. Sci. Agric. Sin. 2017, 50, 1865–1875. [Google Scholar] [CrossRef]
- Pritts, M.P.; Reickenberg, R.L. Dynamics of nutrient uptake from foliar fertilizers in red raspberry (Rubus idaeus L.). J. Am. Soc. Hortic. Sci. 1996, 121, 158–163. [Google Scholar] [CrossRef]
- Tóth, B.; Moloi, M.J.; Mousavi, S.M.N.; Illés, Á.; Bojtor, C.; Szőke, L.; Nagy, J. The evaluation of the effects of Zn, and amino acid-containing foliar fertilizers on the physiological and biochemical responses of a Hungarian fodder corn hybrid. Agronomy 2022, 12, 1523. [Google Scholar] [CrossRef]
- Fan, X.; Du, Z.; Cui, X.; Ji, W.; Ma, J.; Li, X.; Wang, X.; Zhao, H.; Liu, B.; Guo, F.; et al. Preharvest methyl salicylate treatment enhance the chilling tolerance and improve the postharvest quality of apricot during low temperature storage. Postharvest Biol. Technol. 2021, 177, 111535. [Google Scholar] [CrossRef]
- Kumar, N.; Tokas, J.; Raghavendra, M.; Singal, H.R. Impact of exogenous salicylic acid treatment on the cell wall metabolism and ripening process in postharvest tomato fruit stored at ambient temperature. Int. J. Food Sci. Technol. 2021, 56, 2961–2972. [Google Scholar] [CrossRef]
- Hanif, A.; Ahmad, S.; Shahzad, S.; Liaquat, M.; Anwar, R. Postharvest application of salicylic acid reduced decay and enhanced storage life of papaya fruit during cold storage. Food Meas. 2020, 14, 3078–3088. [Google Scholar] [CrossRef]
- Tang, Y.; Kuang, J.; Wang, F.; Chen, L.; Hong, K.; Xiao, Y.; Xie, H.; Lu, W.; Chen, J. Molecular characterization of PR and WRKY genes during SA- and MeJA-induced resistance against Colletotrichum musae in banana fruit. Postharvest Biol. Technol. 2013, 79, 62–68. [Google Scholar] [CrossRef]
- Gomes, E.P.; Vanz Borges, C.V.; Monteiro, G.C.; Filiol Belin, M.A.; Minatel, I.O.; Pimentel Junior, A.; Tecchio, M.A.; Lima, G.P.P. Preharvest salicylic acid treatments improve phenolic compounds and biogenic amines in ‘Niagara Rosada’ table grape. Postharvest Biol. Technol. 2021, 176, 111505. [Google Scholar] [CrossRef]
- Wang, L.B.; Li, X.H.; Bai, J.H.; Luo, H.B.; Jin, C.H.; Hui, J.; Yu, Z.F. Residual impact of methyl salicylate fumigation at the breaker stage on C6 volatile biopathway in red tomato fruit. J. Food Process. Preserv. 2017, 41, 13285. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Z.; Su, Y.; Liu, D.; Ye, X. Effect of salicylic acid treatment on postharvest quality, antioxidant activities, and free polyamines of asparagus. J. Food Sci. 2011, 76, S126–S132. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.T.; Kang, J.W.; Liu, Y.X.; Guo, M.R.; Chen, G.G. Effect of salicylic acid treatment on antioxidant capacity and endogenous hormones in winter jujube during shelf life. Food Chem. 2022, 397, 133788. [Google Scholar] [CrossRef]
- Sayyari, M.; Esna-Ashari, M.; Tarighi, T.H. Impacts of salicylic acid, chitosan, and salicyloyl chitosan on quality preservation and microbial load reduction in strawberry fruits during cold storage. J. Food Process. Preserv. 2022, 46, e16710. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, C.; Xie, J. Effect of salicylic acid treatment on alleviating postharvest chilling injury of ‘Qingnai’ plum fruit. Postharvest Biol. Technol. 2011, 62, 115–120. [Google Scholar] [CrossRef]
- Cai, H.; Yuan, X.; Pan, J.; Li, H.; Wu, Z.; Wang, Y. Biochemical and proteomic analysis of grape berries (Vitis labruscana) during cold storage upon postharvest salicylic acid treatment. J. Agric. Food Chem. 2014, 62, 10118–10125. [Google Scholar] [CrossRef]
- Li, J.Z.; Min, D.D.; Li, Z.L.; Fu, X.D.; Zhao, X.M.; Wang, J.H.; Zhang, X.H.; Li, F.J.; Li, X.A.; Guo, Y.Y. Regulation of sugar metabolism by methyl jasmonate to improve the postharvest quality of tomato fruit. J. Plant Growth Regul. 2022, 41, 1615–1626. [Google Scholar] [CrossRef]
- Gabioud Rebeaud, S.; Cioli, L.; Cotter, P.-Y.; Christen, D. Cultivar, maturity at harvest and postharvest treatments influence softening of apricots. Postharvest Biol. Technol. 2023, 195, 112134. [Google Scholar] [CrossRef]
- Ren, Y.Y.; Sun, P.P.; Wang, X.X.; Zhu, Z.Y. Degradation of cell wall polysaccharides and change of related enzyme activities with fruit softening in Annona squamosa during storage. Postharvest Biol. Technol. 2020, 166, 111203. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Zhu, X.; Hou, Y.Y.; Wang, X.Y.; Li, X.H. Effects of nitric oxide fumigation treatment on retarding cell wall degradation and delaying softening of winter jujube (Ziziphus jujuba Mill. cv. cv. Dongzao) fruit during storage. Postharvest Biol. Technol. 2019, 156, 110954. [Google Scholar] [CrossRef]
- Shi, Z.J.; Yang, H.Y.; Jiao, J.Y.; Wang, F.; Lu, Y.Y.; Deng, J. Effects of graft copolymer of chitosan and salicylic acid on reducing rot of postharvest fruit and retarding cell wall degradation in grapefruit during storage. Food Chem. 2019, 283, 92–100. [Google Scholar] [CrossRef]
- Brummell, D.A. Cell wall disassembly in ripening fruit. Funct. Plant Biol. 2006, 33, 103–119. [Google Scholar] [CrossRef]
- Sinha, A.; Gill, P.P.S.; Jawandha, S.K.; Grewal, S.K. Composite coating of chitosan with salicylic acid retards pear fruit softening under cold and supermarket storage. Food Res. Int. 2022, 160, 111724. [Google Scholar] [CrossRef]
- Li, Y.L.; He, H.; Hou, Y.Y.; Kelimu, A.; Wu, F.; Zhao, Y.T.; Shi, L.; Zhu, X. Salicylic acid treatment delays apricot (Prunus armeniaca L.) fruit softening by inhibiting ethylene biosynthesis and cell wall degradation. Sci. Hortic. 2022, 300, 111061. [Google Scholar] [CrossRef]


| Indicators | PC1 | PC2 |
|---|---|---|
| Firmness | 0.23071 | −0.03907 |
| Weight loss | −0.22551 | −0.08505 |
| Transverse diameter loss rate | −0.23215 | 0.03392 |
| Longitudinal diameter loss rate | −0.22527 | 0.00497 |
| L* value | 0.22644 | 0.01097 |
| a* value | −0.22678 | −0.00648 |
| b* value | 0.22127 | 0.09139 |
| TSS | −0.08195 | 0.50447 |
| TA | 0.13174 | −0.41801 |
| SS | −0.06772 | 0.49485 |
| AsA | 0.2239 | 0.0589 |
| Total phenol | 0.23044 | 0.02615 |
| Flavonoid | −0.06574 | 0.07779 |
| SP | 0.19798 | −0.09027 |
| ABTS free radical scavenging rate | 0.22943 | −0.02986 |
| DPPH free radical scavenging rate | 0.22988 | 8.8183 × 10−5 |
| Starch | 0.22905 | 0.07139 |
| Cellulose | 0.23061 | 0.05648 |
| Protopectin | 0.23136 | 0.01815 |
| Soluble pectin | −0.22685 | −0.10698 |
| Total amylase activity | −0.21989 | 0.00355 |
| α-amylase activity | −0.2188 | −0.13681 |
| Cx | −0.1669 | −0.02606 |
| PG | −0.04918 | −0.49871 |
| β-glu | −0.14691 | 0.00473 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, Y.; Li, B.; Zhang, P.; Yang, L.; Zhao, X.; Tan, Y. Preharvest Foliar Spraying Combined with Postharvest Salicylic Acid Treatment Regulates Panzao (Ziziphus jujuba Mill. cv. ‘Jingcang1’) Fruit Quality and Softening during Storage. Horticulturae 2023, 9, 1260. https://doi.org/10.3390/horticulturae9121260
Geng Y, Li B, Zhang P, Yang L, Zhao X, Tan Y. Preharvest Foliar Spraying Combined with Postharvest Salicylic Acid Treatment Regulates Panzao (Ziziphus jujuba Mill. cv. ‘Jingcang1’) Fruit Quality and Softening during Storage. Horticulturae. 2023; 9(12):1260. https://doi.org/10.3390/horticulturae9121260
Chicago/Turabian StyleGeng, Yang, Binbin Li, Ping Zhang, Lian Yang, Xiaomei Zhao, and Yupeng Tan. 2023. "Preharvest Foliar Spraying Combined with Postharvest Salicylic Acid Treatment Regulates Panzao (Ziziphus jujuba Mill. cv. ‘Jingcang1’) Fruit Quality and Softening during Storage" Horticulturae 9, no. 12: 1260. https://doi.org/10.3390/horticulturae9121260
APA StyleGeng, Y., Li, B., Zhang, P., Yang, L., Zhao, X., & Tan, Y. (2023). Preharvest Foliar Spraying Combined with Postharvest Salicylic Acid Treatment Regulates Panzao (Ziziphus jujuba Mill. cv. ‘Jingcang1’) Fruit Quality and Softening during Storage. Horticulturae, 9(12), 1260. https://doi.org/10.3390/horticulturae9121260