Physiological Responses of Pak Choi (Brassica rapa Subsp. Chinensis) Genotypes to Salt Tolerance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Cultivation Environment
2.2. NaCl Treatment Conditions
2.3. Electrolyte Leakage Content Test
2.4. Measurement of the Maximum Quantum Yield of Photosystem II (Fv/Fm)
2.5. Measurement of Growth Parameters
2.6. Statistical Analyses
3. Results
3.1. Changes in Electrolyte Leakage Content by NaCl Treatment
3.2. Changes in the Maximum Quantum Yield of Photosystem II According to NaCl Treatment
3.3. Changes in Growth Characteristics According to NaCl Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pant, A.P.; Radovich, T.J.K.; Hue, N.V.; Miyasaka, S.C. Pak Choi (Brassica rapa, Chinensis Group) Yield, Phytonutrient Content, and Soil Biological Properties as Affected by Vermicompost-to-water Ratio Used for Extraction. Hortscience 2012, 47, 395–402. [Google Scholar] [CrossRef]
- Zou, L.; Tan, W.K.; Du, Y.; Lee, H.W.; Liang, X.; Lei, J.; Striegel, L.; Weber, N.; Rychlik, M.; Ong, C.N. Nutritional metabolites in Brassica rapa subsp. chinensis var. parachinensis (choy sum) at three different growth stages: Microgreen, seedling and adult plant. Food Chem. 2021, 357, 129535. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef]
- An, R.H.; Luo, S.F.; Zhou, H.S.; Zhang, Y.T.; Zhang, L.G.; Hu, H.L.; Li, P.X. Effects of hydrogen-rich water combined with vacuum precooling on the senescence and antioxidant capacity of pakchoi (Brassica rapa subsp. Chinensis). Sci. Hortic. 2021, 289, 110469. [Google Scholar] [CrossRef]
- Kumpanalaisatit, M.; Setthapun, W.; Sintuya, H.; Jansri, S.N. Efficiency improvement of ground-mounted solar power generation in agrivoltaic system by cultivation of bok choy (Brassica rapa subsp. chinensis L.) under the panels. Int. J. Renew. Energy Dev. 2022, 11, 103. [Google Scholar] [CrossRef]
- Service, K.S.I. Census of Agriculture, Forestry, and Fisheries. 2023. Available online: http://kostat.go.kr (accessed on 10 July 2023).
- Kim, S.-H.; Jang, D.-C.; Zebro, M.; Heo, J.-Y. Effects of various combinations of red and blue LEDs on seed germination and growth in Indian spinach (Basella alba L.). Italus Hortus 2022, 29, 196–205. [Google Scholar] [CrossRef]
- Jia, K.; Yan, C.; Yan, H.; Gao, J. Physiological responses of turnip (Brassica rapa L. subsp. rapa) seedlings to salt stress. HortScience 2020, 55, 1567–1574. [Google Scholar] [CrossRef]
- Svyantek, A.; Wang, Z.; Rana, B.; Tatar, I.; Auwarter, C.; Hatterman-Valenti, H. Performance of hydroponic pak choi (Brassica rapa subsp. chinensis) under elevated sodium conditions. In III International Symposium on Soilless Culture and Hydroponics: Innovation and Advanced Technology for Circular Horticulture; ISHS: Leuven, Belgium, 2021; pp. 133–140. [Google Scholar]
- Wang, C.-F.; Han, G.-L.; Qiao, Z.-Q.; Li, Y.-X.; Yang, Z.-R.; Wang, B.-S. Root Na+ content negatively correlated to salt tolerance determines the salt tolerance of Brassica napus L. inbred seedlings. Plants 2022, 11, 906. [Google Scholar] [CrossRef]
- Furmańczyk, E.; Tartanus, M.; Holtz, T.; Kelderer, M.; Malusa, E. Soil nutrient availability of new organic fertilizers formulations. In the Eco-fruit, Proceedings of the 19th International Conference on Organic Fruit-Growing; University of Hohenheim: Hohenheim, Germany, 2020; pp. 181–183. [Google Scholar]
- Lepp, B.; Zikeli, S.; Hartmann, T.E.; Buchleither, S.; Möller, K. Improving Fertilisation Strategies in Organic Apple Cultivation. In Proceedings of the XIX International Conference on Organic Fruit Growing, Hohenheim, Germany, 17–19 February 2020; pp. 179–180. [Google Scholar]
- Peng, Q.; Guo, L.; Ali, F.; Li, J.; Qin, S.Y.; Feng, P.Y.; Liang, D.L. Influence of Pak choi plant cultivation on Se distribution, speciation and bioavailability in soil. Plant Soil 2016, 403, 331–342. [Google Scholar] [CrossRef]
- Sun, H.; Wei, C.; Xu, W.; Yang, J.; Wang, X.; Qiu, Y. Characteristics of salt contents in soils under greenhouse conditions in China. Environ. Sci. Pollut. Res. Int. 2019, 26, 3882–3892. [Google Scholar] [CrossRef]
- Hanin, M.; Ebel, C.; Ngom, M.; Laplaze, L.; Masmoudi, K. New Insights on Plant Salt Tolerance Mechanisms and Their Potential Use for Breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar] [CrossRef]
- Basak, N.; Rai, A.K.; Sundha, P.; Meena, R.L.; Bedwal, S.; Yadav, R.K.; Sharma, P.C. Assessing soil quality for rehabilitation of salt-affected agroecosystem: A comprehensive review. Front. Environ. Sci. 2022, 10, 935785. [Google Scholar] [CrossRef]
- Afzal, M.Z.; Jia, Q.; Ibrahim, A.K.; Niyitanga, S.; Zhang, L.W. Mechanisms and Signaling Pathways of Salt Tolerance in Crops: Understanding from the Transgenic Plants. Trop. Plant Biol. 2020, 13, 297–320. [Google Scholar] [CrossRef]
- Shang, H.Q.; Shen, G.M. Effect of ammonium/nitrate ratio on pak choi (Brassica chinensis L.) photosynthetic capacity and biomass accumulation under low light intensity and water deficit. Photosynthetica 2018, 56, 1039–1046. [Google Scholar] [CrossRef]
- Xiong, X.; Chang, L.Y.; Khalid, M.; Zhang, J.J.; Huang, D.F. Alleviation of Drought Stress by Nitrogen Application in Brassica campestris ssp. Chinensis L. Agronomy 2018, 8, 66. [Google Scholar] [CrossRef]
- Maluin, F.N.; Hussein, M.Z.; Wayayok, A.; Nik Ibrahim, N.N.L.; Hashim, N. Chitosan nanoparticles as a sustainable alternative nutrient formulation in hydroponically grown Brassica rapa subsp. chinensis (L.) Hanelt microgreen and its adult vegetable. Arch. Agron. Soil Sci. 2022, 69, 2401–2412. [Google Scholar] [CrossRef]
- Upadhyay, T.K. Impact of hydroponics: Present and future perspective for farmer’s welfare. J. Environ. Sci. Technol. 2019, 5, 19–26. [Google Scholar]
- Wu, Y.L.; Huang, B.Z.; Peng, X.X.; Zhang, J.J. Development of an in vitro hydroponic system for studying the interaction between banana plantlet and Fusarium oxysporum f. sp. cubense. Plant Cell Tiss. Org. 2021, 146, 107–114. [Google Scholar] [CrossRef]
- Nhut, D.T.; Ngan, H.T.M.; Mai, N.T.N.; Tung, H.T. In Vitro Hydroponic Culture System in Plant Micropropagation. In Plant Tissue Culture: New Techniques and Application in Horticultural Species of Tropical Region; Springer: Berlin/Heidelberg, Germany, 2022; pp. 191–206. [Google Scholar]
- Zhao, Z.; Zhang, W.; Liu, Y.; Li, S.; Yao, W.; Sun, X.; Li, S.; Ma, L.; Sun, J.; Yang, Q.; et al. De novo hydroponics system efficiency for the cuttings of alfalfa (Medicago sativa L.). Physiol. Mol. Biol. Plants 2021, 27, 1413–1421. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Lee, C.-M.; Kwon, Y.-H.; Park, H.-M.; Jeung, J.-U.; Park, H.-S.; Baek, M.-K.; Ha, S.-K.; Mo, Y. Days to heading and culm length variation of Korean rice varieties in different environments. Korean Soc. Breed. Sci. 2020, 52, 389–397. [Google Scholar] [CrossRef]
- Lee, S.; Lee, K.J.; Lee, S.; Yoo, E.; Cho, G.-T.; Koh, H.-J. Comparison of Agronomic and Seed Traits of Common Bean (Phaseolus vulgaris L.) Germplasm from Korea, Bulgaria, and El Salvador. Korean Soc. Breed. Sci. 2022, 54, 8–15. [Google Scholar] [CrossRef]
- Julkowska, M.M.; Klei, K.; Fokkens, L.; Haring, M.A.; Schranz, M.E.; Testerink, C. Natural variation in rosette size under salt stress conditions corresponds to developmental differences between Arabidopsis accessions and allelic variation in the LRR-KISS gene. J. Exp. Bot. 2016, 67, 2127–2138. [Google Scholar] [CrossRef] [PubMed]
- Sutinen, M.-L.; Palta, J.P.; Reich, P.B. Seasonal differences in freezing stress resistance of needles of Pinus nigra and Pinus resinosa: Evaluation of the electrolyte leakage method. Tree Physiol. 1992, 11, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.Y.; Feng, D.; Niu, X.; Mitchell-Olds, T.; Van Tienderen, P.H.; Tomes, D.; Schranz, M.E. Identification of quantitative trait loci and a candidate locus for freezing tolerance in controlled and outdoor environments in the overwintering crucifer B oechera stricta. Plant Cell Environ. 2014, 37, 2459–2469. [Google Scholar] [CrossRef]
- Hniličková, H.; Hnilička, F.; Orsák, M.; Hejnák, V. Effect of salt stress on growth, electrolyte leakage, Na+ and K+ content in selected plant species. Plant Soil Environ. 2019, 65, 90–96. [Google Scholar] [CrossRef]
- Ahmad, I.; Rana, R.M.; Hassan, M.U.; Khan, M.A.; Sajjad, M. Association mapping for abiotic stress tolerance using heat-and drought-related syntenic markers in okra. Mol. Biol. Rep. 2022, 49, 11409–11419. [Google Scholar] [CrossRef]
- Alam, M.S.; Tester, M.; Fiene, G.; Mousa, M.A.A. Early growth stage characterization and the biochemical responses for salinity stress in tomato. Plants 2021, 10, 712. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Zhu, Y.-F.; Wu, Y.-X.; Hu, Y.; Jia, X.-M.; Zhao, T.; Cheng, L.; Wang, Y.-X. Tolerance of two apple rootstocks to short-term salt stress: Focus on chlorophyll degradation, photosynthesis, hormone and leaf ultrastructures. Acta Physiol. Plant. 2019, 41, 87. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Cho, M.C.; Yang, E.Y.; Lee, J.G. Response to salt stress in lettuce: Changes in chlorophyll fluorescence parameters, phytochemical contents, and antioxidant activities. Agronomy 2020, 10, 1627. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Cho, M.C.; Lee, J.G. Evaluation of chlorophyll fluorescence parameters and proline content in tomato seedlings grown under different salt stress conditions. Hortic. Environ. Biotechnol. 2020, 61, 433–443. [Google Scholar] [CrossRef]
- Okon, O.G. Effect of salinity on physiological processes in plants. In Microorganisms in Saline Environments: Strategies and Functions; Springer: Berlin/Heidelberg, Germany, 2019; pp. 237–262. [Google Scholar] [CrossRef]
- Memon, S.A.; Hou, X.; Wang, L.J. Morphlogical analysis of salt stress response of pak choi. Electron. J. Environ. Agric. Food Chem. 2010, 248–254. [Google Scholar]
- Bhattarai, S.; Biswas, D.; Fu, Y.-B.; Biligetu, B. Morphological, physiological, and genetic responses to salt stress in alfalfa: A review. Agronomy 2020, 10, 577. [Google Scholar] [CrossRef]
- Majidi-Mehr, A.; Amiri-Fahliani, R. Evaluation of reaction of some rice (Oryza sativa L.) genotypes to salinity stress at seedling stage. Environ. Stress. Crop Sci. 2020, 13, 1293–1306. [Google Scholar] [CrossRef]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Giordano, M.; Petropoulos, S.A.; Rouphael, Y. Response and defence mechanisms of vegetable crops against drought, heat and salinity stress. Agriculture 2021, 11, 463. [Google Scholar] [CrossRef]
- de Oliveira, A.B.; Alencar, N.L.M.; Gomes-Filho, E. Comparison between the water and salt stress effects on plant growth and development. Responses Org. Water Stress 2013, 4, 67–94. [Google Scholar] [CrossRef]
- Safdar, H.; Amin, A.; Shafiq, Y.; Ali, A.; Yasin, R.; Shoukat, A.; Hussan, M.U.; Sarwar, M.I. A review: Impact of salinity on plant growth. Nat. Sci. 2019, 17, 34–40. [Google Scholar] [CrossRef]
| Genotype | Electrolyte Leakage (%) | Fv/Fm | ||||
|---|---|---|---|---|---|---|
| Control | 100 mM Salt | Fold Change | Control | 100 mM Salt | % | |
| IT112271(1) | 11.3 ± 1.2 | 44.0 ± 4.7 | 3.89 | 0.81 ± 0.1 | 0.54 ± 2.7 | −33.3 |
| IT135403(2) | 14.7 ± 2.3 | 38.4 ± 3.3 | 2.61 | 0.79 ± 0.4 | 0.71 ± 1.8 | −10.1 |
| IT135406(3) | 11.2 ± 1.1 | 55.7 ± 4.2 | 4.97 | 0.81 ± 0.1 | 0.44 ± 1.9 | −45.7 |
| IT135441(4) | 13.5 ± 1.1 | 37.1 ± 3.3 | 2.75 | 0.81 ± 0.1 | 0.73 ± 1.6 | −9.9 |
| IT166982(5) | 14.9 ± 0.8 | 43.7 ± 2.1 | 2.93 | 0.81 ± 0.1 | 0.49 ± 0.5 | −39.5 |
| IT166983(6) | 11.5 ± 1.8 | 35.7 ± 2.6 | 3.10 | 0.81 ± 0.3 | 0.68 ± 0.4 | −16.1 |
| IT185735(7) | 10.1 ± 2.4 | 28.3 ± 1.4 | 2.80 | 0.81 ± 0.1 | 0.78 ± 0.2 | −3.7 |
| IT188180(8) | 10.5 ± 0.9 | 49.6 ± 4.2 | 4.72 | 0.79 ± 0.2 | 0.40 ± 0.9 | −49.4 |
| IT188181(9) | 14.5 ± 0.1 | 36.5 ± 2.7 | 2.52 | 0.79 ± 0.1 | 0.64 ± 1.2 | −18.9 |
| IT210064(10) | 14.3 ± 0.8 | 38.3 ± 4.2 | 2.68 | 0.80 ± 0.3 | 0.74 ± 1.8 | −7.5 |
| IT262109(11) | 12.3 ± 1.2 | 27.0 ± 2.3 | 2.20 | 0.82 ± 0.3 | 0.79 ± 0.2 | −3.7 |
| IT279432(12) | 11.0 ± 0.9 | 26.2 ± 2.0 | 2.38 | 0.81 ± 0.1 | 0.78 ± 0.1 | −3.7 |
| IT280392(13) | 13.4 ± 2.0 | 39.2 ± 3.5 | 2.93 | 0.81 ± 0.1 | 0.69 ± 0.4 | −14.8 |
| IT280393(14) | 14.9 ± 1.3 | 39.9 ± 3.6 | 2.68 | 0.83 ± 0.5 | 0.71 ± 0.3 | −14.5 |
| IT280394(15) | 13.5 ± 2.2 | 34.4 ± 2.3 | 2.55 | 0.79 ± 0.2 | 0.72 ± 0.0 | −8.9 |
| IT280395(16) | 10.4 ± 0.4 | 44.5 ± 3.4 | 4.28 | 0.82 ± 0.2 | 0.48 ± 0.5 | −41.5 |
| IT280396(17) | 9.9 ± 0.9 | 35.7 ± 4.7 | 3.61 | 0.83 ± 0.2 | 0.71 ± 0.3 | −14.5 |
| IT280397(18) | 13.2 ± 2.1 | 37.4 ± 3.1 | 2.83 | 0.81 ± 0.1 | 0.70 ± 0.3 | −13.6 |
| IT280398(19) | 11.7 ± 0.3 | 37.2 ± 3.8 | 3.18 | 0.80 ± 0.3 | 0.72 ± 0.2 | −10.0 |
| IT293143(20) | 12.4 ± 1.7 | 35.4 ± 2.7 | 2.85 | 0.81 ± 0.4 | 0.73 ± 0.1 | −9.9 |
| IT293144(21) | 12.5 ± 1.6 | 32.7 ± 0.9 | 2.62 | 0.81 ± 0.3 | 0.69 ± 0.2 | −14.8 |
| IT301979(22) | 14.2 ± 1.1 | 40.7 ± 1.3 | 2.87 | 0.80 ± 0.2 | 0.64 ± 0.4 | −20.0 |
| IT301980(23) | 10.4 ± 0.8 | 39.5 ± 6.3 | 3.80 | 0.81 ± 0.3 | 0.62 ± 0.4 | −23.5 |
| IT304044(24) | 13.3 ± 2.2 | 33.8 ± 3.4 | 2.54 | 0.81 ± 0.1 | 0.76 ± 1.6 | −6.2 |
| Average | 12.5 ± 1.3 | 38.0 ± 3.2 | 3.10 | 0.81 ± 0.2 | 0.66 ± 0.75 | −18.1 |
| Genotype | Fresh Weight (g) | |||||
|---|---|---|---|---|---|---|
| Scion | Rootstock | |||||
| Control | 100 mM Salt | % | Control | 100 mM Salt | % | |
| IT112271(1) | 23.19 ± 0.4 | 17.98 ± 0.4 | −22.5 | 2.79 ± 0.6 | 2.28 ± 0.5 | −18.3 |
| IT135403(2) | 9.23 ± 0.6 | 5.26 ± 0.7 | −43.0 | 1.71 ± 0.4 | 1.17 ± 0.5 | −31.6 |
| IT135406(3) | 25.72 ± 1.0 | 16.74 ± 0.4 | −34.9 | 2.56 ± 1.0 | 1.84 ± 0.7 | −28.1 |
| IT135441(4) | 14.81 ± 0.9 | 10.56 ± 0.9 | −28.7 | 1.38 ± 0.5 | 0.99 ± 0.7 | −28.3 |
| IT166982(5) | 34.79 ± 0.4 | 29.44 ± 0.4 | −15.4 | 4.84 ± 0.6 | 3.78 ± 0.5 | −21.9 |
| IT166983(6) | 15.76 ± 0.9 | 11.66 ± 0.7 | −26.0 | 3.27 ± 1.0 | 2.16 ± 0.8 | −33.9 |
| IT185735(7) | 31.14 ± 0.7 | 27.84 ± 0.4 | −10.6 | 4.68 ± 0.7 | 3.73 ± 0.5 | −20.3 |
| IT188180(8) | 39.40 ± 0.8 | 21.91 ± 0.9 | −44.4 | 4.99 ± 0.7 | 3.45 ± 0.8 | −30.9 |
| IT188181(9) | 55.52 ± 0.5 | 46.78 ± 0.5 | −15.7 | 11.78 ± 0.3 | 8.84 ± 0.4 | −25.0 |
| IT210064(10) | 32.79 ± 0.5 | 28.43 ± 0.6 | −13.3 | 4.92 ± 0.5 | 3.84 ± 0.5 | −22.0 |
| IT262109(11) | 25.9 ± 1.2 | 24.70 ± 1.0 | −4.6 | 6.31 ± 1.1 | 5.43 ± 1.0 | −13.9 |
| IT279432(12) | 12.23 ± 0.5 | 12.07 ± 0.6 | −1.3 | 2.04 ± 0.3 | 1.96 ± 0.5 | −3.9 |
| IT280392(13) | 37.72 ± 0.5 | 33.49 ± 0.5 | −11.2 | 4.48 ± 0.5 | 3.87 ± 0.5 | −13.6 |
| IT280393(14) | 14.95 ± 0.6 | 8.99 ± 0.7 | −39.9 | 3.49 ± 0.7 | 2.39 ± 0.7 | −31.5 |
| IT280394(15) | 18.68 ± 0.3 | 13.38 ± 0.4 | −28.4 | 2.82 ± 0.3 | 2.34 ± 0.4 | −17.0 |
| IT280395(16) | 17.56 ± 0.5 | 13.14 ± 0.4 | −25.2 | 1.89 ± 0.4 | 0.87 ± 0.4 | −54.0 |
| IT280396(17) | 11.79 ± 0.6 | 7.72 ± 0.8 | −34.5 | 1.57 ± 0.8 | 0.87 ± 0.8 | −44.6 |
| IT280397(18) | 31.75 ± 0.6 | 22.94 ± 0.5 | −27.7 | 4.85 ± 0.7 | 3.84 ± 0.6 | −20.8 |
| IT280398(19) | 11.84 ± 0.5 | 9.88 ± 0.7 | −16.6 | 2.19 ± 0.5 | 1.78 ± 0.6 | −18.7 |
| IT293143(20) | 25.51 ± 0.8 | 20.16 ± 0.9 | −21.0 | 4.03 ± 1.0 | 3.05 ± 0.9 | −24.3 |
| IT293144(21) | 16.21 ± 0.7 | 13.97 ± 0.6 | −13.8 | 2.97 ± 0.5 | 2.51 ± 0.5 | −15.5 |
| IT301979(22) | 15.70 ± 0.7 | 8.84 ± 0.8 | −43.7 | 2.26 ± 0.7 | 1.01 ± 0.8 | −55.3 |
| IT301980(23) | 43.78 ± 0.8 | 37.89 ± 0.8 | −13.5 | 5.38 ± 0.7 | 4.76 ± 0.7 | −11.5 |
| IT304044(24) | 38.42 ± 0.9 | 27.23 ± 0.6 | −29.1 | 5.13 ± 0.7 | 3.97 ± 0.6 | −22.6 |
| Average | 25.18 ± 0.7 | 19.63 ± 0.6 | −23.5 | 3.85 ± 0.6 | 2.95 ± 0.6 | −25.3 |
| Genotype | Dry Weight (g) | |||||
|---|---|---|---|---|---|---|
| Scion | Rootstock | |||||
| Control | 100 mM Salt | % | Control | 100 mM Salt | % | |
| IT112271(1) | 1.86 ± 1.7 | 1.31 ± 2.6 | −29.6 | 0.19 ± 2.7 | 0.08 ± 2.7 | −57.9 |
| IT135403(2) | 0.98 ± 1.6 | 0.53 ± 3.3 | −45.9 | 0.17 ± 3.6 | 0.07 ± 3.4 | −58.8 |
| IT135406(3) | 1.89 ± 4.0 | 1.28 ± 4.3 | −32.3 | 0.19 ± 3.6 | 0.04 ± 4.4 | −78.9 |
| IT135441(4) | 1.44 ± 1.6 | 0.99 ± 3.9 | −31.3 | 0.15 ± 3.8 | 0.03 ± 4.3 | −80.0 |
| IT166982(5) | 3.13 ± 2.2 | 2.57 ± 3.7 | −17.9 | 0.25 ± 3.0 | 0.13 ± 3.4 | −48.0 |
| IT166983(6) | 1.29 ± 4.0 | 1.05 ± 3.5 | −18.6 | 0.18 ± 3.7 | 0.09 ± 4.6 | −50.0 |
| IT185735(7) | 2.98 ± 1.5 | 2.53 ± 3.6 | −15.1 | 0.45 ± 4.7 | 0.32 ± 4.5 | −28.9 |
| IT188180(8) | 3.67 ± 1.2 | 2.91 ± 2.3 | −20.7 | 0.19 ± 3.8 | 0.06 ± 3.5 | −68.4 |
| IT188181(9) | 4.29 ± 3.1 | 3.64 ± 4.3 | −15.2 | 0.23 ± 3.8 | 0.13 ± 4.7 | −43.5 |
| IT210064(10) | 2.08 ± 2.3 | 1.87 ± 3.7 | −10.1 | 0.25 ± 2.9 | 0.11 ± 3.3 | −56.0 |
| IT262109(11) | 2.15 ± 1.5 | 2.04 ± 3.3 | −5.1 | 0.31 ± 2.7 | 0.28 ± 4.6 | −9.7 |
| IT279432(12) | 0.93 ± 4.4 | 0.84 ± 4.3 | −9.7 | 0.19 ± 1.7 | 0.15 ± 4.2 | −21.1 |
| IT280392(13) | 2.52 ± 3.0 | 2.24 ± 4.5 | −11.1 | 0.18 ± 3.2 | 0.12 ± 3.8 | −33.3 |
| IT280393(14) | 1.11 ± 3.4 | 0.77 ± 4.2 | −30.6 | 0.18 ± 3.4 | 0.08 ± 4.8 | −55.6 |
| IT280394(15) | 1.41 ± 1.2 | 1.08 ± 2.5 | −23.4 | 0.14 ± 2.5 | 0.09 ± 2.5 | −35.7 |
| IT280395(16) | 1.35 ± 2.7 | 1.08 ± 4.7 | −20.0 | 0.09 ± 4.9 | 0.02 ± 4.8 | −77.8 |
| IT280396(17) | 0.88 ± 1.6 | 0.72 ± 4.9 | −18.2 | 0.10 ± 3.1 | 0.05 ± 3.9 | −50.0 |
| IT280397(18) | 2.42 ± 1.6 | 1.98 ± 2.6 | −18.2 | 0.19 ± 3.6 | 0.08 ± 3.1 | −57.9 |
| IT280398(19) | 1.10 ± 1.1 | 0.87 ± 3.1 | −20.9 | 0.17 ± 3.5 | 0.08 ± 4.3 | −52.9 |
| IT293143(20) | 1.81 ± 2.1 | 1.31 ± 4.6 | −27.6 | 0.22 ± 3.3 | 0.17 ± 4.4 | −22.7 |
| IT293144(21) | 1.21 ± 3.0 | 1.00 ± 3.9 | −17.4 | 0.14 ± 5.0 | 0.09 ± 4.5 | −35.7 |
| IT301979(22) | 1.25 ± 1.3 | 0.76 ± 1.9 | −39.2 | 0.12 ± 4.0 | 0.08 ± 2.9 | −33.3 |
| IT301980(23) | 3.94 ± 5.0 | 3.21 ± 4.2 | −18.5 | 0.26 ± 3.3 | 0.11 ± 3.7 | −57.7 |
| IT304044(24) | 3.57 ± 2.7 | 2.63 ± 4.4 | −26.3 | 0.27 ± 3.6 | 0.08 ± 3.8 | −70.4 |
| Average | 2.05 ± 2.4 | 1.63 ± 3.7 | −21.8 | 0.20 ± 3.48 | 0.11 ± 3.9 | −49.3 |
| Genotype | Number of Leaves | Leaf Area (mm2) | ||||
|---|---|---|---|---|---|---|
| Control | 100 mM Salt | % | Control | 100 mM Salt | % | |
| IT112271(1) | 9.3 ± 0.5 | 7.8 ± 0.6 | −16.1 | 123.7 ± 6.9 | 84.7 ± 5.4 | −31.5 |
| IT135403(2) | 7.6 ± 0.5 | 6.3 ± 0.3 | −17.1 | 108.3 ± 7.9 | 65.5 ± 3.6 | −39.5 |
| IT135406(3) | 11.2 ± 0.3 | 8.9 ± 0.3 | −20.5 | 139.5 ± 6.7 | 75.1 ± 3.2 | −46.2 |
| IT135441(4) | 8.4 ± 1.0 | 7.6 ± 0.5 | −9.5 | 177.6 ± 12.9 | 160.9 ± 7.9 | −9.4 |
| IT166982(5) | 12.5 ± 1.0 | 11.3 ± 1.0 | −9.6 | 154.2 ± 10.1 | 137.5 ± 5.8 | −10.8 |
| IT166983(6) | 9.7 ± 0.3 | 7.9 ± 0.3 | −18.6 | 166.1 ± 6.7 | 110.9 ± 5.8 | −33.2 |
| IT185735(7) | 7.9 ± 0.5 | 6.8 ± 0.5 | −13.9 | 123.7 ± 9.7 | 94.2 ± 5.8 | −23.8 |
| IT188180(8) | 13.4 ± 0.6 | 9.2 ± 0.3 | −31.3 | 173.3 ± 13.6 | 96.7 ± 6.4 | −44.2 |
| IT188181(9) | 11.6 ± 0.3 | 10.7 ± 0.3 | −7.8 | 215.6 ± 4.0 | 200.9 ± 6.6 | −6.8 |
| IT210064(10) | 8.4 ± 1.0 | 7.3 ± 0.5 | −13.1 | 182.5 ± 3.6 | 153.6 ± 5.8 | −15.8 |
| IT262109(11) | 10.1 ± 0.9 | 9.8 ± 0.3 | −2.97 | 148.3 ± 10.9 | 138.1 ± 5.8 | −6.9 |
| IT279432(12) | 9.5 ± 0.5 | 9.2 ± 0.3 | −3.16 | 117.8 ± 10.5 | 109.3 ± 5.5 | −7.2 |
| IT280392(13) | 11.3 ± 0.5 | 9.9 ± 0.7 | −12.4 | 163.3 ± 10.2 | 148.0 ± 6.0 | −9.4 |
| IT280393(14) | 9.5 ± 0.7 | 7.8 ± 1.1 | −17.9 | 127.4 ± 5.5 | 75.5 ± 4.4 | −40.7 |
| IT280394(15) | 12.3 ± 0.8 | 10.7 ± 0.6 | −13.0 | 112.9 ± 10.9 | 86.8 ± 3.6 | −23.1 |
| IT280395(16) | 13.1 ± 0.5 | 11.3 ± 0.4 | −13.7 | 109.4 ± 14.3 | 81.9 ± 4.5 | −25.1 |
| IT280396(17) | 9.2 ± 0.6 | 7.9 ± 0.3 | −14.1 | 106.5 ± 11.6 | 73.6 ± 8.0 | −30.9 |
| IT280397(18) | 14.8 ± 0.6 | 12.6 ± 0.6 | −14.9 | 119.5 ± 6.8 | 80.8 ± 5.0 | −32.4 |
| IT280398(19) | 13.2 ± 0.4 | 11.9 ± 0.7 | −9.9 | 86.1 ± 2.4 | 68.8 ± 9.6 | −20.1 |
| IT293143(20) | 10.7 ± 0.5 | 9.6 ± 0.5 | −10.3 | 134.2 ± 19.3 | 109.2 ± 3.6 | −18.6 |
| IT293144(21) | 9.7 ± 0.5 | 8.4 ± 0.3 | −13.4 | 175.7 ± 5.9 | 144.2 ± 8.0 | −17.9 |
| IT301979(22) | 8.6 ± 0.2 | 7.3 ± 0.3 | −15.1 | 138.6 ± 8.8 | 87.4 ± 8.6 | −36.9 |
| IT301980(23) | 11.8 ± 0.2 | 10.7 ± 0.3 | −9.3 | 189.2 ± 8.1 | 172.2 ± 3.9 | −9.0 |
| IT304044(24) | 12.6 ± 0.2 | 10.6 ± 0.3 | −15.9 | 167.4 ± 6.4 | 115.7 ± 4.3 | −30.9 |
| Average | 10.7 ± 0.5 | 9.23 ± 0.5 | −13.5 | 144.2 ± 8.9 | 111.31 ± 5.7 | −23.8 |
| 100 mM Salt | Fv/Fm (%) | Fresh Weight (%) | Dry Weight (%) | Number of Leaves (%) | Leaf Area (%) | ||
|---|---|---|---|---|---|---|---|
| Scion | Rootstock | Scion | Rootstock | ||||
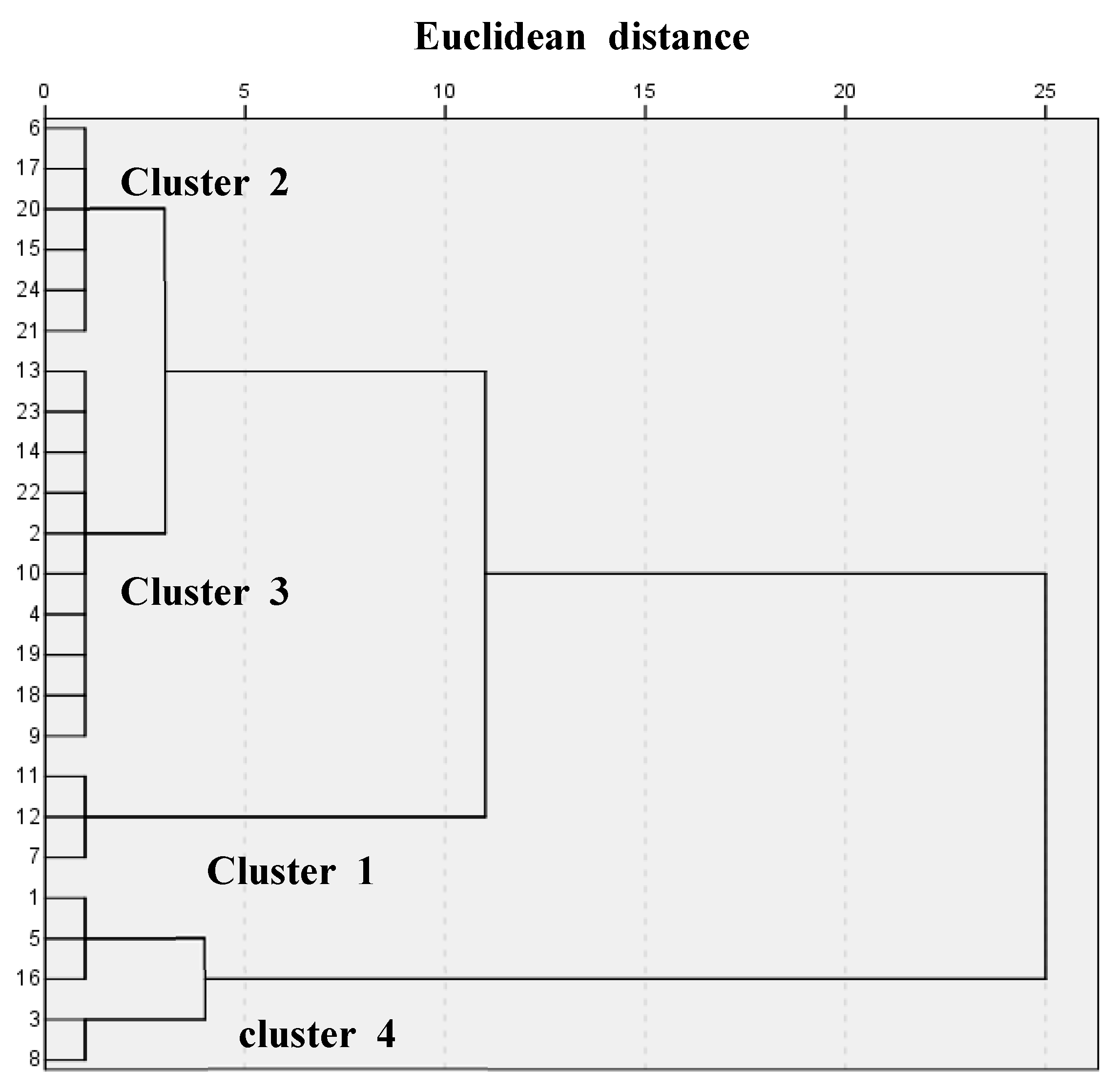
| Cluster 1 | 3.70 c | 5.50 b | 12.70 b | 9.97 b | 19.90 c | 6.68 c | 112.63 b |
| Cluster 2 | 11.73 b | 25.47 a | 26.32 ab | 21.92 a | 44.08 b | 14.22 ab | 25.77 ab |
| Cluster 3 | 14.28 b | 25.33 a | 25.83 ab | 24.10 a | 52.90 ab | 12.70 bc | 22.00 ab |
| Cluster 4 | 43.13 a | 31.13 a | 30.68 a | 23.53 a | 66.57 a | 20.42 a | 33.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-k.; Kim, S.-H.; Lee, J.-H.; Kim, K.-Y.; Sim, J.-E.; Jang, D.-C.; Park, S.-M. Physiological Responses of Pak Choi (Brassica rapa Subsp. Chinensis) Genotypes to Salt Tolerance. Horticulturae 2023, 9, 1161. https://doi.org/10.3390/horticulturae9111161
Park H-k, Kim S-H, Lee J-H, Kim K-Y, Sim J-E, Jang D-C, Park S-M. Physiological Responses of Pak Choi (Brassica rapa Subsp. Chinensis) Genotypes to Salt Tolerance. Horticulturae. 2023; 9(11):1161. https://doi.org/10.3390/horticulturae9111161
Chicago/Turabian StylePark, Han-kyeol, Si-Hong Kim, Joo-Hwan Lee, Kyeong-Yeon Kim, Jeong-Eun Sim, Dong-Cheol Jang, and Sung-Min Park. 2023. "Physiological Responses of Pak Choi (Brassica rapa Subsp. Chinensis) Genotypes to Salt Tolerance" Horticulturae 9, no. 11: 1161. https://doi.org/10.3390/horticulturae9111161
APA StylePark, H.-k., Kim, S.-H., Lee, J.-H., Kim, K.-Y., Sim, J.-E., Jang, D.-C., & Park, S.-M. (2023). Physiological Responses of Pak Choi (Brassica rapa Subsp. Chinensis) Genotypes to Salt Tolerance. Horticulturae, 9(11), 1161. https://doi.org/10.3390/horticulturae9111161






