Abstract
In this study, the solid culture method, and Plantform™ and SETIS™ temporary immersion bioreactor systems were used comparatively to propagate, root, and acclimatize ‘Grande Naine’ and ‘Azman’ banana varieties for rapid, cheap, and mass production in in vitro conditions. Micropropagation rate, plant height, number of leaves, and fresh and dry weight parameters were investigated in the micropropagation stage across eight subcultures. Rooting rate, plant height, number of leaves, number of roots/plant, root length, fresh and dry weight parameters were investigated in the rooting stage. Photosynthetic pigment analyses and stoma examinations were performed throughout all stages. In the micropropagation stage, a 20% increase in the Plantform™ system, a 12% increase in the SETIS™ system in ‘Grande Naine’, an 82% increase in the Plantform™ system, and a 98% increase in SETIS™ system in ‘Azman’ were determined compared to the solid culture. At the rooting stage, higher data were obtained from bioreactor systems than solid culture. Plants from bioreactor systems acclimatized faster and developed healthier in the greenhouse stage. It was determined that stomata were more active, and pigment accumulation was higher in bioreactor systems. Genetic variations across subcultures are among the most critical issues in banana clonal propagation. Leaf samples were taken from each system, and plant variation was investigated using SSR (Simple Sequence Repeat) markers. No variation was observed from the initial stage to the greenhouse stage. As a result, it has been determined that bioreactor systems are an essential alternative for the mass production of bananas.
1. Introduction
For years, classical tissue culture techniques have been used to produce seedlings and saplings in many plant species through micropropagation. However, recent years have introduced modern tissue culture techniques, such as bioreactor systems, as an alternative to classical techniques. Bioreactor systems offer cost, labor, and time savings, especially in mass production [,]. Plant production in a bioreactor is achieved by temporary immersion in liquid nutrients and regular aeration. This provides faster growth and improved plant quality than conventional gelled medium []. The concept of automatic plant tissue culture systems was introduced in 1985 [], and it has become convenient and easily accessible today with various companies’ standardization and mass production of different bioreactor systems. Although the number of designed systems with similar working principles is increasing, handmade bioreactors are also commonly used.
Bananas (Musa spp.) are the world’s most important fruit crop in acreage and production. Among all agricultural commodities, it is the seventh largest traded commodity in the world, following wheat, maize, soybean, rice, barley, and sugar. As a tropical fruit originating from Asia, bananas are a popular and convenient fruit worldwide, as they are affordable, nutritious, and available throughout the year []. While world banana production increased by 14% in the last ten years (2011–2021), it increased by 425% in Türkiye []. Parallel to this significant increase in production, the need for a significant amount of high-quality banana saplings has emerged. The Cavendish banana is the most widely commercialized type, accounting for about 47% of global production. Among the Cavendish bananas, the Grand Naine and Dwarf Cavendish are the most traded internationally []. The ‘Azman’ variety has good market value because it is fragrant, delicious, and durable to transport. This banana variety’s plants are more resistant to low temperatures than other varieties and are vigorous and productive []. It is grown under the ecology of Türkiye and as an alternative to the ‘Grande Naine’ variety.
The use of Plantform™ and SETIS™ systems in different plant species is common in the literature. Studies on the use of bioreactor systems in banana micropropagation are still limited. This study aimed to evaluate the effects of different culture systems on the in vitro propagation of two different banana varieties. In addition to in vitro micropropagation, rooting, acclimatization performances, stoma examinations, pigment contents, and genetic stability analyses were made to determine the most efficient production method.
2. Materials and Methods
2.1. Plant Materials and In Vitro Establishment
‘Grande Naine’ and ‘Azman’ banana varieties were used in the study. The varieties’ 8-month-old rhizomes (100 pieces of each) were collected from the greenhouses, pre-cleaned, and brought to the laboratory. The rhizomes were cut down slowly to not damage the growth tip and made ready for sterilization. The rhizomes prepared for sterilization were brought into sterile cabinets. Explants were treated with 0.05% HgCl2 (mercuric chloride) + a few drops of Tween 20 for 10 min, then rinsed thrice with sterile water. The explants were cut 0.5 cm long and wide, soaked in 70% ethanol for one minute, then treated with 10% NaClO (sodium hypochlorite) + Tween 20 solution for 10 min, and rinsed with sterile water. This step has been repeated twice []. After sterilization, shoot tips were transferred to culture dishes containing one mg L−1 BAP (6-Benzilaminopurin) and MS nutrient medium []. We added 30 g L−1 sucrose and 8 g L−1 agar to the nutrient media. Shoot tips were cultured in the dark and at 25 °C ambient temperature during the micropropagation stages. Plants were subcultured thrice every four weeks to obtain plant material for micropropagation experiments in solid culture, and Plantform™ and SETIS™ systems.
2.2. Comparison of Different Culture Systems in the Micropropagation Stage
Compared to the classic solid culture method with Plantform™ [] and SETIS™ [], temporary immersion bioreactor systems were used. Glass culture vessels with a diameter of 8 cm and a height of 10 cm were used in the solid culture method. Following the culture containers, lids close to transparent, allowing light to pass, are preferred. In the solid culture method, 50 mL of nutrient medium and five plants were placed in the culture dishes. In bioreactor systems, 50 plants were placed in each culture vessel, and 500 mL of nutrient medium was used. Thus, 10 mL of nutrient medium was used for each plant. The micropropagation stage used the MS nutrient medium containing 2 mg L−1 BAP. Plantlets transferred to the propagation media were subjected to 16 h of light and 8 h of dark, 25 °C culture conditions (with the light intensity of 42 μmol/m2s). Plantlets were subcultured eight times, every four weeks in solid culture and every six weeks in bioreactor systems. The immersion frequencies were applied for 10 min every 8 h in the Plantform™ system and 2 min every 4 h in the SETIS™ system. After six weeks of cultures, 20 plants from each culture system were randomly selected with three replications, and plant height, proliferation coefficient, number of leaves, and fresh and dry weight parameters were examined. In addition, chlorophyll contents and stomata were examined in leaf samples taken from each subculture.
2.3. Comparison of Different Culture Systems in the Rooting Stage
Rooting experiments were conducted using an MS medium containing 1 mg L−1 IBA (Indole-3-butyric acid). In the rooting stage of plants in temporary immersion bioreactor systems, immersion periods of 10 min were applied every eight hours in the Plantform™ system and 2 min every four hours in the SETIS™ system, as in the micropropagation experiments. After eight weeks, plant height, rooting rate, root length, root number, fresh weight, and dry weight parameters were examined in 50 randomly selected plants from each culture system.
2.4. Photosynthetic Pigment and Total Carotenoid Analysis
To analyze chlorophyll a, chlorophyll b, total chlorophyll, and total carotenoids, 0.2 g of leaf samples were taken from each subculture from each system at the micropropagation, rooting, and greenhouse stages. The samples were homogenized in 15 mL of 80% acetone solution using an ultrathorax device under dim light. The resulting solution was passed through 0.22 µm microfilters and read in the spectrophotometer at 470, 645, 652, and 663 nm wavelengths. The obtained values were calculated using the coefficients Lichtenthaler [] provided.
2.5. Stoma Investigations
Stomata were examined on the lower and upper surfaces of leaves from plants grown in solid culture and bioreactor systems at different stages of growth. Transparent nail varnish was applied to the surfaces of the leaves, creating a mold of the stomata to conduct the examination. The mold was transferred onto a transparent tape and placed on a microscope slide []. Stomata were counted and measured using a microscope at different magnifications. Stomata density was calculated at 40× magnification by counting the number of stomata in a given area, usually measured in mm2. Stomata width and length were measured at 100× magnification, while stomata opening was also observed at this magnification.
2.6. Ex Vitro Acclimatization
Before the plants obtained from the solid culture rooting experiments were transferred to external conditions, the culture containers were opened gradually, and the preliminary acclimation process was carried out in the laboratory. The plants were transferred to the vials without pre-exercising in the bioreactor systems. After the plants obtained from the solid culture were wholly removed from the agar with water, they were transferred directly to vials containing a 1:1 ratio of peat/perlite mixture in the greenhouse since there was a liquid culture in the bioreactor systems. The plants were gradually acclimated to the external conditions under the mini plastic tunnel. Plantlets from solid culture and temporary immersion bioreactor systems were observed in individual vials. Plant height and number of leaves were measured 12 weeks after the plants acclimatized to the external environment.
2.7. Determination of Genetic Stability of Plants
In micropropagation experiments using classical and new-generation tissue culture methods, SSR analyses determined whether there was variation at the end of subcultures, rooting stage, and plants acclimatized to external conditions. In the micropropagation stage, at the end of each subculture, 8 samples (8 solid cultures, 8 Plantform™, and 8 SETIS™), three rootings (1 solid culture, 1 Plantform™, and 1 SETIS™), and three greenhouses (1 solid culture, 1 Plantform™, and 1 SETIS™) completed the adaptation. DNA analyses were conducted in 90 plants for each variety, with three replications from each of the 30 samples. Two types of bananas were used in the study. For this reason, SSR analyses were carried out with 180 plants. DNA isolation from the obtained plants was carried out using the DNAeasy Plant Mini Kit (Qiagen, Germany) method. SSR analyses were performed with the isolated DNAs. PCR (Polymerase Chain Reaction) reactions were prepared with a total volume of 20 µL, and a Li-Cor gel system (Li-Cor DNA analyzer 4300-Biosciences, Germany) was used while images were taken of the PCR products. Thirteen microsatellite primers developed specifically for bananas were used in SSR analysis [] (Table 1).

Table 1.
Primers and sequence information used to determine the genetic stability of plants.
2.8. Experimental Plan and Statistical Analysis
All of the experiments for micropropagation and rooting of banana plants were established according to the “factorial order in random plots” experimental design with three replications. Analysis of variance was performed with the data obtained in the study. The LSD test determined differences between significant means. The JMP 5.01 program [] was used for statistical analysis.
3. Results
3.1. Assessing the Impact of Different Culture Methods on Shoot Multiplication
The results obtained in the micropropagation stage showed significant differences according to the varieties and between the applied culture systems (Table 2). System selectivity among varieties has been observed (Figure 1). For the ‘Grande Naine’ variety, the highest shoot multiplication was obtained with an average of 4.32 shoots per explant in the Plantform™ system, while 4.01 shoots per explant were obtained in the SETIS™ system. The lowest shoot multiplication was obtained in solid culture application with 3.58 shoots per explant. In the ‘Azman’ variety, differences of up to two-fold were observed between solid culture and SETIS™ system. The highest shoot multiplication was obtained at 4.08 shoots per explant in the SETIS™ system. The lowest shoot multiplication was obtained in solid culture application with 2.06 shoots per explant. In the Plantform™ system, the highest shoot multiplication was obtained in the ‘Grande Naine’ variety, while the highest yield in the SETIS™ system was obtained in the ‘Azman’ variety. The longest plant height data for the ‘Grande Naine’ variety was obtained at 4.38 cm in the solid culture application, while plant height data of 4.06 cm and 3.88 cm were obtained in the Plantform™ system and the SETIS™ system, respectively.

Table 2.
Effects of different culture systems on in vitro micropropagation of banana varieties. Means with different letters are significantly different according to the LSD test (p ≤ 0.01).

Figure 1.
Plants obtained from different culture systems in the multiplication stage. All plants are 6-week-old plants obtained from media containing MS + 2 mg L−1 BAP (ruler 15 cm).
Similarly, the longest plant height data was obtained at 3.98 cm in the solid culture medium for the ‘Azman’ variety. The lowest plant height data were obtained in the SETIS™ system for the ‘Grande Naine’ variety and in the Plantform™ system for the ‘Azman’ variety. The plants showed more robust growth in the bioreactor systems (Figure 2). The highest fresh weight data for ‘Grande Naine’ were obtained in the Plantform™ and SETIS™ systems (1.89 and 1.85). The lowest data were obtained in the solid culture application for both varieties. However, better results were obtained in the SETIS™ system (1.73 g) compared to the ‘Azman’ variety’s Plantform™ and solid culture method. When examining the dry weight data, the ‘Grande Naine’ variety showed the highest value of 0.22 g in the Plantform™ system. The highest dry weight datum of 0.18 g for the ‘Azman’ variety was obtained from the SETIS™ system. Conversely, the solid culture application observed the lowest dry weight datum of 0.09 g.

Figure 2.
Development of plants in different culture systems. (a) Solid culture initiation stage. (b) Two-week-old plants in solid culture. (c,d) Plants that have reached the transfer stage in solid culture. (e,f) Plantform™ initiation stage. (g,h) Plants obtained after 6 weeks of culture in Plantform™ system. (i) SETIS™ initiation stage. (j) SETIS™ system and growth of plants in 3-week culture. (k) Plants obtained after 6 weeks of culture in SETIS™ system.
3.2. Assessing the Impact of Different Culture Methods on Rooting
The results obtained during the rooting stage showed significant differences among the varieties and between the applied culture systems (Table 3). The highest plant height recorded was 8.51 cm, obtained through SETIS™ application in the ‘Grande Naine’ variety. The lowest plant height recorded in the solid culture application was 5.45 cm. For the ‘Azman’ variety, the highest plant height of 7.79 cm was achieved through the SETIS™ application, while the lowest plant height recorded was 5.28 cm in the solid culture application. During the rooting stage, the plants exhibited more robust growth in the bioreactor systems compared to the solid culture application, with the SETIS™ system showing particular selectivity (Figure 3 and Figure 4). In the number of roots/plant data, the highest data was obtained in the ‘Azman’ variety SETIS™ application with 9.92 number of roots/plant. For the ‘Grande Naine’ variety, the highest value was obtained in the SETIS™ application with 9.18 number of roots/plant, indicating the equal importance of this parameter in both varieties. The lowest number of roots/plant for both varieties was observed in the solid culture application. The longest roots were observed in the ‘Grande Naine’ variety, with 13.47 cm in the SETIS™ application. In comparison, the longest root length in the ‘Azman’ variety was recorded as 12.03 cm in the Plantform™ system. The shortest root length data for both varieties were obtained from the solid culture application. The lowest root length data were obtained from solid culture applications for both varieties. The highest data were obtained in the Plantform™ application of the ‘Grande Naine’ variety, with 6.63 leaves in the leaf count examinations. The lowest number of leaves was obtained from the ‘Azman’ variety Plantform™ application. The highest number of leaves in the ‘Azman’ variety was obtained from solid culture application. When the fresh weight data were examined, the highest datum was obtained with 3.21 g wet weight in the ‘Azman’ variety SETIS™ application. The lowest wet weight data were obtained from the ‘Azman’ variety solid culture application. The highest wet weight data for the ‘Grande Naine’ variety were obtained from SETIS™ (2.94 g) and Plantform™ (2.84 g) systems.

Table 3.
Effects of different culture systems on in vitro rooting of banana varieties. Means with different letters are significantly different according to the LSD test (p ≤ 0.01). Differences between non-letterization values are insignificant.
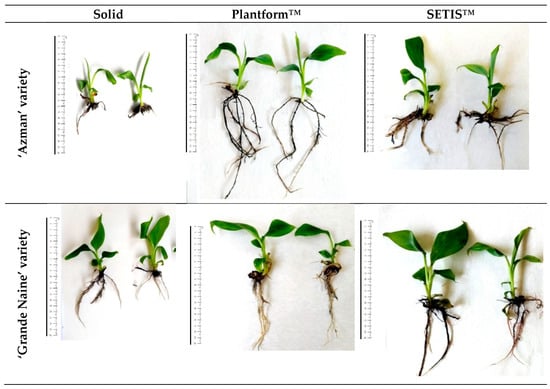
Figure 3.
Plants obtained from different culture systems at the rooting stage after eight weeks (ruler 20 cm).

Figure 4.
The development of plants in the rooting stage. (a–c) Solid culture initial and rooting stages in Grande Naine variety. (d,e) Plants obtained from Plantform™ rooting experiments in Azman variety. (f,g) Plants obtained from Plantform™ rooting experiments in Grande Naine variety. (h) SETIS™ system and growth of rooting stage plants. (i) Plants obtained from SETIS™ rooting experiments in Azman variety (j,k) Plants obtained from SETIS™ rooting experiments in Grande Naine variety.
3.3. Stomata Examinations
Significant differences emerged between the culture systems and varieties used in the stoma examinations conducted on the anterior and posterior surfaces of the leaf (Table 4). When the stomatal density on the front surface of the leaf was examined, the highest stoma numbers were obtained in the SETIS™ system as 18.73/mm2 for the ‘Grande Naine’ variety and 20.01/mm2 for the ‘Azman’ variety. When the stomatal density on the front surface of the leaf was examined, the highest stomata numbers were obtained in solid culture application for both varieties. In bioreactor systems, the number of stomata per mm2 was generally similar at the same significance level. When examining the stomatal openings on the anterior and posterior surfaces of the leaf, it was observed that the stomata of plants obtained from bioreactor systems were similarly more active. In solid culture micropropagation experiments, it was observed that although the plants generally contained more stomata per mm2, the stomata were more closed. On the other hand, the plants obtained from bioreactor systems had a lower stomatal density per mm2, but their stomata were more active.

Table 4.
Effects of different culture systems on in vitro stomatal activity of banana varieties. Means with different letters are significantly different according to the LSD test (p ≤ 0.01). The stoma area used to create stoma profiles is given as an average.
Stomata profiles were created by taking the averages of the obtained width–length measurements (Figure 5). In stoma profiles, it was observed that the stomata on the anterior surface of the leaf were smaller and more rounded, while the stomata on the posterior surface of the leaf were more elliptical and larger. When stomata profiles on the back surface of the leaf were examined, it was observed that stomata had a larger area in bioreactor systems.
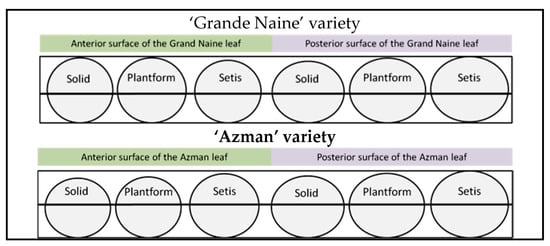
Figure 5.
Stomatal profiles of plants obtained from different culture systems.
3.4. Assessing the Impact of Different Culture Methods on Acclimatization
During the acclimatization stage to external conditions, the plants showed a 100% survival percentage in all treatments in both varieties (Table 5). In the greenhouse stage, the highest plant height was obtained from plants from the ‘Azman’ variety SETIS™ system at 23.91 cm. The lowest plant height datum was obtained with 22.18 cm from plants obtained from the Plantform™ system and 15.73 cm from the solid culture system. The highest plant height data were obtained in SETIS™ (23.42 cm) and Plantform™ (22.87 cm) systems in the ‘Grande Naine’ variety, and the lowest plant height data were obtained in plants obtained from the solid culture (18.35 cm) method. When the number of leaves of the plants in greenhouse conditions was examined, the highest number was obtained in the solid culture (8.73) application in the ‘Grande Naine’ variety and the SETIS™ system in the ‘Azman’ variety.

Table 5.
Effects of different culture systems on acclimatization of banana varieties. Means with different letters are significantly different according to the LSD test (p ≤ 0.01).
3.5. Effect of Different Culture Systems on Photosynthetic Pigment Contents
Photosynthetic pigment content analyses were evaluated separately at micropropagation, rooting, and greenhouse stages (Figure 6). During the micropropagation stage, in the analysis conducted using shoots obtained from different culture systems, the highest chlorophyll-a content was measured in the SETIS™ system, as 0.546 mg g−1 FW in the ‘Grande Naine’ variety and 0.564 mg g−1 FW in the ‘Azman’ variety. In the Plantform™ system, a slight decrease in chlorophyll-a content was observed (0.512 mg g−1 FW and 0.425 mg g−1 FW), while the lowest content was measured in solid culture applications, with 0.409 mg g−1 FW and 0.345 mg g−1 FW. The highest chlorophyll b content in the SETIS™ system was obtained as 0.251 mg g−1 FW in the ‘Azman’ variety. Decreases in chlorophyll b content occurred in Plantform™ (0.160 mg g−1 FW) and solid culture (0.116 mg g−1 FW) applications. While the highest chlorophyll b content was obtained in SETIS™ (0.216 mg g−1 FW) and Plantform™ systems (0.200 mg g−1 FW) in ‘Grande Naine,’ decreases were observed in solid culture (0.167 mg g−1 FW) application. Similarly, the highest values in total chlorophyll contents were obtained in the SETIS™ system. There was some reduction in the Plantform™ system, but the lowest contents were obtained in the solid culture application.
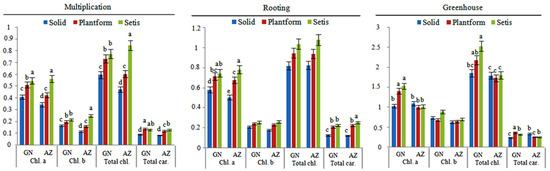
Figure 6.
The effect of different culture systems on photosynthetic pigment contents (mg g−1 FW) of banana varieties during micropropagation, rooting, and greenhouse stages. Different letters within a bar denote statistically significant differences according to the LSD test at p ≤ 0.01. Differences between non-letter bars are insignificant. (GN: Grande Naine variety; AZ: Azman variety; Chl. a: Chlorophyll a; Chl. b: Chlorophyll b; Total chl.: Total chlorophyll; Total car.: Total carotenoid).
When comparing chlorophyll contents during the rooting stage, the highest values were observed in the ‘Grande Naine’ variety in the SETIS™ system, with 0.744 mg g−1 FW. In the Plantform™ system, the value was 0.712 mg g−1 FW, while the lowest value of 0.579 mg g−1 FW was obtained in the solid culture method. In the ‘Azman’ variety, the highest values were obtained in the SETIS™ system with 0.779 mg g−1 FW, followed by the Plantform™ system with 0.675 mg g−1 FW. The solid culture method obtained the lowest value of 0.501 mg g−1 FW. When examining chlorophyll b contents, the highest data were obtained from the bioreactor systems. However, these results were found to be statistically insignificant. While the total chlorophyll levels were also insignificant, similar to chlorophyll b contents, higher values were measured in the bioreactor systems. The highest results for total carotenoid contents were obtained in the SETIS™ (0.223 mg g−1 FW) and Plantform™ (0.206 mg g−1 FW) systems for the ‘Grande Naine’ variety. There was a significant decrease in total carotenoid content in the solid culture (0.123 mg g−1 FW). Similarly, for the ‘Azman’ variety, the highest values were obtained in the SETIS™ (0.251 mg g−1 FW) and Plantform™ (0.221 mg g−1 FW) systems, while a decrease was observed in the solid culture (0.118 mg g−1 FW) application.
While the highest chlorophyll contents of the ‘Grande Naine’ variety were determined in plants obtained from SETIS™ (1.535 mg g−1 FW) and Plantform™ (1.411 mg g−1 FW) systems in greenhouse stage plants, comparable results were obtained in the ‘Azman’ variety in all applications. Differences in chlorophyll b contents between application and varieties were not found to be significant. Similarly, high values were measured in total chlorophyll contents in plants obtained from SETIS™ (2.537 mg g−1 FW) and Plantform™ (2.186 mg g−1 FW) systems in the ‘Grande Naine’ variety. In contrast, similar results were obtained in the ‘Azman’ variety in all applications. While the highest values in total carotenoid contents were obtained in the Plantform™ (0.373 mg g−1 FW) system in the ‘Grande Naine’ variety, it was obtained in solid culture (0.339 mg g−1 FW) applications and the SETIS™ (262 mg g−1 FW) system in the ‘Azman’ variety.
3.6. Genetic Stability Analysis Results
For genetic stability tests, SSR analyses were performed with DNA samples obtained from plant materials at the initial, solid culture micropropagation and rooting stage, Plantform™ micropropagation and rooting stage, SETIS™ micropropagation and rooting stage, and greenhouse stage. As a result of the analysis using 13 SSR primers, no variation was found in the starting material and eight subculture micropropagation stages and in the plants in the rooting stage and greenhouse conditions. An example of the obtained gel image is presented in Figure 7.
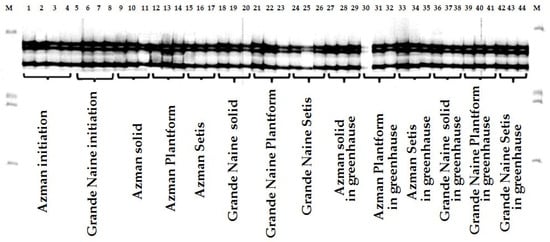
Figure 7.
Gel images of Maocen13 primer: ‘Azman’ (AZ) and ‘Grande Naine’ (GN) initiation samples and root and greenhouse stages, M: 50-350 bp DNA ladder.
4. Discussion
4.1. Assessing the Impact of Different Culture Methods on Shoot Multiplication
As a result of the research, it has been conclusively established that bioreactor systems represent an indispensable alternative for the large-scale cultivation of bananas, encompassing both of the banana varieties examined in the study. Noceda et al. [] reported that temporary immersion bioreactor systems strengthen micro-shoots and increase the multiplication rate in banana in vitro propagation. Wilken et al. [] reached the results of 4.2 shoots/plants in a 5-L culture pot and 3 shoots/plants in a 40 L culture bowl for the ‘Grande Naine’ banana variety in the bioreactor system they developed. Morena et al. [] reported that large-scale production of banana in vitro propagation can be performed using bioreactor systems. They explained that using the Rita bioreactor system significantly increases the multiplication rate and other parameters. However, they used 500 mL of nutrient medium for 10 plants in the Plantform™ system and 200 mL for four plants in the Rita bioreactor system. Bello Bello et al. [] investigated in vitro micropropagation and rooting possibilities of the ‘Grande Naine’ banana variety by comparing bioreactor systems with liquid culture and solid culture medium.
Our study obtained the lowest values in the multiplication rate data in liquid culture and solid culture applications, 2.60 and 2.86 shoots/plants. The highest reproduction coefficient was reached in the SETIS™ system with 7.30 shoots/plants. Researchers used 4 mg L−1 BA, and 50 mL of nutrient medium was used for each plant. In this study, the highest multiplication rate data were obtained in the Plantform™ system of ‘Grande Naine,’ 4.32 shoots/plant, and the ‘Azman’ variety, 4.03 shoots/plants in the SETIS™ system. It used 2 mg L−1 BA, and 10 mL of nutrient medium was used for each plant. The use of bioreactor systems similarly resulted in increases in the multiplication rate. In large-scale production, 50 mL of nutrient medium per plant can incur a significant cost. Uma et al. [] compared the bioreactor system they developed for banana in vitro propagation with the solid culture method and obtained 2.7 times more shoots. Abdulmalik et al. [] reported significant increases in shoot propagation and rooting percentages in the bioreactor system they designed for banana in vitro propagation. Similar to using bioreactor systems in the study, a significant increase in the multiplication rate was achieved. In this study, the multiplication rate of the ‘Azman’ variety increased up to twice using bioreactor systems.
The plant height data showed that the plants that gave a large number of shoots were shorter than those that gave a small number of shoots because they directed their energy toward shoot development. Roels et al. [] reported that using a bioreactor system in the in vitro propagation of bananas significantly increased the growth coefficient and the highest data in plant height measurements were obtained using the solid culture method. Bello-Bello et al. [] achieved the highest results in plant height measurements with the solid culture method. Similarly, in this study, while a significant increase was observed in the shoot multiplication in bioreactor systems, higher results were obtained with the solid culture method in the plant height parameter.
In the micropropagation stage, fresh and dry weight data increases were observed with a greater shoot yield and mass increase in strongly developing shoots in bioreactor systems. In their study, Ramírez-Mosqueda and Bello-Bello [], investigating the in vitro regeneration possibilities of the vanilla plant, reached the highest wet and dry weight data using the SETIS™ system. Hwang et al. [], in their study of the Golden Bel chrysanthemum plant, obtained the highest fresh and dry weight data in the SETIS™ system. Park and Jeong [] reported increases of up to 3 times in the fresh weight of plants obtained from temporary immersion bioreactor systems. Zhang et al. [] reported the biomass and alkaloid ratios of the medicinal plant Dendrobium nobile Lindl. increased from 2 to 10 times compared to solid culture using the Bioreactor system. Saptari et al. [] obtained up to a 40% increase in rates of viability and steviol glycoside yield in the bioreactor system in the stevia plant. In this study, using bioreactors, the wet weight data increased by 23% in the ‘Grande Naine’ variety and 61% in the ‘Azman’ variety compared to the solid culture method. Bioreactor systems increased dry weight data up to twice in the ‘Azman’ variety.
4.2. Assessing the Impact of Different Culture Methods on Rooting
In rooting experiments using bioreactor systems, extremely high results were obtained for both varieties in all critical parameters compared to solid culture. Leyva-Ovalle et al. [] reported that they did not need another protocol for rooting when the plants formed roots in the micropropagation stages using the TIS bioreactor system in the orchid species. This study observed the rooting tendency in banana plants even at the micropropagation stage in bioreactor systems. By turning to this advantage in the rooting stage, vital developments were observed in both the plant and root parts. Wilken et al. [] reported a high rooting rate in bioreactor systems for in vitro ‘Grande Naine’ banana variety rooting. Bello Bello et al. [] found that the highest root number per plant was reached, with 5.33 in plantlets obtained from the SETIS™ system. In SETIS™, partial immersion, and semi-solid culture systems, plantlets with the highest root length were obtained with an average length of 6 cm. This study obtained quite a high root number (9.55) and root length (11.13) data compared to other studies in the plants obtained from the SETIS™ system. Uma et al. [] reported that rooting percentage and number of roots per shoot increased significantly in banana plants using the temporary immersion bioreactor system. Costa et al. [] reported that the rooting properties of the Dwarf Cavendish banana variety were significantly increased by the use of silicon elements and the temporary immersion bioreactor system compared to the solid culture method. By evaluating the data we have obtained and similar studies in the literature, bioreactor systems significantly improve the in vitro rooting properties of the banana plant.
Temporary immersion bioreactor systems increase biomass in vitro to supply root-derived substances, producing solid and numerous roots. Pavlov and Bley [] obtained biomass increase and maximum betalain yield from capillary root culture of radish plant using RITA temporary immersion bioreactor. Mišic et al. [] achieved approximately 2–4 times higher biomass production rate and up to 8 times higher total secoiridoid glycoside production rate in RITA® bioreactors for the production of secoiridoid glycosides, which are of immense importance to the food and pharmaceutical industry. The wet weight values obtained in the rooting experiments in this study prove that using a bioreactor system significantly increases biomass. According to the solid culture method, fresh weight data showed an increase of 30% in the ‘Grande Naine’ variety and 179% in the ‘Azman’ variety.
4.3. Effect of Different Culture Systems on Stoma Examinations and Photosynthetic Pigment Contents
Yang and Yeh [] investigated in vitro leaf anatomy and ex vitro photosynthetic contents of linden plants obtained from solid media and temporary immersion systems. They reported that plants obtained from the bioreactor system produced thicker leaf chlorenchyma and aquifer parenchyma, lower stomatal density, and more epicuticular wax than plants in solid media. Park and Jeong [] reported the highest chlorophyll content and the most normal stoma formation using temporary immersion bioreactor systems in the in vitro propagation of clove plants. In this study, during the solid culture micropropagation experiments, it was observed that although the plants generally contained more stomata per mm2, the stomata were more closed, while the plants obtained from bioreactor systems contained fewer stomata per mm2, but the stomata were more active. Width and length measurements of the stomata were taken during the micropropagation trials. Although there was no significant structural difference, it was observed that stomata were larger in bioreactor systems.
Additionally, the stomata on the anterior surface of the leaf were smaller and more rounded, while the stomata on the posterior surface of the leaf were more elliptical and larger. Uma et al. [] obtained the highest chlorophyll a, b, a/b ratio and carotenoid contents from the TIB bioreactor system in banana in vitro propagation. Ramírez-Mosqueda and Iglesias-Andreu [], in their study investigating the in vitro growth possibilities of the vanilla plant, found that the highest pigment accumulation (chlorophyll a, b, and a + b) was obtained in the BIT® bioreactor system, and the highest carotenoid content was obtained in the BIG® bioreactor system. Bello-Bello et al. [] evaluated the chlorophyll content of banana plants. They measured the highest chlorophyll value in the semi-solid medium and the lowest in the TIB bioreactor system. The highest chlorophyll b value was determined in the SETIS™ bioreactor system. This study obtained the lowest chlorophyll contents in the solid culture method as 0.406 mg g−1 in ‘Grande Naine’ and 0.386 mg g−1 in the ‘Azman’ variety. The highest contents were reached at 0.546 mg g−1 in the ‘Grande Naine’ variety and 0.564 mg g−1 in the ‘Azman’ variety in the SETIS™ bioreactor system. Similarly, the highest values in chlorophyll b and total chlorophyll contents were measured in the SETIS™ system. In all measurements, pigment accumulation was measured much higher than the values obtained by the researchers.
4.4. Genetic Stability Analysis Results
SSR analysis was not employed for somaclonal variation in bananas by far. In their study, Bairu et al. [] observed an increasing trend in somaclonal variation in the Zelig banana variety with the progression of proliferation cycles and the concentration of BA, reaching a substantial 72% variation in the 10th subculture with a 7.5 mg L−1 BA application. On the other hand, Korneva et al. [] reported a modest 1.55% somaclonal variation in Barraganete banana (AAB) plants cultivated under controlled greenhouse conditions. These plants were derived from embryogenic cell suspensions and somatic embryos obtained through a temporary immersion system. Safarpour et al. [] conducted genetic stability tests using Random Amplified Polymorphic DNA (RAPD) analyses. They reported a significant 23.46% polymorphism in the in vitro propagation of the ‘Grande Naine’ banana variety, utilizing 4.5 mg L−1 BAP. In the present study, we employed 2 mg L−1 of BA as a growth regulator and conducted micropropagation experiments over eight successive subcultures, each lasting between 4 and 6 weeks. Notably, no morphological or genetic variations were detected from the initial stages of micropropagation to the greenhouse stage. It is worth noting that the banana genome size is reported to be approximately 523 megabases []. While the analyses conducted in this study yield valuable insights, the advent of next-generation sequencing technologies holds the potential to unveil more subtle mutations in the banana genome.
5. Conclusions
System selectivity among varieties has been observed. For the ‘Grande Naine’ variety, the highest shoot multiplication was obtained from the Plantform™ system. The highest shoot multiplication of the ‘Azman’ variety was obtained in the SETIS™ system. Besides the success in micropropagation, excellent results were also obtained in the rooting phase. In the rooting stage, both cultivars obtained better results in the SETIS™ system. The vigorous plants obtained continued their development in the greenhouse stage. As a result, it was determined that temporary immersion bioreactor systems used for ‘Grande Naine’ and ‘Azman’ banana cultivars are essential alternatives for in vitro banana production. Variety system selectivity was also observed. The most efficient production method can be determined by comparing different systems in different varieties.
Author Contributions
Conceptualization, Y.A.K. methodology, M.H.E., D.D., B.B., Ö.Ş. and Y.A.K.; data curation, M.H.E.; writing—original draft preparation, M.H.E., Y.A.K. and Ö.Ş.; writing—review and editing, Ö.Ş.; visualization, M.H.E.; supervision, Y.A.K.; project administration, Y.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Scientific and Technological Research Council of Türkiye (TUBITAK), TEYDEB-1505 University-Industry Cooperation Program (Project Number: 5190040).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank Eugene Steele, professional English Editor of Erciyes University, for the English language editing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aka Kaçar, Y.; Biçen, B.; Şimşek, Ö.Z.; Dönmez, D.; Erol, M. Evaluation and comparison of a new type of temporary immersion system (TIS) bioreactors for myrtle (Myrtus communis L.). Appl. Ecol. Environ. Res. 2020, 18, 1611–1620. [Google Scholar] [CrossRef]
- Şimşek, Ö.; Dönmez, D.; Sarıdaş, M.A.; Acar, E.; Kaçar, Y.A.; Kargı, S.P.; İzgü, T. In vitro and ex vitro propagation of Turkish myrtles through conventional and plantform bioreactor systems. PeerJ 2023, 11, e16061. [Google Scholar] [CrossRef] [PubMed]
- Plantform. 2023. Available online: https://www.plantform.se/pub/ (accessed on 8 August 2023).
- Tisserat, B.; Vandercook, C.E. Development of an automated plant culture system. Plant Cell Tiss. Org. 1985, 5, 107–117. [Google Scholar] [CrossRef]
- Wardhan, H.; Das, S.; Gulati, A. Banana and mango value chains. In Agricultural Value Chains in India: Ensuring Competitiveness, Inclusiveness, Sustainability, Scalability, and Improved Finance; Springer Nature: Berlin/Heidelberg, Germany, 2022; pp. 99–143. [Google Scholar] [CrossRef]
- Faostat. 2023. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 28 July 2023).
- Voora, V.; Larrea, C.; Bermudez, S. Global Market Report: Bananas; International Institute for Sustainable Development: Winnipeg, MB, Canada, 2020. [Google Scholar]
- Saridaş, M.A.; Kargi, S.P.; Bayiroğlu, B.M.; Şeyma, Y.A. New ecology for banana production of Turkey. Yuzuncu Yil Univ. J. Agric. Sci. 2017, 27, 370–377. [Google Scholar] [CrossRef]
- Kacar, Y.A.; Biçen, B.; Varol, I.; Mendi, Y.Y.; Serçe, S.; Çetiner, S. Gelling agents and culture vessels affect in vitro multiplication of banana plantlets. Genet. Mol. Res. 2010, 9, 416–424. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Vervit. 2023. Available online: http://www.vervit.be/products/technologies/setis-platform (accessed on 8 August 2023).
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef]
- Mattos, L.A.; Amorim, E.P.; Amorim, V.B.; Cohen, K.D.; Ledo, C.A.; Silva, S.D. Agronomical and molecular characterization of banana germplasm. Pesqui. Agropecu. Bras. 2010, 45, 146–154. [Google Scholar] [CrossRef]
- Crouch, J.H.; Crouch, H.K.; Constandt, H.; Van Gysel, A.; Breyne, P.; Van Montagu, M.; Jarret, R.L.; Ortiz, R. Comparison of PCR-based molecular marker analyses of Musa breeding populations. Mol. Breed. 1999, 5, 233–244. [Google Scholar] [CrossRef]
- Lagoda, P.J.L.; Noyer, J.L.; Baurens, F.C.; Lanaud, C.; Dambier, D. Abundance and Distribution of Simple Sequence Repeats in the Musaceae Family. Microsatellite Markers to Map the Banana Genome; IAEA: Vienna, Austria, 1995. [Google Scholar]
- Creste, S.; Tulmann Neto, A.; Vencovsky, R.; de Oliveira Silva, S.; Figueira, A. Genetic diversity of Musa diploid and triploid accessions from the Brazilian banana breeding program estimated by microsatellite markers. Genet. Resour. Crop Evol. 2004, 51, 723–733. [Google Scholar] [CrossRef]
- Oriero, C.E.; Odunola, O.A.; Lokko, Y.; Ingelbrecht, I. Analysis of B-genome derived simple sequence repeat (SSR) markers in Musa spp. Afr. J. Biotechnol. 2006, 5, 126–128. [Google Scholar]
- JMP®; Version <5.01>; SAS Institute Inc.: Cary, NC, USA, 1989–2023.
- Noceda, C.; Vargas, A.; Roels, S.; Cejas, I.; Santamaría, E.; Escalona, M.; Debergh, P.; Rodríguez, R.; Sandoval, J.; Cañal, M.J. Field performance and (epi) genetic profile of plantain (Musa AAB) clone ‘CEMSA ¾’plants micropropagated by temporary immersion systems. Sci. Hortic. 2012, 46, 65–75. [Google Scholar] [CrossRef]
- Wilken, D.; Jiménez Gonzalez, E.; Gerth, A.; Gómez-Kosky, R.; Schumann, A.; Claus, D. Effect of immersion systems, lighting, and TIS designs on biomass increase in micropropagating banana (Musa spp. cv. ‘Grande Naine’ AAA). Vitr. Cell. Dev. Biol. Plant 2014, 50, 582–589. [Google Scholar] [CrossRef]
- Moreno, A.; Bernal, Á.; Ugarte, F.; Lima, K.; Coig, M.; Sánchez, C.; Aldrey, A.; Vidal, N. Use of liquid medium and biofortificants for improving micropropagation and acclimation of Musa AAA cv. Williams. In Proceedings of the he 5th International Conference of the IUFRO Unit 2.09.02: Clonal Trees in the Bioeconomy Age: Opportunities and Challenges, Coimbra, Portugal, 10–15 September 2018. [Google Scholar]
- Bello-Bello, J.J.; Cruz-Cruz, C.A.; Pérez-Guerra, J.C. A new temporary immersion system for commercial micropropagation of banana (Musa AAA cv. Grand Naine). Vitro Cell. Dev. Biol. Plant 2019, 55, 313–320. [Google Scholar] [CrossRef]
- Uma, S.; Karthic, R.; Kalpana, S.; Backiyarani, S.; Saraswathi, M.S. A novel temporary immersion bioreactor system for large scale multiplication of banana (Rasthali AAB—Silk). Sci. Rep. 2021, 11, 20371. [Google Scholar] [CrossRef] [PubMed]
- Abdulmalik, M.M.; Usman, I.S.; Nasir, A.U.; Sani, L.A. Micropropagation of banana (Musa spp.) using temporary immersion bioreactor system. Bayero J. Pure Appl. Sci. 2020, 12, 197–200. [Google Scholar] [CrossRef]
- Roles, S.; Escalona, M.; Cejas, I.; Noceda, C.; Rodriguez, R.; Canal, M.J.; Sandoval, J.; Debergh, P. Optimization of plantain (Musa AAB) micropropagation by temporary immersion system. Plant Cell Tiss. Org. 2005, 82, 57–66. [Google Scholar] [CrossRef]
- Ramírez-Mosqueda, M.A.; Bello-Bello, J.J. SETIS™ bioreactor increases in vitro multiplication and shoot length in vanilla (Vanilla planifolia Jacks. Ex Andrews). Acta Physiol. Plant. 2021, 43, 52. [Google Scholar] [CrossRef]
- Hwang, H.D.; Kwon, S.H.; Murthy, H.N.; Yun, S.W.; Pyo, S.S.; Park, S.Y. Temporary immersion bioreactor system as an efficient method for mass production of in vitro plants in horticulture and medicinal plants. Agronomy 2022, 12, 346. [Google Scholar] [CrossRef]
- Thi, L.T.; Park, Y.G.; Jeong, B.R. Growth and development of carnation ‘Dreambyul’plantlets in a temporary immersion system and comparisons with conventional solid culture methods. Vitro Cell. Dev. Biol. Plant. 2019, 55, 539–548. [Google Scholar] [CrossRef]
- Zhang, B.; Niu, Z.; Li, C.; Hou, Z.; Xue, Q.; Liu, W.; Ding, X. Improving large-scale biomass and total alkaloid production of Dendrobium nobile Lindl. using a temporary immersion bioreactor system and MeJA elicitation. Plant Methods 2022, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Saptari, R.T.; Esyanti, R.R.; Putranto, R.A. Daminozide enhances the vigor and steviol glycoside yield of stevia (Stevia rebaudiana Bert.) propagated in temporary immersion bioreactors. Plant Cell Tiss. Org. 2022, 149, 257–268. [Google Scholar] [CrossRef]
- Leyva-Ovalle, O.R.; Bello-Bello, J.J.; Murguía-González, J.; Núñez-Pastrana, R.; Ramírez-Mosqueda, M.A. Micropropagation of Guarianthe skinneri (Bateman) Dressler et WE Higging in temporary immersion systems. 3 Biotech 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.N.; Rúbio Neto, A.; Chagas, E.A.; Chagas, P.C.; Pasqual, M.; Vendrame, W.A. Influence of silicon and in vitro culture systems on the micropropagation and acclimatization of “Dwarf Cavendish” banana. Acta Scientiarum. Agronomy 2020, 43, 1–6. [Google Scholar] [CrossRef]
- Pavlov, A.; Bley, T. Betalains biosynthesis by Beta vulgaris L. hairy root culture in a temporary immersion cultivation system. Process Biochem. 2006, 41, 848–852. [Google Scholar] [CrossRef]
- Mišić, D.; Šiler, B.; Skorić, M.; Djurickovic, M.S.; Živković, J.N.; Jovanović, V.; Giba, Z. Secoiridoid glycosides production by Centaurium maritimum (L.) Fritch hairy root cultures in temporary immersion bioreactor. Process Biochem. 2013, 48, 1587–1591. [Google Scholar] [CrossRef]
- Yang, S.H.; Yeh, D.M. In vitro leaf anatomy, ex vitro photosynthetic behaviors and growth of Calathea orbifolia (Linden) Kennedy plants obtained from semi-solid medium and temporary immersion systems. Plant Cell Tiss. Org. 2008, 93, 201–207. [Google Scholar] [CrossRef]
- Ramírez-Mosqueda, M.A.; Iglesias-Andreu, L.G. Evaluation of different temporary immersion systems (BIT®, BIG, and RITA®) in the micropropagation of Vanilla planifolia Jacks. Vitro Cell. Dev. Biol. Plant 2016, 52, 154–160. [Google Scholar] [CrossRef]
- Bairu, M.W.; Fennell, C.W.; van Staden, J. The effect of plant growth regulators on somaclonal variation in Cavendish banana (Musa AAA cv. ‘Zelig’). Sci. Hortic. 2006, 108, 347–351. [Google Scholar] [CrossRef]
- Korneva, S.; Flores, J.; Santos, E.; Piña, F.; Mendoza, J. Plant regeneration of plantain ‘Barraganete’from somatic embryos using a temporary immersion system. Biotecnol. Apl. 2013, 30, 267–270. [Google Scholar]
- Safarpour, M.; Sinniah, U.R.; Subramaniam, S.; Swamy, M.K. A novel technique for Musa acuminata Colla ‘Grand Naine’(AAA) micropropagation through transverse sectioning of the shoot apex. Vitro Cell. Dev. Biol. Plant 2017, 53, 226–238. [Google Scholar] [CrossRef]
- D’hont, A.; Denoeud, F.; Aury, J.M.; Baurens, F.C.; Carreel, F.; Garsmeur, O.; Noel, B.; Bocs, S.; Droc, G.; Rouard, M.; et al. The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature 2012, 488, 213–217. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).