Hydrocolloid Coatings as a Pre-Frying Treatment in the Production of Low-Fat Banana Chips
Abstract
:1. Introduction
2. Materials and Methods
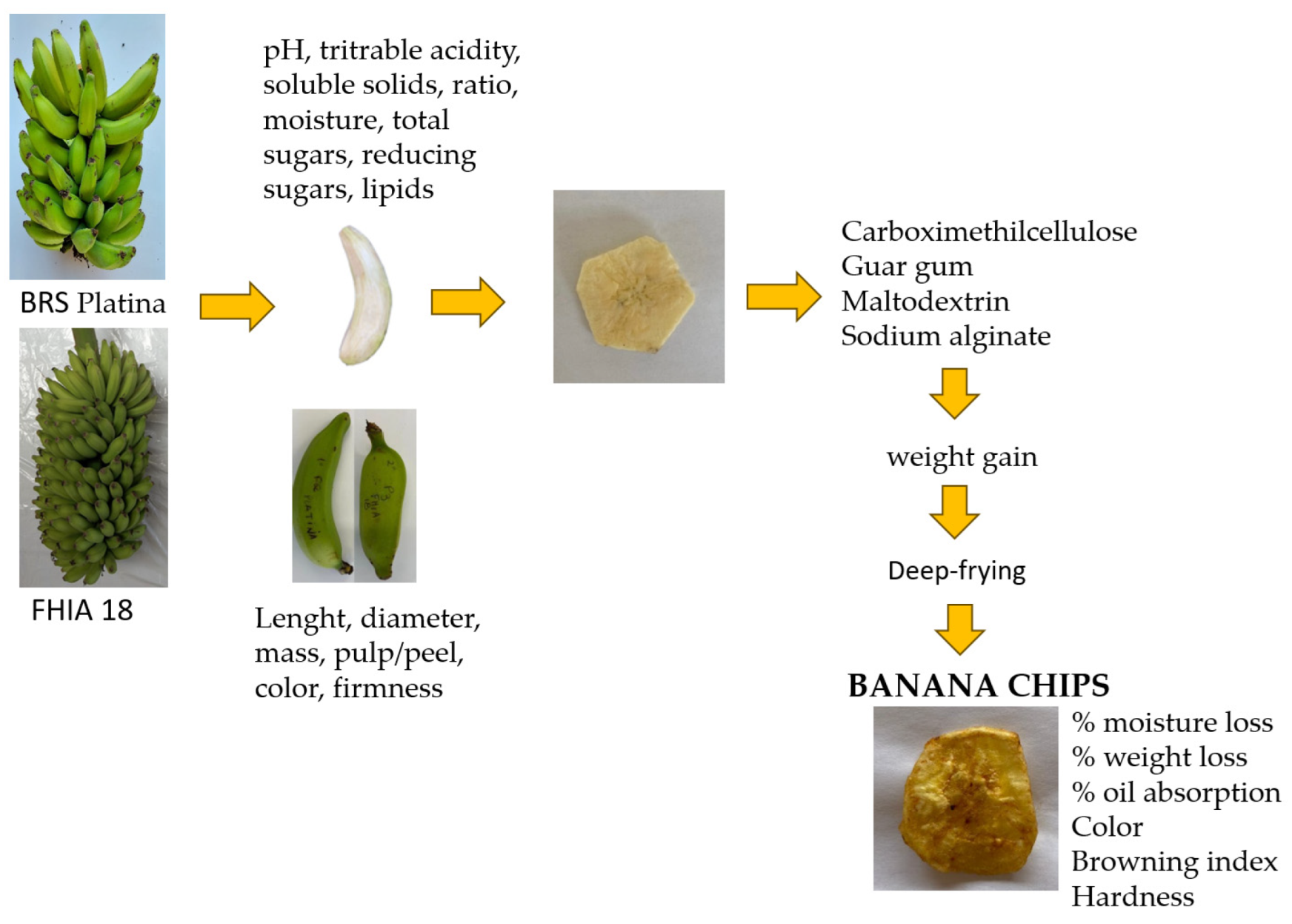
2.1. Orchard and Fruit Harvest
2.2. Characteristics of Unripe Fruits of Banana Cultivars
2.3. Production of Banana Chips
2.4. Characteristics of Banana Chips
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Unripe Fruits of Banana Cultivars
3.2. Characteristics of Banana Chips
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO—Food and Agriculture Research of United Nations. Faostat: Food and Agriculture Data. 2023. Available online: https://www.fao.org/faostat/en/#home (accessed on 5 July 2023).
- FAO. Food and Agriculture Organization of the United Nations. The State of Food and Agriculture 2019. Moving Forward on Food Loss and Waste Reduction. Available online: https://www.fao.org/3/ca6030en/ca6030en.pdf (accessed on 5 July 2023).
- Santos, K.L.; Panizzon, J.; Cenci, M.M.; Grabowski, G.; Jahno, V.D. Food losses and waste: Reflections on the current Brazilian scenario. Braz. J. Food Technol. 2020, 23, e2019134. [Google Scholar] [CrossRef]
- Moraes, N.V.; Lermen, F.H.; Echeveste, M.E.S. A systematic literature review on food waste/loss prevention and minimization methods. J. Environ. Manag. 2021, 286, 112268. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.L.; Souza, L.S. O Cultivo da Bananeira, 2nd ed.; Embrapa Mandioca e Fruticultura: Cruz das Almas, Brazil, 2004; p. 279. [Google Scholar]
- Al-Dairi, M.; Pathare, P.B.; Al-Yahyai, R.; Jayasuriya, H.; Al-Attabi, Z. Postharvest quality, technologies, and strategies to reduce losses along the supply chain of banana: A review. Trends Food Sci. Technol. 2023, 134, 177–191. [Google Scholar] [CrossRef]
- Dale, V.V.; Palghadmal, S.M.; Gajbhiye, S.K.; Gawade, A.S.; CV Pujari, C.V. Suitability of cultivars for making banana chips. Pharm. Innov. J. 2022, 11, 3984–3987. Available online: https://www.thepharmajournal.com/archives/2022/vol11issue12/PartAW/11-12-180-418.pdf (accessed on 5 July 2023).
- Nomura, E.S.; Moraes, W.S.; Damatto, E.R., Jr.; Fuzitani, E.J.; Saes, L.A.; Amorim, E.P.; Silva, S.O. Evaluation of banana genotypes over two crop cycles under subtropical conditions in the Ribeira Valley, São Paulo, Brazil. Acta Hortic. 2013, 986, 61–70. [Google Scholar] [CrossRef]
- Leonel, M.; Leonel, S.; dos Santos, T.P.R.; Souza, J.M.A.; Martins, R.C.; da Silva, M.S.C. Agronomic yield and starch properties of banana cultivars. Pesqui. Agropecuária Bras. 2021, 56, e02491. [Google Scholar] [CrossRef]
- Singthong, J.; Thongkaew, C. Using hydrocolloids to decrease oil absorption in banana chips. LWT-Food Sci. Technol. 2009, 42, 1199–1203. [Google Scholar] [CrossRef]
- Elkhalifa, A.E.O.; Hassan, A.M.; Zei, M.E.A. Analytical quality and acceptability of baked and fried banana chips. J. Hum. Nutr. 2014, 2, 1052. [Google Scholar]
- Pinzón, M.I.F.; Montoya, J.L.; Lucas, J.C.A. Evaluation of quality parameters of chips 12 varieties of bananas AAB genotype under deep frying. Vitae 2016, 23, S531–S535. [Google Scholar]
- Sumonsiri, N.; Imjaijit, S.; Padboke, T. Effect of guar gum and glycerol on oil absorption and qualities of banana chips. Int. Food Res. J. 2020, 27, 529–535. Available online: http://www.ifrj.upm.edu.my/27%20(03)%202020/DONE%20-%2014%20-%20IFRJ19520.R1.pdf (accessed on 10 June 2023).
- Panang, C.; Thikeaw, K.; Soubsub, K.; Olanwanit, W.; Phahom, T. A correlation between oil measurement methods and the application of principal component analysis for selecting the best pre-frying treatment of reduced-fat banana chips. J. Food Meas. Charact. 2023, 17, 5402–5411. [Google Scholar] [CrossRef]
- Ananey-Obiri, D.; Matthews, L.; Azahrani, M.H.; Ibrahim, S.A.; Galanakis, C.M.; Tahergorabi, R. Application of protein-based edible coatings for fat uptake reduction in deep-fat fried foods with an emphasis on muscle food proteins. Trends Food Sci. Technol. 2018, 80, 167–174. [Google Scholar] [CrossRef]
- Kurek, M.; Ščetar, M.; Galić, K. Edible coatings to minimize fat uptake in deep fat fried products: A review. Food Hydrocoll. 2017, 71, 225–235. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Adhikari, B. Recent developments in frying technologies applied to fresh foods. Trends Food Sci. Technol. 2020, 98, 68–81. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, M.; Chitrakar, B.; Zhang, W. Reduction of oil uptake with osmotic dehydration and coating pre-treatment in microwave-assisted vacuum fried potato chips. Food Biosci. 2021, 39, 100825. [Google Scholar] [CrossRef]
- Sothornvit, R. Edible coating and post-frying centrifuge step effect on quality of vacuum-fried banana chips. J. Food Eng. 2011, 107, 319–325. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, T.; Fan, D.; Li, J.; Fan, L. The description of oil absorption behavior of potato chips during the frying. LWT-Food Sci. Technol. 2018, 96, 119–126. [Google Scholar] [CrossRef]
- Li, J.-M.; Nie, S.-P. The functional and nutritional aspects of hydrocolloids in foods. Food Hydrocoll. 2016, 53, 46–61. [Google Scholar] [CrossRef]
- Zhang, N.; Zhou, Q.; Fan, D.; Xiao, J.; Zhao, Y.; Cheng, K.-W.; Wang, M. Novel roles of hydrocolloids in foods: Inhibition of toxic Maillard reaction products formation and attenuation of their harmful effects. Trends Food Sci. Technol. 2021, 111, 706–715. [Google Scholar] [CrossRef]
- Suyatma, N.E.; Ulfah, K.; Prangdimurti, E.; Ishikawa, Y. Effect of blanching and pectin coating as pre-frying treatments to reduce acrylamide formation in banana chips. Int. Food Res. J. 2015, 22, 936–942. [Google Scholar]
- Shamla, L.; Nisha, P. Acrylamide formation in plantain (Musa paradisiaca) chips influenced by different ripening stages: A correlation study with respect to reducing sugars, amino acids and phenolic content. Food Chem. 2017, 222, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- EMBRAPA (Empresa Brasileira de Pesquisa Agropecuária). Sistema Brasileiro de Classificação de Solos, 3rd ed.; Centro Nacional de Pesquisa de Solos: Rio de Janeiro, Brazil, 2013; 353p. [Google Scholar]
- Amorim, E.P.; Santos-Cerejo, J.A. BRS Platina: Variedade de Bananeira Tipo Prata Resistente ao Mal do Panamá; Embrapa Mandioca e Fruticultura: Cruz das Almas, Brazil, 2012; 2p, Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/71994/1/BRS-Platina-variedade-de-bananeira-4XWB.pdf (accessed on 10 June 2023).
- Castricini, A.; Coelho, E.F.; Rodrigues, M.G.V.; Coutinho, R.C. Caracterização pós-colheita de frutos de bananeira ‘BRS Platina’ de primeiro ciclo, sob regulação do déficit de irrigação. Rev. Bras. Frutic. 2012, 34, 1013–1021. [Google Scholar] [CrossRef]
- Gaidashova, S.V.; Karemera, F.; Karamura, E.B. Agronomic performance of introduced banana varieties in lowlands of Rwanda. Afr. Crop Sci. J. 2008, 16, 9–16. [Google Scholar] [CrossRef]
- Nijuguna, J.; Nguthi, F.; Wepukhulu, S.; Wambugu, F.; Gitau, D.; Karuoya, M.; Karamura, D.A. Introduction and evaluation of improved banana cultivars for agronomic and yield characteristics in Kenya. Afr. Crop Sci. J. 2008, 16, 35–40. [Google Scholar] [CrossRef]
- Leonel, S.; Bolfarini, A.C.B.; Souza, J.M.A.; Leonel, M.; Ferreira, R.B.; Putti, F.F.; Tecchio, M.A. Agronomic performance of Banana ‘FHIA 18′ in response to phosphate fertilization. Agron. J. 2020, 112, 2033–2046. [Google Scholar] [CrossRef]
- CIE L*a*b* Color Scale. Applications Note. In sight on Color. In HunterLab Technical Manual; Hunter Lab.: Reston, VA, USA, 2008; Volume 8, pp. 1–4. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2007; p. 2. [Google Scholar]
- PBMH e PIF—Programa Brasileiro para a Modernização da Horticultura e Produção Integrada de Frutas. Normas de Classificação de Banana; CEAGESP: São Paulo, Brazil, 2006. Available online: https://ceagesp.gov.br/wp-content/uploads/2015/07/banana.pdf (accessed on 14 June 2023).
- Aquino, C.F.; Salomão, L.C.C.; Cecon, P.R.; Siqueira, D.L.; Ribeiro, S.M.R. Physical, chemical and morphological characteristics of banana cultivars depending on maturation stages. Rev. Caatinga 2017, 30, 87–96. [Google Scholar] [CrossRef]
- Cho, B.-H.; Koseki, S. Determination of banana quality indices during the ripening process at different temperatures using smartphone images and an artificial neural network. Sci. Hortic. 2021, 288, 110382. [Google Scholar] [CrossRef]
- Sanaeifar, A.; Bakhshipour, A.; de la Guardia, M. Prediction of banana quality indices from color features using support vector regression. Talanta 2016, 148, 54–61. [Google Scholar] [CrossRef]
- Adão, R.C.; Glória, M.B.A. Bioactive amines and carbohydrate changes during ripening of ‘Prata’ banana (Musa acuminata x M. balbisiana). Food Chem. 2005, 90, 705–711. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A. Colour measurement and analysis in fresh and processed foods: A Review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Bolfarini, A.C.B.; Souza, J.M.A.; Putti, F.F.; Silva, M.S.; Ferreira, R.B.; Leonel, M.; Tecchio, M.A.; Leonel, S. Physicochemical characteristics of unripe and ripe banana ‘FHIA 18’ submitted to phosphorus fertilizer over three production cycles. Semin. Ciênc. Agrár. 2020, 41, 33–48. [Google Scholar] [CrossRef]
- Silva, M.S.C.; Leonel, S.; Ferreira, A.F.A.; Souza, J.M.A.; Oliveira Junior, M.A.; Martins, R.C.; Ferreira, R.B.; de Souza Silva, M. Banana bunch cover: Evaluation of promising bag materials. Comun. Sci. 2022, 13, e3741. [Google Scholar] [CrossRef]
- Vaitkevičien, N.; Jarienė, E.; Kulaitienė, J.; Levickienė, D. The physico-chemical and sensory characteristics of coloured-flesh potato chips: Influence of cultivar, slice, thickness and frying temperature. Appl. Sci. 2022, 12, 1211. [Google Scholar] [CrossRef]
- Fellows, P.J. Food Processing Technology; Principles and Practice; Artmed: São Paulo, Brazil, 2006; 608p. [Google Scholar]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar Gum: Processing, Properties and Food Applications—A Review. J. Food Sci. Technol. 2011, 51, 409–418. [Google Scholar] [CrossRef]
- Hadnađev, M.; Hadnađev, T.D.; Dokić, L.; Pajin, B.; Torbica, A.; Šarić, L.; Ikonić, P. Physical and sensory aspects of maltodextrin gel addition used as fat replacers in confectionery filling systems. LWT-Food Sci. Technol. 2014, 59, 495–503. [Google Scholar] [CrossRef]
- Soto, M.; Pérez, A.M.; Servant, A.; Vaillant, F.; Achir, N. Monitoring and modelling of physicochemical properties of papaya chips during vacuum frying to control their sensory attributes and nutritional value. J. Food Eng. 2021, 299, 110514. [Google Scholar] [CrossRef]
- Rezagholizade-Shirvan, A.; Kalantarmahdavi, M.; Amiryousef, M.R. Evaluation of the effect of basil seed gum, tragacanth gum, pectin, and coating formulation with corn flour on oil absorption and sensory properties of watermelon rind chips. Heliyon 2023, 9, e16976. [Google Scholar] [CrossRef]
- Martínez, D.F.; Castellanos, F.J.; Bravo, H.E. Application of edible coatings in green plantain slices subjected to deep-fat frying. Ing. Compet. 2015, 17, 91–99. [Google Scholar]
- Paramasivam, S.K.; David, A.K.; Somasundaram, S.M.; Suthanthiram, B.; Shiva, K.N.; Subbaraya, U. Influence of food hydrocolloids on the structural, textural and chemical characteristics of low-fat banana chips. Food Sci. Technol. Int. 2021, 28, 203–215. [Google Scholar] [CrossRef]
- Iglesias-Carres, L.; Racine, K.C.; Chadwick, S.; Nunn, C.; Kalambur, S.B.; Neilson, A.P.; Ferruzzi, M.G. Mechanism of off-color formation in potato chips fried in oil systems containing ascorbic acid as a stabilizer. LWT-Food Sci. Technol. 2023, 179, 114682. [Google Scholar] [CrossRef]
- Alimi, B.A.; Shittu, T.A.; Sanni, L.O.; Arowolo, T.A. Effect of pre-drying and hydrocolloid type on colour and textural properties of coated fried yam chips. Niger. Food J. 2013, 31, 97–102. [Google Scholar] [CrossRef]
- Al-Asmar, A.; Naviglio, D.; Giosafatto, C.; Mariniello, L. Hydrocolloid-based coatings are effective at reducing acrylamide and oil content of French fries. Coatings 2018, 8, 147. [Google Scholar] [CrossRef]


| Parameter | Cultivars | CV (%) | MSD | |
|---|---|---|---|---|
| BRS Platina | FHIA 18 | |||
| Fruit length (cm) | 16.76 ± 0.75 a | 13.50 ± 0.43 b | 8.73 | 2.78 |
| Fruit diameter (peduncle) (mm) | 39.18 ± 1.49 a | 23.50 ± 1.12 b | 3.04 | 2.14 |
| Fruit diameter (middle of the fruit) (mm) | 40.57 ± 1.27 a | 30.01 ± 1.41 b | 5.92 | 4.70 |
| Fruit diameter (apex) (mm) | 36.15 ± 0.97 a | 27.00 ± 1.22 b | 6.74 | 4.79 |
| Fruit mass (g) | 131.00 ± 2.37 a | 59.73 ± 1.44 b | 1.98 | 4.25 |
| Peel mass (g) | 42.42 ± 0.40 a | 23.63 ± 0.71 b | 1.73 | 1.29 |
| Pulp mass (g) | 74.12 ± 1.71 a | 30.65 ± 0.72 b | 5.50 | 6.48 |
| Pulp/peel | 1.75 ± 0.06 a | 1.32 ± 0.04 b | 3.63 | 0.28 |
| Firmness whole fruit (peduncle) (N) | 109.37 ± 0.39 a | 89.95 ± 0.74 b | 6.46 | 1.45 |
| Firmness whole fruit (middle of the fruit) (N) | 112.50 ± 0.62 a | 88.70 ± 0.73 b | 9.61 | 2.17 |
| Firmness whole fruit (apex) (N) | 110.92 ± 0.42 a | 88.85 ± 0.72 b | 7.48 | 1.69 |
| Peel firmness (peduncle) (N) | 70.37 ± 0.38 a | 51.97 ± 0.56 b | 9.43 | 1.38 |
| Peel firmness (middle of the fruit) (N) | 77.70 ± 0.43 a | 57.00 ± 0.65 b | 4.14 | 0.63 |
| Peel firmness (apex) (N) | 77.50 ± 0.48 a | 54.45 ± 0.61 b | 2.21 | 0.33 |
| Pulp firmness (peduncle) (N) | 60.61 ± 0.49 a | 60.12 ± 0.54 a | 8.70 | 1.18 |
| Pulp firmness (middle of the fruit) (N) | 59.70 ± 0.39 a | 57.10 ± 0.26 b | 4.35 | 1.88 |
| Pulp firmness (apex) (N) | 60.05 ± 0.35 a | 58.52 ± 0.23 b | 4.17 | 0.56 |
| L* peel | 48.22 ± 2.12 a | 48.90 ± 2.21 a | 6.81 | 7.44 |
| a* peel | −13.52 ± 0.58 a | −14.60 ± 1.04 a | 10.6 | 3.34 |
| b* peel | 26.10 ± 0.92 a | 26.90 ± 0.67 a | 4.21 | 2.51 |
| C* | 30.62 ± 2.53 a | 29.42 ± 1.12 a | 7.02 | 4.43 |
| h* | 118.34 ± 1.71 a | 117.38 ± 1.45 a | 4.12 | 2.19 |
| L* pulp | 83.03 ± 3.28 a | 84.19 ± 2.93 a | 2.92 | 5.50 |
| a* pulp | 1.91 ± 0.15 a | −1.02 ± 0.09 a | 4.61 | 0.54 |
| b* pulp | 29.85 ± 1.03 a | 18.65 ± 0.58 b | 4.57 | 2.49 |
| C* | 29.68 ± 2.50 a | 18.68 ± 0.72 b | 4.03 | 2.84 |
| h* | 86.49 ± 1.20 b | 93.07 ± 0.80 a | 5.37 | 3.79 |
| Parameter | Cultivars | CV (%) | MSD | |
|---|---|---|---|---|
| BRS Platina | FHIA 18 | |||
| Moisture (%) | 66.28 ± 1.64 a | 67.24 ± 0.52 a | 1.94 | 2.92 |
| Total sugars (%) | 1.70 ± 0.04 b | 2.04 ± 0.03 a | 2.66 | 0.11 |
| Reducing sugars (%) | 1.04 ± 0.03 a | 1.10 ± 0.07 a | 3.42 | 0.33 |
| Lipids (%) | 0.45 ± 0.04 a | 0.11 ± 0.02 b | 15.75 | 0.10 |
| pH | 5.37 ± 0.09 a | 5.63 ± 0.13 a | 3.18 | 0.09 |
| Titratable acidity (mg/100 g) | 0.47 ± 0.11 a | 0.26 ± 0.09 b | 5.22 | 0.043 |
| Soluble solids (°Brix) | 3.85 ± 0.18 b | 4.32 ± 0.16 a | 3.63 | 0.99 |
| Ratio | 8.19 ± 0.13 b | 16.61 ± 0.10 a | 4.13 | 3.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.S.P.d.; Leonel, M.; Jesus, P.R.R.d.; Leonel, S.; Fernandes, A.M.; Ouros, L.F.d. Hydrocolloid Coatings as a Pre-Frying Treatment in the Production of Low-Fat Banana Chips. Horticulturae 2023, 9, 1139. https://doi.org/10.3390/horticulturae9101139
Santos JSPd, Leonel M, Jesus PRRd, Leonel S, Fernandes AM, Ouros LFd. Hydrocolloid Coatings as a Pre-Frying Treatment in the Production of Low-Fat Banana Chips. Horticulturae. 2023; 9(10):1139. https://doi.org/10.3390/horticulturae9101139
Chicago/Turabian StyleSantos, Júlia Silva Pereira dos, Magali Leonel, Paulo Ricardo Rodrigues de Jesus, Sarita Leonel, Adalton Mazetti Fernandes, and Lucas Felipe dos Ouros. 2023. "Hydrocolloid Coatings as a Pre-Frying Treatment in the Production of Low-Fat Banana Chips" Horticulturae 9, no. 10: 1139. https://doi.org/10.3390/horticulturae9101139
APA StyleSantos, J. S. P. d., Leonel, M., Jesus, P. R. R. d., Leonel, S., Fernandes, A. M., & Ouros, L. F. d. (2023). Hydrocolloid Coatings as a Pre-Frying Treatment in the Production of Low-Fat Banana Chips. Horticulturae, 9(10), 1139. https://doi.org/10.3390/horticulturae9101139







