Cucumber Bioassay and HPLC Analysis to Detect Diuron Residues in Remineralized Soils Following Canavalia ensiformis Cultivation as a Phytoremediator
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Area and Soils Used
2.2. Experimental Design and Treatments
2.3. Biometric Evaluations and Injury Level of Canavalia ensiformis
2.4. Analysis of Bioavailable Diuron Residues in Soil Solution Using a Bioassay
2.5. Analysis of Diuron Residues by High Performance Liquid Chromatography
2.6. Analysis of Diuron Residues by High Performance Liquid Chromatography
2.7. Statistical Analysis
3. Results
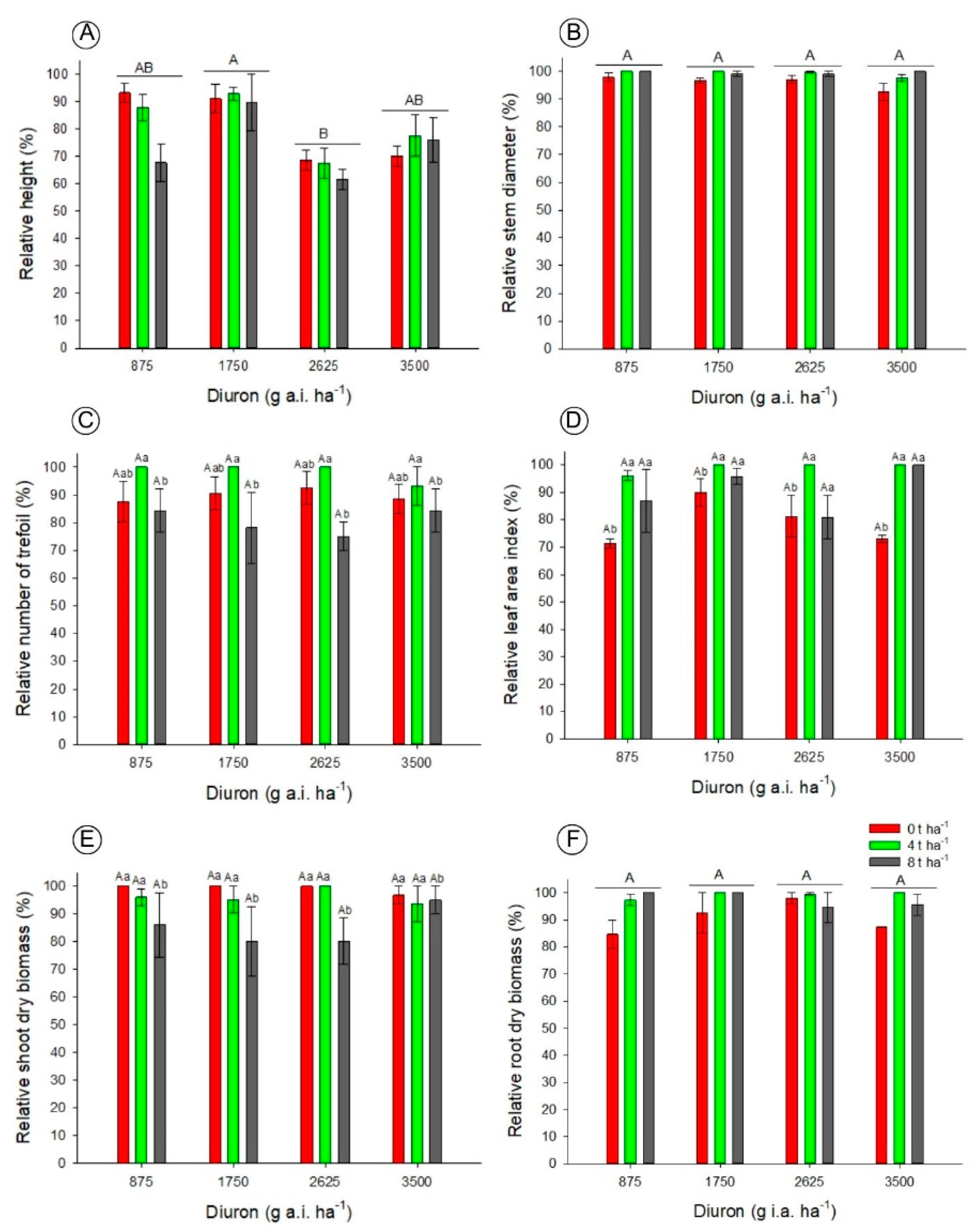
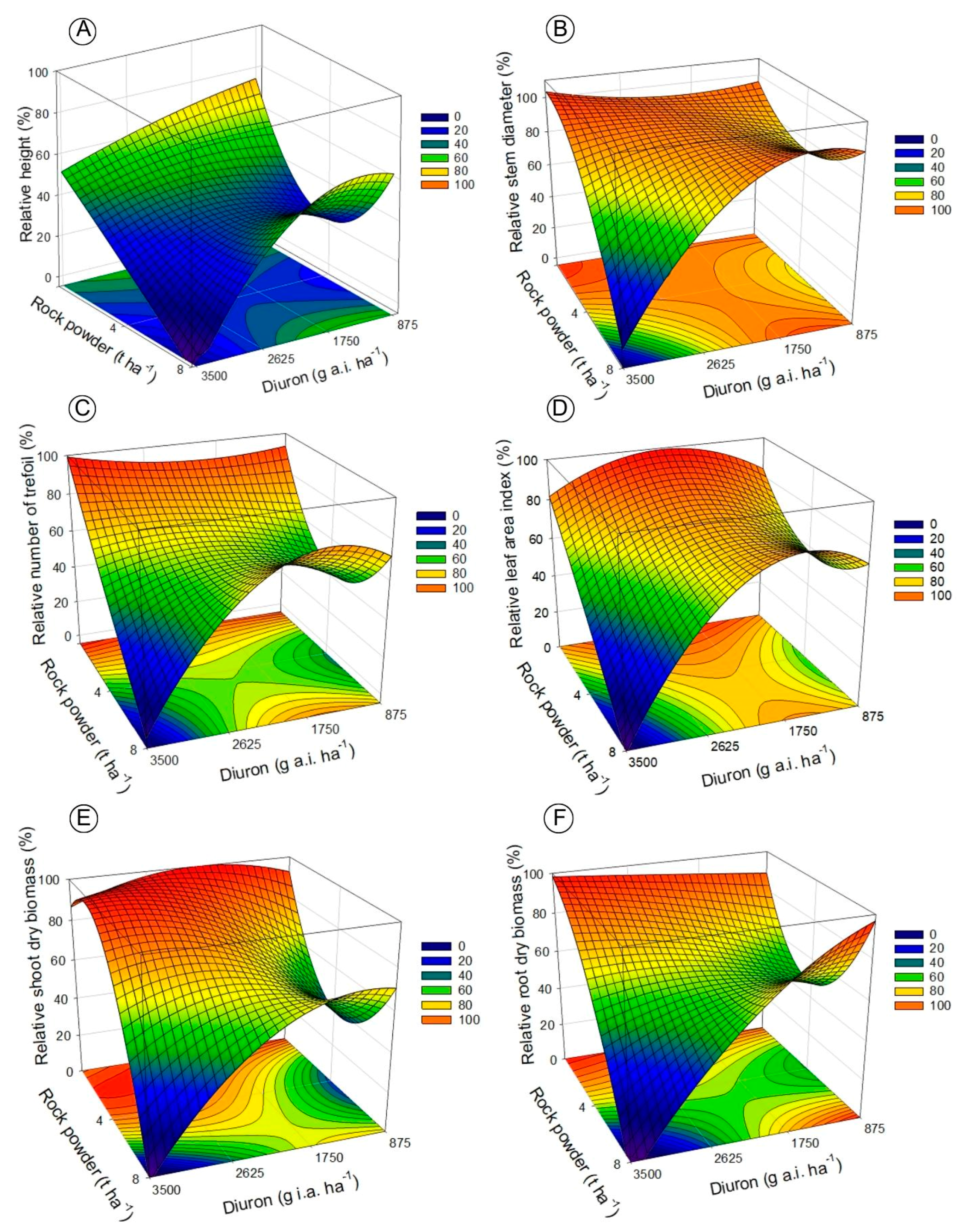
3.1. Biometric and Injury Level Analyses of Canavalia ensiformis in Oxisol and Inceptisol
3.2. Diuron Residue Analysis with Bioassay in Oxisol and Inceptisol
3.3. Analysis of Diuron Residue Analyzed using High Performance Liquid Chromatography
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. Pesticides Use, Pesticides Trade and Pesticides Indicators–Global, Regional and Country Trends, 1990–2020. FAOSTAT Anal. Briefs 2022, 46, 1–13. [Google Scholar] [CrossRef]
- Matzrafi, M.; Seiwert, B.; Reemtsma, T.; Rubin, B.; Peleg, Z. Climate Change Increases the Risk of Herbicide-Resistant Weeds Due to Enhanced Detoxification. Planta 2016, 244, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M.N.; Barceló, D. Persistence of Pesticides-Based Contaminants in the Environment and Their Effective Degradation Using Laccase-Assisted Biocatalytic Systems. Sci. Total Environ. 2019, 695, 133896. [Google Scholar] [CrossRef] [PubMed]
- Mendes, K.F.; Régo, A.P.J.; Takeshita, V.; Tornisielo, V.L. Water Resource Pollution by Herbicide Residues. In Biochemical Toxicology: Heavy Metals and Nanomaterials; BoD—Books on Demand: Norderstedt, Germany, 2020; ISBN 978-1-78984-696-6. [Google Scholar]
- Li, J.; Zhang, W.; Lin, Z.; Huang, Y.; Bhatt, P.; Chen, S. Emerging Strategies for the Bioremediation of the Phenylurea Herbicide Diuron. Front. Microbiol. 2021, 12, 686509. [Google Scholar] [CrossRef] [PubMed]
- Silva Moretto, J.A.; Rueda Furlan, J.P.; Tonelli Fernandes, A.F.; Bauermeister, A.; Lopes, N.P.; Stehling, E.G. Alternative Biodegradation Pathway of the Herbicide Diuron. Int. Biodeterior. Biodegrad. 2019, 143, 104716. [Google Scholar] [CrossRef]
- Muendo, B.M.; Shikuku, V.O.; Getenga, Z.M.; Lalah, J.O.; Wandiga, S.O.; Rothballer, M. Adsorption-Desorption and Leaching Behavior of Diuron on Selected Kenyan Agricultural Soils. Heliyon 2021, 7, e06073. [Google Scholar] [CrossRef]
- Alba, L.-M.; Esmeralda, M.; Jaime, V. Enhanced Biodegradation of Phenylurea Herbicides by Ochrobactrum Anthrophi CD3 Assessment of Its Feasibility in Diuron-Contaminated Soils. Int. J. Environ. Res. Public Health 2022, 19, 1365. [Google Scholar] [CrossRef]
- PPDB—Banco de Dados de Propriedades de Pesticidas. Available online: http://sitem.herts.ac.uk/aeru/ppdb/ (accessed on 27 October 2023).
- Calegari, R.P.; Mendes, K.F.; Martins, B.C.; Pimpinato, R.F.; Baptista, A.S.; Tornisielo, V.L. Removal of Diuron and Hexazinone from Public Water Supply Using a Filter System. Planta Daninha 2018, 36, e018183662. [Google Scholar] [CrossRef]
- Chen, B.; Wang, X. Combined Approach for Determining Diuron in Sugarcane and Soil: Ultrasound-Assisted Extraction, Carbon Nanotube-Mediated Purification, and Gas Chromatography–Electron Capture Detection. J. Food Sci. 2019, 84, 2402–2411. [Google Scholar] [CrossRef]
- Da Rocha, M.S.; Arnold, L.L.; Dodmane, P.R.; Pennington, K.L.; Qiu, F.; De Camargo, J.L.V.; Cohen, S.M. Diuron Metabolites and Urothelial Cytotoxicity: In Vivo, In Vitro and Molecular Approaches. Toxicology 2013, 314, 238–246. [Google Scholar] [CrossRef]
- Behrens, D.; Rouxel, J.; Burgeot, T.; Akcha, F. Comparative Embryotoxicity and Genotoxicity of the Herbicide Diuron and Its Metabolites in Early Life Stages of Crassostrea Gigas: Implication of Reactive Oxygen Species Production. Aquat. Toxicol. 2016, 175, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Manonmani, G.; Sandhiya, L.; Senthilkumar, K. Mechanism and Kinetics of Diuron Oxidation by Hydroxyl Radical Addition Reaction. Environ. Sci. Pollut. Res. 2020, 27, 12080–12095. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Arshad, M.; Springael, D.; SøRensen, S.R.; Bending, G.D.; Devers-Lamrani, M.; Maqbool, Z.; Martin-Laurent, F. Abiotic and Biotic Processes Governing the Fate of Phenylurea Herbicides in Soils: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1947–1998. [Google Scholar] [CrossRef]
- Chang, J.; Fang, W.; Chen, L.; Zhang, P.; Zhang, G.; Zhang, H.; Liang, J.; Wang, Q.; Ma, W. Toxicological Effects, Environmental Behaviors and Remediation Technologies of Herbicide Atrazine in Soil and Sediment: A Comprehensive Review. Chemosphere 2022, 307, 136006. [Google Scholar] [CrossRef]
- Canle López, M.; Fernández, M.I.; Rodríguez, S.; Santaballa, J.A.; Steenken, S.; Vulliet, E. Mechanisms of Direct and TiO2-Photocatalysed UV Degradation of Phenylurea Herbicides. ChemPhysChem 2005, 6, 2064–2074. [Google Scholar] [CrossRef]
- Guimarães, A.C.D.; Mendes, K.F.; Dos Reis, F.C.; Campion, T.F.; Christoffoleti, P.J.; Tornisielo, V.L. Role of Soil Physicochemical Properties in Quantifying the Fate of Diuron, Hexazinone, and Metribuzin. Environ. Sci. Pollut. Res. 2018, 25, 12419–12433. [Google Scholar] [CrossRef]
- da Silva Teófilo, T.M.; Mendes, K.F.; Fernandes, B.C.C.; de Oliveira, F.S.; Silva, T.S.; Takeshita, V.; de Freitas Souza, M.; Tornisielo, V.L.; Silva, D.V. Phytoextraction of Diuron, Hexazinone, and Sulfometuron-Methyl from the Soil by Green Manure Species. Chemosphere 2020, 256, 127059. [Google Scholar] [CrossRef]
- Park, J.M.; Jhung, S.H. Polyaniline-Derived Carbons: Remarkable Adsorbents to Remove Atrazine and Diuron Herbicides from Water. J. Hazard. Mater. 2020, 396, 122624. [Google Scholar] [CrossRef]
- Procópio, S.d.O.; Santos, J.B.; Pires, F.R.; Silva, A.A.; Santos, E.A.; Cargnelutti Filho, A. Development of Bean Plants in Soil Contaminated with Trifloxysulfuron-Sodium after Stizolobium Aterrimum and Canavalia Ensiformis Cultivation. Planta Daninha 2007, 25, 87–96. [Google Scholar] [CrossRef]
- Ferraço, M.; Pires, F.R.; Belo, A.F.; Celin, A.; Bonomo, R. Effect of Population Density of Canavalia Ensiformis on the Phytoremediation of Soil Contaminated with Sulfentrazone. Rev. Ciência Agronômica 2017, 48, 32–40. [Google Scholar] [CrossRef]
- Mendes, K.F.; Maset, B.A.; Mielke, K.C.; Sousa, R.N.D.; Martins, B.A.B.; Tornisielo, V.L. Phytoremediation of Quinclorac and Tebuthiuron-Polluted Soil by Green Manure Plants. Int. J. Phytoremediation 2021, 23, 474–481. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Silva, M.; de Souza, H.B.F.; de Brito Bispo, J.; da Hora Góes, N.; Rodrigues, T.C.; de Santana Cerqueira, L.R.; Santana, A.N.; Dias, I.A. Desafios e Estratégias Da Fitorremediação No Meio Ambiente. Pesqui. Agrárias E Ambient. 2021, 6. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Moreira, B.R.d.A.; Montagnolli, R.N.; Prado, E.P.; Viana, R.D.S.; Tomaz, R.S.; Cruz, J.M.; Bidoia, E.D.; Frias, Y.A.; Lopes, P.R.M. Green Manure Species for Phytoremediation of Soil with Tebuthiuron and Vinasse. Front. Bioeng. Biotechnol. 2021, 8, 613642. [Google Scholar] [CrossRef]
- Theodoro, S.H.; de Paula Medeiros, F.; Ianniruberto, M.; Jacobson, T.K.B. Soil Remineralization and Recovery of Degraded Areas: An Experience in the Tropical Region. J. S. Am. Earth Sci. 2021, 107, 103014. [Google Scholar] [CrossRef]
- Brazilian Soil Classification System.—Portal Embrapa. Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1094001/brazilian-soil-classification-system (accessed on 4 October 2023).
- Mendes, K.F.; Goulart, B.F.; Possamai, A.C.S.; Inoue, M.H.; de Matos, A.K.A.; Tschope, M.C. Leaching of hexazinone and mixture hexazinone + diuron in columns of soils with distinct textures. Agro@Mbiente -Line 2013, 7, 218–224. [Google Scholar] [CrossRef][Green Version]
- Madalão, J.C.; de Souza, M.F.; Silva, A.A.; Silva, D.V.; Jakelaitis, A.; Pereira, G.A.M. Action of Canavalia Ensiformis in Remediation of Contaminated Soil with Sulfentrazone. Bragantia 2017, 76, 292–299. [Google Scholar] [CrossRef]
- Inmetro – Instituto Nacional de Metrologia, Normalização e Qualidade Industrial. Orientações sobre Validação de Métodos de Ensaios Químicos, DOC-CGCRE-008. 2020. Available online: http://www.inmetro.gov.br/credenciamento/organismos/doc_organismos.asp?torganismo=calibensaios (accessed on 23 September 2023).
- Queiroz, S.d.N.; Ferracini, V.L.; Rosa, M.A.; Cerdeira, A.L. Validação de Método, Por CLAE, Para Determinação de Hexazinone e Diuron Em Solo. In Reunião anual da Sociedade Brasileira de Química 29., 2006, Águas de Lindóia. Resumos; SBQ: Águas de Lindóia, Brazil, 2006. [Google Scholar]
- Anvisa, A.N.d.V.S. Resolucao. RDC N° 4. Dispõe Sobre Os Critérios Para a Realização de Estudos de Resíduos de Agrotóxicos Para Fins de Registro de Agrotóxicos No Brasil. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2012/res0004_18_01_2012.html (accessed on 27 October 2023).
- Refatti, J.P.; de Avila, L.A.; Agostinetto, D.; Manica-Berto, R.; Bundt, A.D.C.; Elgueira, D.B. Efeito Da Calagem Na Lixiviação de Imazethapyr e Imazapyr Em Solo de Cultivo de Arroz Irrigado. Ciência Rural 2014, 44, 1008–1014. [Google Scholar] [CrossRef]
- Fontecha-Cámara, M.A.; López-Ramón, M.V.; Álvarez-Merino, M.A.; Moreno-Castilla, C. Effect of Surface Chemistry, Solution pH, and Ionic Strength on the Removal of Herbicides Diuron and Amitrole from Water by an Activated Carbon Fiber. Langmuir 2007, 23, 1242–1247. [Google Scholar] [CrossRef]
- Castro Neto, M.D.D.; Souza, M.D.F.; Silva, D.V.; Faria, A.T.; Da Silva, A.A.; Pereira, G.A.M.; De Freitas, M.A.M. Leaching of Imidazolinones in Soils under a Clearfield System. Arch. Agron. Soil Sci. 2017, 63, 897–906. [Google Scholar] [CrossRef]
- Antonious, G.F.; Turley, E.T.; Hill, R.R. Impact of Soil Amendments on Metribuzin and DCPA Half-Lives and Mobility into Agricultural Runoff Water. J. Environ. Sci. Health Part B 2014, 49, 313–323. [Google Scholar] [CrossRef]
- das Chagas, P.S.F.; Souza, M.d.F.; Freitas, C.D.M.; de Mesquita, H.C.; Silva, T.S.; dos Santos, J.B.; Passos, A.B.R.d.J.; Medeiros, R.d.C.A.d.; Silva, D.V. Increases in pH, Ca2+, and Mg2+ Alter the Retention of Diuron in Different Soils. Catena 2020, 188, 104440. [Google Scholar] [CrossRef]
- El-Nahhal, Y.; Hamdona, N. Adsorption, Leaching and Phytotoxicity of Some Herbicides as Single and Mixtures to Some Crops. J. Assoc. Arab Univ. Basic Appl. Sci. 2017, 22, 17–25. [Google Scholar] [CrossRef]
- El-Nahhal, Y.; Abadsa, M.; Affifi, S. Adsorption of Diuron and Linuron in Gaza Soils. Am. J. Anal. Chem. 2013, 4, 94–99. [Google Scholar] [CrossRef]
- López-Piñeiro, A.; Cabrera, D.; Albarrán, Á.; Peña, D. Cumulative and Residual Effects of De-Oiled Two-Phase Olive Mill Waste Application to Soil on Diuron Sorption, Leaching, Degradation, and Persistence. Chemosphere 2010, 78, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Smernik, R.J.; Kookana, R.S. The Effects of Organic Matter–Mineral Interactions and Organic Matter Chemistry on Diuron Sorption across a Diverse Range of Soils. Chemosphere 2015, 119, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Z.; Wu, X.; Gui, W.; Zhu, G. Adsorption and Desorption Behavior of Herbicide Diuron on Various Chinese Cultivated Soils. J. Hazard. Mater. 2010, 178, 462–468. [Google Scholar] [CrossRef]
- Giori, F.G.; Tornisielo, V.L.; Pellegrino Cerri, C.E.; Regitano, J.B. Sugarcane Straw Management and Soil Attributes on Alachlor and Diuron Sorption in Highly Weathered Tropical Soils. J. Environ. Sci. Health Part B 2014, 49, 352–360. [Google Scholar] [CrossRef]
- Sun, C.; Xu, Y.; Hu, N.; Ma, J.; Sun, S.; Cao, W.; Klobučar, G.; Hu, C.; Zhao, Y. To Evaluate the Toxicity of Atrazine on the Freshwater Microalgae Chlorella Sp. Using Sensitive Indices Indicated by Photosynthetic Parameters. Chemosphere 2020, 244, 125514. [Google Scholar] [CrossRef]
- Radwan, D.E.M.; Mohamed, A.K.; Fayez, K.A.; Abdelrahman, A.M. Oxidative Stress Caused by Basagran® Herbicide Is Altered by Salicylic Acid Treatments in Peanut Plants. Heliyon 2019, 5, e01791. [Google Scholar] [CrossRef]
- Mendes, K.F.; Silva, A.A. Classificação, Seletividade e Mecanismos de Ação de Herbicidas. In Plantas Daninhas; Oficina de Textos: São Paulo, Brazil, 2022; Volume 2. [Google Scholar]
- Weber, J.B.; Wilkerson, G.G.; Reinhardt, C.F. Calculating Pesticide Sorption Coefficients (Kd) Using Selected Soil Properties. Chemosphere 2004, 55, 157–166. [Google Scholar] [CrossRef]
- Agbaogun, B.K.; Fischer, K. Adsorption of Phenylurea Herbicides by Tropical Soils. Environ. Monit. Assess. 2020, 192, 212. [Google Scholar] [CrossRef] [PubMed]

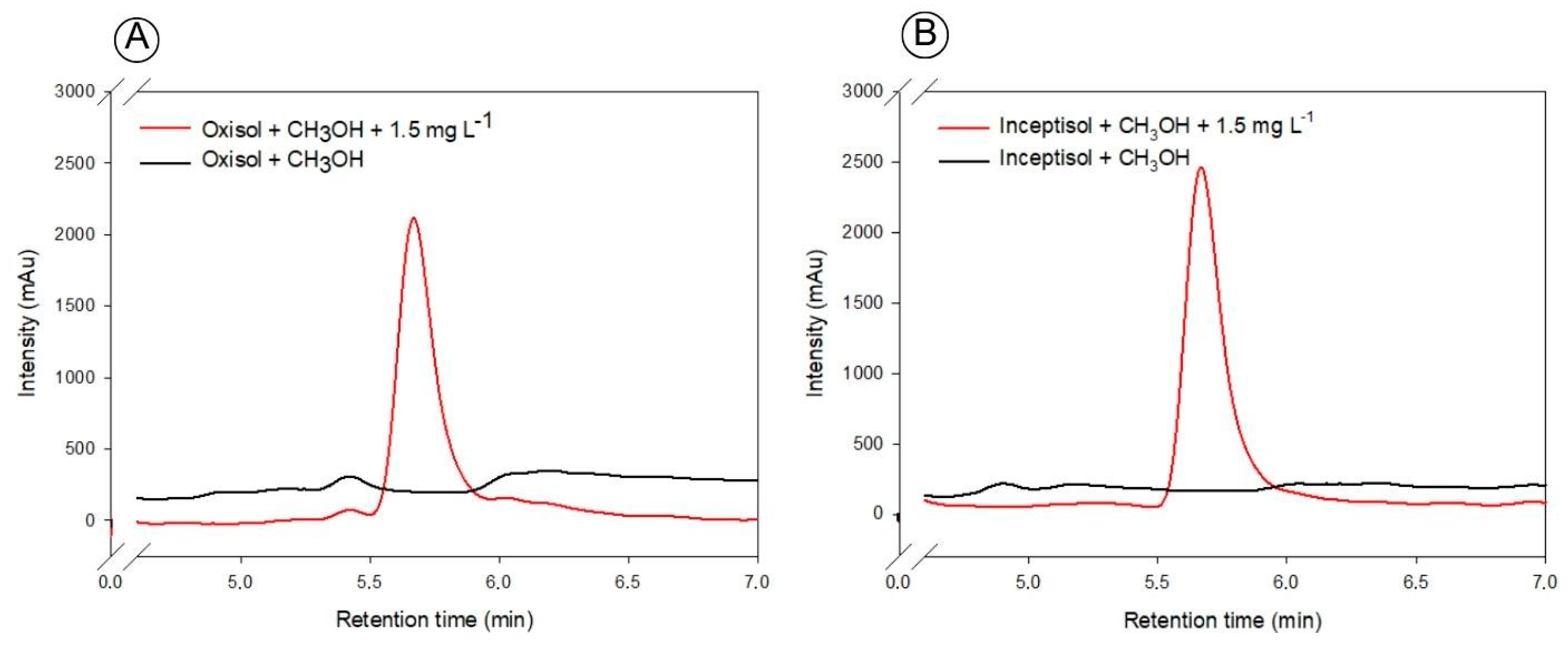

| Applied Concentration (mg kg−1) | Precision | Accuracy | ||
|---|---|---|---|---|
| CV (%) | R (%) | |||
| Oxisol | Inceptisol | Oxisol | Inceptisol | |
| 0.08 | 6.78 | 7.22 | 80.25 | 93.69 |
| 0.16 | 6.78 | 5.83 | 82.94 | 93.41 |
| 0.30 | 6.68 | 7.95 | 89.60 | 102.88 |
| Soils—Doses of Rock Powder | Diuron Doses (g a.i. ha−1) | LoQ (mg kg−1) | |||
|---|---|---|---|---|---|
| 875 | 1750 | 2625 | 3500 | ||
| Concentration Found (mg kg−1) (HPLC) | |||||
| Oxisol—0 t ha−1 | 0.03 | 0.03 | 0.03 | 0.06 | 0.096 |
| Oxisol—4 t ha−1 | 0.03 | 0.03 | 0.04 | 0.08 | |
| Oxisol—8 t ha−1 | 0.04 | 0.04 | 0.04 | 0.06 | |
| Inceptisol—0 t ha−1 | 0.04 | 0.04 | 0.08 * | 0.11 * | 0.073 |
| Inceptisol—4 t ha−1 | 0.05 | 0.04 | 0.04 | 0.08 * | |
| Inceptisol—8 t ha−1 | 0.03 | 0.04 | 0.04 | 0.09 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araujo, G.R.; da Silva, L.B.X.; Vaz, V.; Borges, M.P.d.S.; Spolidorio, E.S.; Mendes, K.F. Cucumber Bioassay and HPLC Analysis to Detect Diuron Residues in Remineralized Soils Following Canavalia ensiformis Cultivation as a Phytoremediator. Horticulturae 2023, 9, 1251. https://doi.org/10.3390/horticulturae9121251
Araujo GR, da Silva LBX, Vaz V, Borges MPdS, Spolidorio ES, Mendes KF. Cucumber Bioassay and HPLC Analysis to Detect Diuron Residues in Remineralized Soils Following Canavalia ensiformis Cultivation as a Phytoremediator. Horticulturae. 2023; 9(12):1251. https://doi.org/10.3390/horticulturae9121251
Chicago/Turabian StyleAraujo, Grazielle Rodrigues, Laryssa Barbosa Xavier da Silva, Valter Vaz, Maiara Pinheiro da Silva Borges, Eduardo Scarpari Spolidorio, and Kassio Ferreira Mendes. 2023. "Cucumber Bioassay and HPLC Analysis to Detect Diuron Residues in Remineralized Soils Following Canavalia ensiformis Cultivation as a Phytoremediator" Horticulturae 9, no. 12: 1251. https://doi.org/10.3390/horticulturae9121251
APA StyleAraujo, G. R., da Silva, L. B. X., Vaz, V., Borges, M. P. d. S., Spolidorio, E. S., & Mendes, K. F. (2023). Cucumber Bioassay and HPLC Analysis to Detect Diuron Residues in Remineralized Soils Following Canavalia ensiformis Cultivation as a Phytoremediator. Horticulturae, 9(12), 1251. https://doi.org/10.3390/horticulturae9121251








