Characterization of Juice Extracted from Ultrasonic-Treated Red Pitaya Flesh
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.1.1. Total Soluble Solid (TSS), pH, Titratable Acidity, Color, and Viscosity
2.1.2. Organic Acids, Total Phenolic Content (TPC), Total Anthocyanin Content (TAC), and 2,2-Diphenyl-1-picrylhydrazyl (DPPH)
2.2. Statistical Analysis
3. Results and Discussion
3.1. Total Soluble Solids, Titratable Acidity, pH, Viscosity, and Color
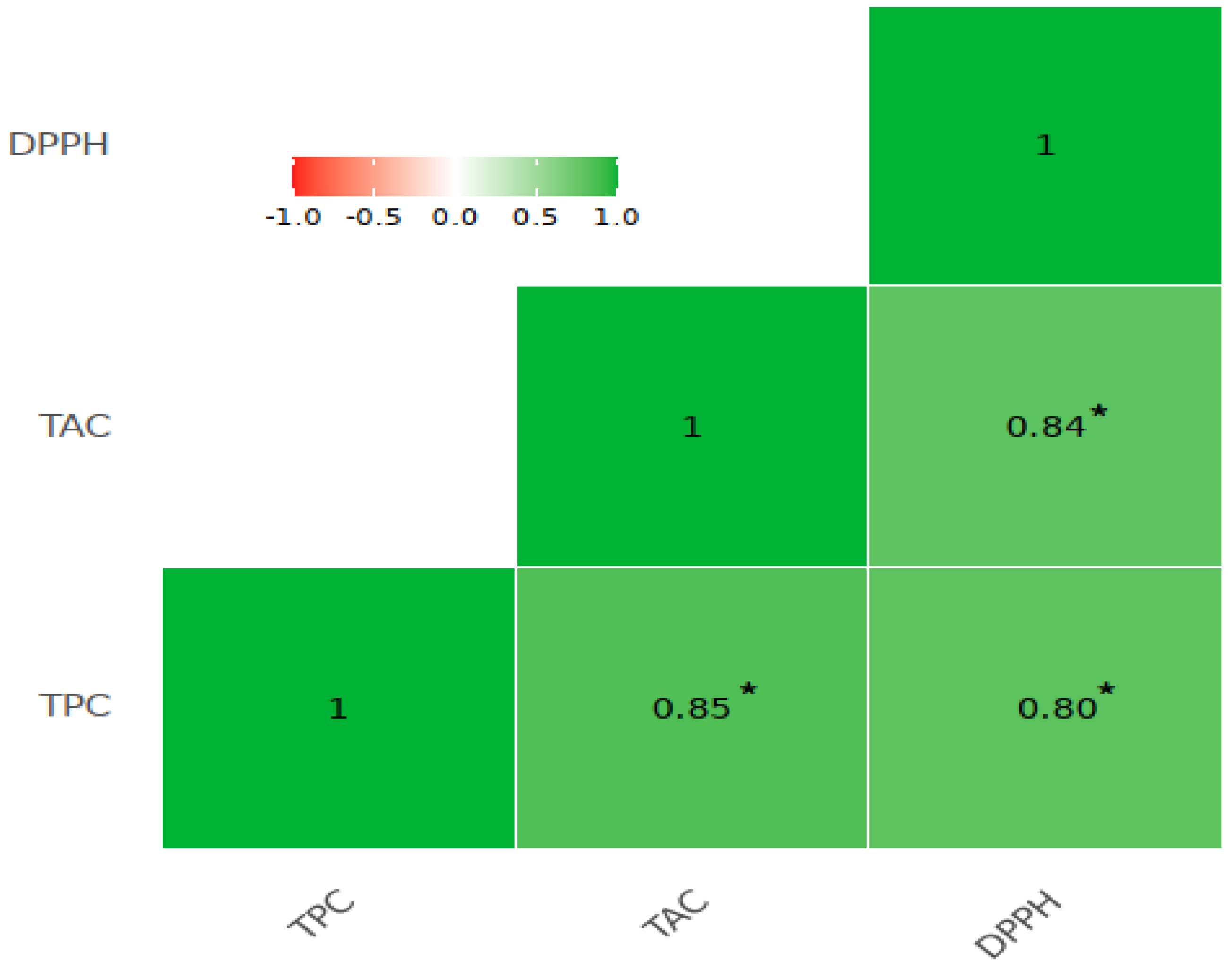
3.2. Organic Acids, Phenolics, Anthocyanins, and DPPH Radical Scavenging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, H.; Zhu, W.; Zhong, K.; Liu, Y. Evaluation of colour stability of clear red pitaya juice treated by thermosonication. LWT-Food Sci. Technol. 2020, 121, 108997. [Google Scholar] [CrossRef]
- Jalgaonkar, K.; Mahawar, M.K.; Bibwe, B.; Kannaujia, P. Postharvest profile, processing and waste utilization of dragon fruit (Hylocereus Spp.): A review. Food Rev. Int. 2022, 38, 733–759. [Google Scholar] [CrossRef]
- Esquivel, P.; Usaga, J.; Schweiggert, R.; Steingass, C.B.; Jiménez, V.M. Effect of Processing on Biofunctionality of Selected Tropical Fruit Juices. ACS Food Sci. Technol. 2022, 2, 455–473. [Google Scholar] [CrossRef]
- Nurul, S.R.; Asmah, R. Variability in nutritional composition and phytochemical properties of red pitaya (Hylocereus polyrhizus) from Malaysia and Australia. Int. Food Res. J. 2014, 21, 1689–1697. [Google Scholar]
- Ramli, N.S.; Brown, L.; Ismail, P.; Rahmat, A. Effects of red pitaya juice supplementation on cardiovascular and hepatic changes in high-carbohydrate, high-fat diet-induced metabolic syndrome rats. BMC Complement. Altern. Med. 2014, 14, 189. [Google Scholar] [CrossRef]
- Widyaningsih, A.; Setiyani, O.; Umaroh, U.; Sofro, M.A.U.; Amri, F. Effect of consuming red dragon fruit (Hylocereus costaricensis) juice on the levels of hemoglobin and erythrocyte among pregnant women. Belitung Nurs. J. 2017, 3, 255–264. [Google Scholar] [CrossRef]
- Radziejewska-Kubzdela, E.; Szwengiel, A.; Ratajkiewicz, H.; Nowak, K. Effect of ultrasound, heating and enzymatic pre-treatment on bioactive compounds in juice from Berberis amurensis Rupr. Ultrason. Sonochem. 2020, 63, 104971. [Google Scholar] [CrossRef]
- Tan, C.X.; Chin, R.; Tan, S.T.; Tan, S.S. Phytochemicals and antioxidant activity of ultrasound-assisted avocado seed extract. Malaysian J. Anal. Sci. 2022, 26, 439–446. [Google Scholar]
- Moraes, D.P.; Ferreira, D.F.; Farias, C.A.; Nehring, P.; Barcia, M.T.; Cichoski, A.J.; Barin, J.S. Sonication of whole blackberries as a pretreatment for the anthocyanin enrichment of juices obtained by pressing. ResearchSqaure, 2022; preprint. [Google Scholar]
- Tan, C.X.; Chong, G.H.; Hamzah, H.; Ghazali, H.M. Optimization of ultrasound-assisted aqueous extraction to produce virgin avocado oil with low free fatty acids. J. Food Process Eng. 2017, 41, e12656. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2007. [Google Scholar]
- Ismail, B.B.; Liu, D.; Pu, Y.; He, Q.; Guo, M. High-intensity ultrasound processing of baobab fruit pulp: Effect on quality, bioactive compounds, and inhibitory potential on the activity of α-amylase and α-glucosidase. Food Chem. 2021, 361, 130144. [Google Scholar] [CrossRef] [PubMed]
- Scherer, R.; Cecília, A.; Rybka, P.; Augusto, C.; Dillenburg, A.; Teixeira, J.; Teixeira, H. Validation of a HPLC method for simultaneous determination of main organic acids in fruits and juices. Food Chem. 2012, 135, 150–154. [Google Scholar] [CrossRef]
- Choo, Y.X.; Teh, L.K.; Tan, C.X. Effects of sonication and thermal pasteurization on the nutritional, antioxidant, and microbial properties of noni juice. Molecules 2023, 28, 313. [Google Scholar] [CrossRef]
- Choo, K.Y.; Kho, C.; Ong, Y.Y.; Thoo, Y.Y.; Lim, R.L.H.; Tan, C.P.; Ho, C.W. Studies on the storage stability of fermented red dragon fruit (Hylocereus polyrhizus) drink. Food Sci. Biotechnol. 2018, 27, 1411–1417. [Google Scholar] [CrossRef]
- Cheng, X.F.; Zhang, M.; Adhikari, B. Changes in quality attributes of strawberry purees processed by power ultrasound or thermal treatments. Food Sci. Technol. Res. 2014, 20, 1033–1041. [Google Scholar] [CrossRef]
- Nayak, P.K.; Basumatary, B.; Chandrasekar, C.M.; Seth, D.; Kesavan, R.K. Impact of thermosonication and pasteurization on total phenolic contents, total flavonoid contents, antioxidant activity, and vitamin C levels of elephant apple (Dillenia indica) juice. J. Food Process Eng. 2020, 43, e13447. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Vanga, S.K.; Raghavan, V. High-intensity ultrasound processing of kiwifruit juice: Effects on the microstructure, pectin, carbohydrates and rheological properties. Food Chem. 2020, 313, 126121. [Google Scholar] [CrossRef]
- Grassino, A.N.; Brnčić, M.; Vikić-Topić, D.; Roca, S.; Dent, M.; Brnčić, S.R. Ultrasound assisted extraction and characterization of pectin from tomato waste. Food Chem. 2016, 198, 93–100. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, H.W.; Ye, J.H.; Wang, J.; Raghavan, V. Ultrasound pretreatment to enhance drying kinetics of kiwifruit (Actinidia deliciosa) slices: Pros and cons. Food Bioprocess Technol. 2019, 12, 865–876. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ahmad, N.; Ahmed, Z.; Siddique, R.; Mehmood, A.; Usman, M.; Zeng, X.A. Effect of dielectric barrier discharge plasma, ultra-sonication, and thermal processing on the rheological and functional properties of sugarcane juice. J. Food Sci. 2020, 85, 3823–3832. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Le, V.V.M. Application of ultrasound to pineapple mash treatment in juice processing. Int. Food Res. J. 2012, 19, 547–552. [Google Scholar]
- Nguyen, C.L.; Nguyen, H.V. Ultrasonic effects on the quality of mulberry juice. Beverages 2018, 4, 56. [Google Scholar] [CrossRef]
- Khoo, H.E.; He, X.M.; Tang, Y.; Li, Z.; Li, C.; Zeng, Y.; Tang, J.; Sun, J. Betacyanins and Anthocyanins in Pulp and Peel of Red Pitaya (Hylocereus polyrhizus cv. Jindu), Inhibition of Oxidative Stress, Lipid Reducing, and Cytotoxic Effects. Front. Nutr. 2022, 9, 894438. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.S.; Tan, S.T.; Tan, C.X. The anti-hypertensive and hypoglycemic potential of bioactive compounds derived from pulasan rind. Processes 2022, 10, 592. [Google Scholar] [CrossRef]
- Le, T.T.; Le, N.L. Antioxidant capacities and betacyanin lc-ms profile of red-fleshed dragon fruit juice (Hylocereus polyrhizus) extracted by ultrasound-assisted enzymatic treatment and optimized by response surface methodology. J. Food Process. Preserv. 2021, 45, e15217. [Google Scholar] [CrossRef]
| Variable | Ultrasonic PreTreatment Time (min) | |||
|---|---|---|---|---|
| 0 | 20 | 40 | 60 | |
| TSS (% Brix) | 4.93 ± 0.06 a | 5.03 ± 0.06 a | 4.93 ± 0.06 a | 5.00 ± 0.00 a |
| TA (%) | 0.08 ± 0.01 a | 0.07 ± 0.01 a | 0.08 ± 0.01 a | 0.08 ± 0.01 a |
| pH | 5.07 ± 0.01 a | 5.08 ± 0.02 a | 5.05 ± 0.01 a | 5.07 ± 0.01 a |
| Viscosity (mP.a.s) | 26.47 ± 0.06 a | 26.73 ± 0.12 b | 26.57 ± 0.06 ab | 26.57 ± 0.06 ab |
| Color | ||||
| L* | 42.42 ± 0.08 a | 41.98 ± 0.09 b | 41.77 ± 0.01 c | 41.60 ± 0.01 d |
| a* | 11.09 ± 0.34 a | 8.87 ± 0.06 b | 7.99 ± 0.02 c | 7.22 ± 0.21 d |
| b* | −5.17 ± 0.15 a | −3.91 ± 0.14 b | −3.26 ± 0.03 c | −2.75± 0.08 d |
| Chroma | 12.23 ± 0.38 a | 8.63 ± 0.03 b | 9.69 ± 0.29 c | 7.72 ± 0.23 d |
| Hue angle | −25.00 ± 0.07 a | −22.19 ± 0.18 b | −23.77 ± 0.17 c | −20.88 ± 0.07 d |
| Browning index | 16.51 ± 0.45 a | 12.36 ± 0.03 b | 13.53 ± 0.33 c | 11.32 ± 0.33 d |
| ∆E | REF | 2.59 ± 0.08 a | 3.69 ± 0.41 b | 4.64 ± 0.02 c |
| Variable | Ultrasonic Pretreatment Time (min) | |||
|---|---|---|---|---|
| 0 | 20 | 40 | 60 | |
| Organic acid (mg/100 g) | ||||
| Citric acid | 689.19 ± 6.67 a | 728.29 ± 35.3 ab | 741.88 ± 21.99 ab | 754.95 ± 13.58 b |
| Succinic acid | 17.37 ± 1.24 a | 19.98 ± 7.31 a | 23.85 ± 2.08 a | 40.50 ± 0.50 b |
| TPC (mg GAE/100 g) | 37.33 ± 0.83 a | 42.67 ± 0.83 b | 43.83 ± 0.83 c | 46.33 ± 0.83 d |
| TAC (mg CGE/100 g) | 1.37 ± 0.00 a | 1.54 ± 0.01 b | 1.78 ± 0.03 c | 1.98 ± 0.04 d |
| DPPH (%) | 12.36 ± 1.82 a | 13.80 ± 1.07 b | 15.55 ± 1.22 c | 17.54 ± 1.47 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, C.X.; Lim, S.W.; Tan, S.S.; Tan, S.T. Characterization of Juice Extracted from Ultrasonic-Treated Red Pitaya Flesh. Horticulturae 2023, 9, 92. https://doi.org/10.3390/horticulturae9010092
Tan CX, Lim SW, Tan SS, Tan ST. Characterization of Juice Extracted from Ultrasonic-Treated Red Pitaya Flesh. Horticulturae. 2023; 9(1):92. https://doi.org/10.3390/horticulturae9010092
Chicago/Turabian StyleTan, Chin Xuan, See Wen Lim, Seok Shin Tan, and Seok Tyug Tan. 2023. "Characterization of Juice Extracted from Ultrasonic-Treated Red Pitaya Flesh" Horticulturae 9, no. 1: 92. https://doi.org/10.3390/horticulturae9010092
APA StyleTan, C. X., Lim, S. W., Tan, S. S., & Tan, S. T. (2023). Characterization of Juice Extracted from Ultrasonic-Treated Red Pitaya Flesh. Horticulturae, 9(1), 92. https://doi.org/10.3390/horticulturae9010092






