Investigation of Growth Factors and Mathematical Modeling of Nutrient Media for the Shoots Multiplication In Vitro of Rare Plants of the Rostov Region
Abstract
1. Introduction
2. Materials and Methods
3. Results
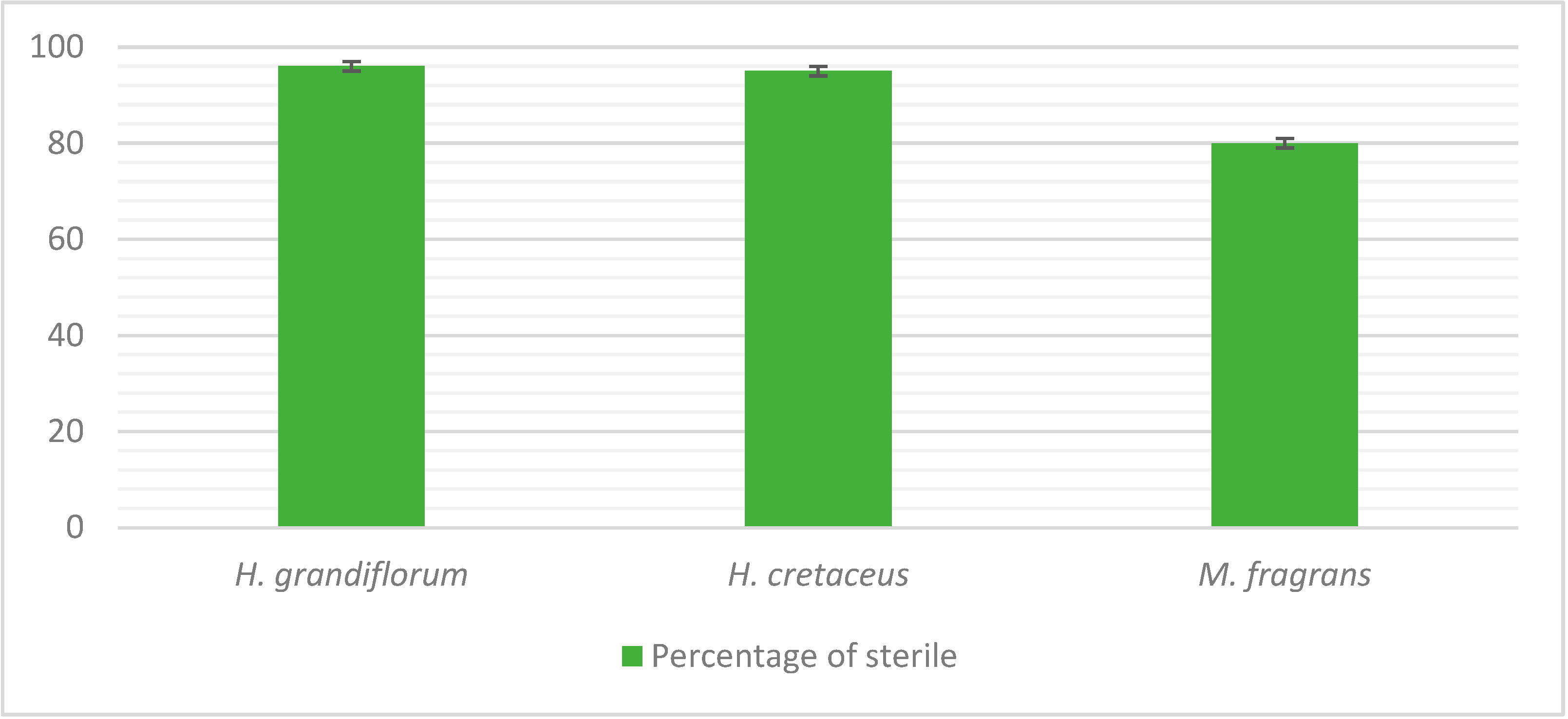
3.1. Sterilization
3.2. Multiplication
3.3. Statistical Analysis
4. Discussion
4.1. Seed Sterilization
4.2. Multiplication of Shoots
4.2.1. Hedysarum Grandiflorum
4.2.2. Hysoppus Cretaceus
4.2.3. Matthiola Fragrans
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Effect | One-Dimensional Significance Criterion for the Number of Shoots Per Plant, pcs. (Data Table 2) Sigma-Limited Parametrization Decomposition of the Hypothesis | ||||
|---|---|---|---|---|---|
| SS | Degrees | MS | F | p | |
| Medium type | 37.580 * | 2 * | 18.790 * | 42.277 * | 0 * |
| Hormone | 2.327 | 2 * | 1.163 | 2.617 | 0.073570 |
| Hormone concentration, mg/L | 18.733 * | 4 * | 4.683 * | 10.538 * | 0 * |
| Medium type * Hormone | 48.953 * | 4 * | 12.238 * | 27.536 * | 0 * |
| Medium type * Hormone concentration, mg/L | 59.453 * | 8 * | 7.432 * | 16.721 * | 0 * |
| Hormone * Hormone concentration, mg/L | 9.240 * | 8 * | 1.155 * | 2.599 * | 0.008249 * |
| Medium type * Hormone * Hormone concentration, mg/L | 26.713 * | 16 * | 1.670 * | 3.757 * | 0.000001 * |
| Error | 380.000 | 855 | 0.444 | ||
| Effect | One-Dimensional Significance Criterion for the Number of Shoots Per Plant. pcs. (Data Table 3) Sigma-Limited Parametrization Decomposition of the Hypothesis | ||||
|---|---|---|---|---|---|
| SS | Degrees | MS | F | p | |
| Medium type | 43.469 * | 2 * | 21.734 * | 19.254 * | 0 * |
| Hormone | 18.729 * | 2 * | 9.364 * | 8.296 * | 0.000270 * |
| Hormone concentration, mg/L | 86.196 * | 4 * | 21.549 * | 19.090 * | 0 * |
| Medium type * Hormone | 90.884 * | 4 * | 22.721 * | 20.128 * | 0 * |
| Medium type * Hormone concentration, mg/L | 61.231 * | 8 * | 7.654 * | 6.780 * | 0 * |
| Hormone * Hormone concentration, mg/L | 30.338 * | 8 * | 3.792 * | 3.359 * | 0.000844 * |
| Medium type * Hormone * Hormone concentration, mg/L | 114.816 * | 16 * | 7.176 * | 6.357 * | 0 * |
| Error | 965.150 | 855 | 1.129 | ||
| Effect | One-Dimensional Significance Criterion for the Number of Shoots Per Plant, pcs. (Data Table 4) Sigma-Limited Parametrization Decomposition of the Hypothesis | ||||
|---|---|---|---|---|---|
| SS | Degrees | MS | F | p | |
| Medium type | 64.642 * | 2 * | 32.321 * | 22.327 * | 0 * |
| Hormone | 88.169 * | 2 * | 44.084 * | 30.453 * | 0 * |
| Hormone concentration, mg/L | 31.473 * | 4 * | 7.868 * | 5.435 * | 0.000253 * |
| Medium type * Hormone | 60.551 * | 4 * | 15.138 * | 10.457 * | 0 * |
| Medium type * Hormone concentration, mg/L | 26.180 * | 8 * | 3.272 * | 2.261 * | 0.021535 * |
| Hormone * Hormone concentration, mg/L | 46.553 * | 8 * | 5.819 * | 4.020 | 0.000106 * |
| Medium type * Hormone * Hormone concentration, mg/L | 81.993 * | 16 * | 5.125 * | 3.540 | 0.000003 * |
| Error | 1237.700 | 855 | 1.448 | ||
References
- Cardoso, J.C.; Sheng Gerald, L.T.; Teixeira da Silva, J.A. Micropropagation in the twenty-first century. In Plant Cell Culture Protocols. Methods in Molecular Biology; Loyola-Vargas, V., Ochoa-Alejo, N., Eds.; Humana Press: New York, NY, USA, 2018; Volume 1815. [Google Scholar] [CrossRef]
- Molkanova, O.; Shirnina, I.; Mitrofanova, I. Conservation and micropropagation of rare and endemic species in genepool collections of the Russian Federation. J. Biotechnol. 2018, 280, S83–S88. [Google Scholar] [CrossRef]
- Chokheli, V.A.; Dmitriev, P.A.; Rajput, V.D.; Bakulin, S.D.; Azarov, A.S.; Varduni, T.V.; Stepanenko, V.V.; Tarigholizadeh, S.; Singh, R.K.; Verma, K.K.; et al. Recent Development in Micropropagation Techniques for Rare Plant Species. Plants 2020, 9, 1733. [Google Scholar] [CrossRef] [PubMed]
- Kamelin, R.V.; Gizatulin, R.R.; Mitvol, O.L.; Amirkhanov, A.M.; Bardunov L.V.; Novikov, V.S.; Orlov, V.A.; Stepanitsky, V.B.; Belanovich, D.M.; Varlygina, T.I.; et al. Red book of the Rostov Region. Ministry of Natural Resources and Ecology of the Rostov Region, 2nd ed.; Association of Scientific Publications KMK: Rostov-on-Don, Russia, 2008. (In Russian) [Google Scholar]
- Erst, A.A.; Zvyagina, N.S.; Novikova, T.I.; Dorogina, O.V. Clonal Micropropagation of a Rare Species Hedysarum theinum Krasnob. (Fabaceae) and Assessment of the Genetic Stability of Regenerated Plants Using ISSR Markers. Russ. J. Genet. 2015, 51, 158–162. [Google Scholar] [CrossRef]
- Akhmetova, A.S.; Zaripova, A.A. In vitro propagation of some species of the genus Hedysarum L. In Biology, Biochemistry and Genetics; News of the Ufa Scientific Center of the Russian Academy of Sciences: Ufa, Russia, 2017; pp. 28–33. [Google Scholar]
- Kaviani, B. Kaviani Behzad Micropropagation of ten weeks (Matthiola incana) and lisianthus (Eustoma grandiflorum) (two ornamental plants) by using kinetin (KIN), naphthalene acetic acid (NAA) and 2,4-dichlorophenoxyacetic acid (2,4-D). Acta Sci. Pol. Hortorum Cultus 2014, 13, 141–154. [Google Scholar]
- Mohajjel, S.H.; Kharrati, S.H. Effects of different hormonal treatments on growth parameters and secondary metabolite production in organ culture of Hyssopus officinalis L. J. Biotechnol. Comput. Biol. Bionanotechnol. 2021, 102, 33–41. [Google Scholar]
- Hossein, B.; Morteza, A.; Hassani, A.; Morad, J.; Rahimi, A. High-Frequency in Vitro Direct Shoot Regeneration from Nodal Explants of Hyssop Plant (Hyssopus officinalis L.). J. Med. Plants By-Prod. 2016, 2, 187–193. [Google Scholar]
- Prakash, J. Micropropagation of ornamental perennials: Progress and problems. Acta Hortic. 2009, 812, 289–294. [Google Scholar] [CrossRef]
- Werbrouck, S.P.O.; van der Jeugt, B.; Dewitte, W.; Prinsen, E.; Van Onckelen, H.A.; Debergh, P.C. The metabolism of benzyladenine in Spathiphyllum floribundum “Schott Petite” in relation to acclimatisation problems. Plant Cell Rep. 1995, 14, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Bairu, M.W.; Stirk, W.A.; Dolezal, K.; Van Staden, J. Optimizing the micropropagation protocol for the endangered Aloe polyphylla: Can meta-topolin and its derivatives serve as replacement for benzyladenine and zeatin? Plant Cell Tissue Organ Cult. 2007, 90, 15–23. [Google Scholar] [CrossRef]
- Strnad, M.; Hanus, J.; Vanek, T.; Kaminek, M.; Ballantine, J.A.; Fussell, B.; Hanke, D.E. Meta-topolin, a highly active aromatic cytokinin from poplar leaves (Populus × canadensis Moench., CV. Robusta. Phytochemistry 1997, 45, 213–218. [Google Scholar] [CrossRef]
- Zlenko, V.A.; Kotikov, I.V.; Volynkin, V.A.; Troshin, L.P. Optimization of nutrient medium composition by the mathematical design of experiment for shoot tip development in four grapevine genotypes. Sci. J. KubSAU 2010, 55, 237–254. Available online: http://ej.kubagro.ru/2010/01/pdf/05.pdf (accessed on 14 September 2022). (In Russian).
- Imachueva, D.R.; Serebryanaya, F.K. Use of Capillary Electrophoresis Method in Determination of Quantitative Content of Mangiferin in Grass of Species of Genus Hedysarum (Hedysarum Caucasicum M.Bieb., Hedysarum Grandiflorum Pall., Hedysarum Daghestanicum Rupr. ex Boiss) of flora of the North Caucasus. Dev. Regist. Med. (Razrabotka i registraciya lekarstvenny`x sredstv) 2021, 10, 90–96. (In Russian) [Google Scholar]
- Shevchuk, O.M.; Korotkov, O.I.; Malaeva, E.V.; Feskov, S.A. Component composition of essential oil in Hyssopus cretaceus Dubj. and Hyssopus officinalis L. Ind. Bot. (Promy`shlennaya botanika) 2019, 19, 49–54. (In Russian) [Google Scholar]
- Kamelin, R.V.; Gizatulin, R.R.; Mitvol, O.L.; Amirkhanov, A.M.; Bardunov, L.V.; Novikov, V.S.; Orlov, V.A.; Stepanitsky, V.B.; Belanovich, D.M.; Varlygina, T.I.; et al. Red Book of the Russian Federation (Plants and Fungi); Association of Scientific Publications KMK: Moscow, Russia, 2008. (In Russian) [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Plant Physiol. 1962, 15, 437–497. [Google Scholar] [CrossRef]
- McCown, B.H.; Lloyd, G. Woody Plant Medium (WPM)—A Mineral Nutrient Formulation for Microculture of Woody Plant Species. HortScience 1981, 15, 437–497. [Google Scholar]
- Lakin, G.F. Biometriya, 4th ed.; Revised and additional; Higher School: Moscow, Russia, 1990; 351p. (In Russian) [Google Scholar]
- Bakulin, S.D. Cultivation of the Red Book plant species of the Rostov region Hedysarum cretaceum Fisch. In Vitro Culture. Materials of the International Youth Scientific Forum “LOMONOSOV-2020”; Aleshkovsky, I.A., Andriyanov, A.V., Antipov, E.A., Eds.; MAKS Press: Moscow, Russia, 2020; Available online: https://lomonosov-msu.ru/archive/Lomonosov_2020/index.htm (accessed on 5 October 2022). (In Russian)
- Bliudneva, E.A.; Kritckaia, T.A.; Kashin, A.S.; Kirillova, I.M. Conservation of Plant Species and Cultivars in Botanical Garden Saratov State University in vitro Collection. Proc. Saratov Univ. Nov. Ser. Ser. Chem. Biol. Ecol. 2014, 14, 48–53. [Google Scholar] [CrossRef]
- Duque, A.S.; Araújo, S.S.; Fevereiro, P.; Barradas, A.; Godinho, B.; Silva, A.R.; Crespo, J.P. Development of protocols for micropropagation of elite genotype forage allogamous legume species. Acta Hortic. 2015, 1083, 409–413. [Google Scholar] [CrossRef]
- Murakami, Y.; Omoto, T.; Asai, I.; Shimomura, K.; Yoshihira, K.; Ishimaru, K. Rosmarinic acid and related phenolics in transformed root cultures of Hyssopus officinalis. Plant Cell Tissue Organ Cult. 1998, 53, 75–78. [Google Scholar] [CrossRef]
- Zayova, E.; Geneva, M.; Stancheva, I.; Dimitrova, L.; Petrova, M.; Hristozkova, M.; Salamon, I. Evaluation of the antioxidant potential of in vitro propagated hyssop (Hyssopus officinalis L.) with different plant growth regulators. Med. Plants 2018, 10, 295–304. [Google Scholar] [CrossRef]
- Zybkina, D.R. Introduction to the Culture of Some Rare and Medicinal Plants of the Lamiaceae Family (Labiaceae): Final Qualifying Work; Belgorod State University: Belgorod, Russia, 2017; 68p. [Google Scholar]
- Maslova, E.; Gulya, N.; Perelugina, T.; Semykina, V.; Kalashnikova, E. Introduction of Hyssopus officinalis L. into in vitro culture to optimize the conditions for obtaining callus tissues and microclonal propagation as a promising metod of innovative agrobiotechnologies. BIO Web Conf. 2021, 30, 05006. [Google Scholar] [CrossRef]
- Golub, N.O.; Cherednichenko, M.Y. In vitro Introduction of Two Matthiola Species. In Proceedings of the 3rd International Symposium on EuroAsian Biodiversity, Minsk, Belarus, 5–8 July 2017. [Google Scholar]
- Akhmetova, A.S. Morphogenesis of Hedysarum argyrophyllum Ledeb. in in vitro culture. Agrochemistry. Plant Growth Regul. 2013, 9, 55–58. (In Russian) [Google Scholar]
- Gamburg, K.Z. A possibility of saving an endangered endemic of the Lake Baikal shore, Hedysarum zundukii Peschkova (Fabaceae Lindl.) using clonal micropropagation. Nat. Sci. 2013, 5, 1289–1297. [Google Scholar]
- Erst, A.A.; Nuzhdina, N.S. In vitro propagation of endemic species Hedysarum chaiyrakanicum (Tuva Republic, Russia) and its widespread congener, H. gmelini (Fabaceae). BIO Web Conf. EDP Sci. 2020, 24, 00021. [Google Scholar] [CrossRef]
- Alieva, Z.M. Reproduction of rare plants of dagestan in vitro. Botany in the modern world. In Proceedings of the XIV Congress of the Russian Botanical Society and the Conference “Botany in the Modern World”, Makhachkala, Russia, 18–23 June 2018; pp. 233–234. (In Russian). [Google Scholar]
- Erst, A.A.; Zheleznichenko, T.V.; Novikova, T.I.; Dorogina, O.V.; Banaev, E.V. Ecological and geographic variability of Hedysarum theinum and features of its propagation in vitro. Contemp. Probl. Ecol. 2014, 7, 67–71. [Google Scholar] [CrossRef]
- Konurbaeva, R.U.; Aldayrbek kyzy, G.; Umralina, A.R. Introduction to in vitro culture and maintenance of in vitro collections of Hedysarum genus species of Kyrgyzstan. News Univ. 2015, 126–131. (In Russian) [Google Scholar]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soyabean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Bennett, I.J.; McDavid, D.A.J.; McComb, J.A. The influence of ammonium nitrate, pH and indole butyric acid on root induction and survival in soil of micropropagated Eucalyptus globulus. Biol. Plant. 2003, 47, 355–360. [Google Scholar] [CrossRef]
- Rahman, M.H.; Haider, S.A.; Hossain, M.; Islam, R. Effect of potassium and ammonium nitrate media on in vitro growth response of potato (Solanum tuberosun L.). Int. J. Biosci. (IJB) 2011, 1, 54–57. [Google Scholar]
- Luciani, G.; Marinangeli, P.A.; Curvetto, N.R. Increasing nitrate/ammonium ratio for improvement of garlic micropropagation. Sci. Hortic. 2001, 87, 11–20. [Google Scholar] [CrossRef]
- Croser, J.S.; Lülsdorf, M.M.; Davies, P.A.; Clarke, H.J.; Bayliss, K.L.; Mallikarjuna, N.; SiddiqueToward, K.H.M. Toward doubled haploid production in the Fabaceae: Progress, constraints, and opportunities. Crit. Rev. Plant Sci. 2006, 25, 139–157. [Google Scholar] [CrossRef]
- Mongomaké, K.; Hilaire, K.T.; Daouda, K.; Michel, Z.; Justin, K.Y.; Sergio, J. Ochatt In vitro plantlets regeneration in Bambara groundnut [Vigna subterranea (L.) Verdc. (Fabaceae)] through direct shoot bud differentiation on hypocotyl and epicotyl cuttings). Afr. J. Biotechnol. 2009, 8, 1466–1473. [Google Scholar]
- Nanova, Z.; Slavova, Y.; Nenkova, D.; Ivanova, I. Microclonal propagation of hyssop (Hyssopus officinalis L.). Bulg. J. Agric. 2007, 13, 213–219. [Google Scholar]
- Rolli, E.; Ricci, A.; Bianchi, A.; Bruni, R. Optimisation ofin vitropropagation of Hyssopus officinalis L. using two-node explants and N-phenyl-N’-benzothiazol-6-yl-urea (PBU), a new urea-type cytokinin. J. Hortic. Sci. Biotechnol. 2011, 86, 141–145. [Google Scholar] [CrossRef]
- Kaviani, B. Micropropagation of Matthiola incana using BA and IBA. Iran. J. Plant Physiol. 2014, 4, 1071–1078. [Google Scholar]
- Kaviani, B.; Hesar, A.A.; Kharabian-Masouleh, A. In vitro propagation of Matthiola incana (Brassicaceae)-an ornamental plant. Plant Omics J. 2011, 4, 435–440. [Google Scholar]
- Werbrouck, S.P.O.; Strnad, M.; Van Onckelen, H.A.; Debergh, P.C. Meta-topolin, an alternative to benzyladenine in tissue culture? Physiol. Plant. 2008, 98, 291–297. [Google Scholar] [CrossRef]
- Valero-Aracama, C.; Kane, M.E.; Wilson, S.B.; Philman, N.L. Substitution of benzyladenine with meta-topolin during shoot multiplication increases acclimatization of difficult- and easy-to-acclimatize sea oats (Uniola paniculata L.) genotypes. Plant Growth Regul. 2009, 60, 43–49. [Google Scholar] [CrossRef]
- Wojtania, A. Effect of meta-topolin on in vitro propagation of Pelargonium hortorum and Pelargonium hederaefolium cultivars. Acta Soc. Bot. Pol. 2010, 79, 101–106. [Google Scholar] [CrossRef]
- Escalona, M.; Cejas, I.; Gonzalez-Olmedo, J.; Capote, I.; Roles, S.; Cañal, M.J.; Rodríguez, R.; Sandoval, J.; Debergh, P. The effect of metatopolin on plantain propagation using a Temporary Immersion Bioreactor. Infomusa 2003, 12, 28–30. [Google Scholar]
- Podwyszyńska, M.; Wojtania, A.; Rojek, A.; Kowalczyk, J. Mikrorozmnażanie perukowca podolskiego [Micropropagation of smoke tree]. In Proceedings of the Polish Conf. “New Technology in Nursery Production”, Poznań-Kórnik, Poland, 8–9 September 2000; pp. 92–95. [Google Scholar]
- Kubaláková, M.; Strnad, M. The effect of aromatic cytokinins on micropropagation and regeneration of sugarbeet in vitro. Biol. Plant 1992, 34, 578–579. [Google Scholar]
- Hemant, L.; Suman, C.; Natascha, T.; Khana, I.A.; ElSohlya, M.A. In vitro mass propagation of Cannabis sativa L.: A protocol refinement using novel aromatic cytokinin meta-topolin and the assessment of eco-physiological, biochemical and genetic fidelity of micropropagated plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 18–26. [Google Scholar] [CrossRef]
- Chauhan, R.D.; Taylor, N.J. Meta-topolin stimulates de novo shoot organogenesis and plant regeneration in cassava. Plant Cell Tissue Organ Cult. 2018, 132, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Elayaraja, D.; Subramanyam, K.; Vasudevan, V.; Sathish, S.; Kasthurirengan, S.; Ganapathi, A.; Manickavasagam, M. Meta-Topolin (mT) enhances the in vitro regeneration frequency of Sesamum indicum (L.). Biocatal. Agric. Biotechnol. 2019, 21, 101320. [Google Scholar] [CrossRef]
- Gentile, A.; Ja`quez Gutie´rrez, M.; Martinez, J.; Frattarelli, A.; Nota, P.; Caboni, E. Effect of meta-Topolin on micropropagation and adventitious shoot regeneration in Prunus rootstocks. Plant Cell Tissue Organ Cult. 2014, 118, 373–381. [Google Scholar] [CrossRef]



| MS | B5 | WPM | |
|---|---|---|---|
| Control | - | - | - |
| BAP | 0.5 | 0.5 | 0.5 |
| 1.0 | 1.0 | 1.0 | |
| 1.5 | 1.5 | 1.5 | |
| 2.0 | 2.0 | 2.0 | |
| mT | 0.5 | 0.5 | 0.5 |
| 1.0 | 1.0 | 1.0 | |
| 1.5 | 1.5 | 1.5 | |
| 2.0 | 2.0 | 2.0 | |
| KT | 0.5 | 0.5 | 0.5 |
| 1.0 | 1.0 | 1.0 | |
| 1.5 | 1.5 | 1.5 | |
| 2.0 | 2.0 | 2.0 |
| MS | B5 | WPM | ||
|---|---|---|---|---|
| Control | 1.00 ± 0.00 * | 1.00 ± 0,00 * | 1.7 ± 0.22 * | |
| mT | 0.5 | 1.00 ± 0.00 * | 2.10 ± 0.16 * | 1.7 ± 0.23 * |
| 1.0 | 1.00 ± 0.00 * | 2.90 ± 0.34 | 1.3 ± 0.11* | |
| 1.5 | 1.00 ± 0.00 * | 2.40 ± 0.15 | 2.00 ± 0.21* | |
| 2.0 | 1.00 ± 0.00 * | 2.45 ± 0.23 | 1.30 ± 0.13* | |
| BAP | 0.5 | 1.75 ± 0.19 * | 1.25 ± 0.01 * | 1.60 ± 0.18 * |
| 1.0 | 2.35 ± 0.26 | 1.60 ± 0.11 * | 1.20 ± 0.09 * | |
| 1.5 | 1.20 ± 0.14 * | 1.30 ± 0.11 * | 1.65 ± 0.20 * | |
| 2.0 | 1.55 ± 0.14 * | 1.70 ± 0.19 * | 1.35 ± 0.15 * | |
| KT | 0.5 | 1.35 ± 0.14 * | 1.55 ± 0.14 * | 1.25 ± 0.10 * |
| 1.0 | 1.10 ± 0.07 * | 1.90 ± 0.1 * | 1.20 ± 0.09 * | |
| 1.5 | 1.15 ± 0.08 * | 1.55 ± 0.14 * | 1.35 ± 0.13 * | |
| 2.0 | 1.40 ± 0.13 * | 2.65 ± 0.23 | 1.35 ± 0.11 * | |
| MS | B5 | WPM | ||
|---|---|---|---|---|
| Control | 1.00 ± 0.00 * | 1.45 ± 0.18 * | 1.80 ± 0.25 * | |
| mT | 0.5 | 1.00 ± 0.00 * | 4.20 ± 0.42 * | 3.00 ± 0.32 * |
| 1.0 | 1.15 ± 0.08 * | 2.05 ± 0.20 * | 3.00 ± 0.36 * | |
| 1.5 | 1.60 ± 0.23 * | 1.90 ± 0.20 * | 2.25 ± 0.19 * | |
| 2.0 | 1.20 ± 0.16 * | 1.85 ± 0.20 * | 3.50 ± 0.52 | |
| BAP | 0.5 | 1.40 ± 0.15 * | 1.60 ± 0.20 * | 3.20 ± 0.46 |
| 1.0 | 2.60 ± 0.22 * | 1.75 ± 0.20 * | 1.95 ± 0.22 * | |
| 1.5 | 2.20 ± 0.21 * | 1.30 ± 0.15 * | 1.45 ± 0.15 * | |
| 2.0 | 2.40 ± 0.23 * | 1.65 ± 0.21 * | 1.00 ± 0.00 * | |
| KT | 0.5 | 2.25 ± 0.19 * | 2.60 ± 0.20 * | 2.00 ± 0.24 * |
| 1.0 | 2.10 ± 0.20 * | 1.85 ± 0.23 * | 2.30 ± 0.24 * | |
| 1.5 | 2.85 ± 0.33 * | 2.25 ± 0.25 * | 2.45 ± 0.26 * | |
| 2.0 | 2.35 ± 0.31 * | 1.80 ± 0.20 * | 2.60 ± 0.22 * | |
| MS | B5 | WPM | ||
|---|---|---|---|---|
| Control | 1.95 ± 0.14 * | 2.90 ± 0.28 | 3.05 ± 0.22 | |
| mT | 0.5 | 2.65 ± 0.27 * | 3.25 ± 0.36 | 3.15 ± 0.30 * |
| 1.0 | 2.75 ± 0.37 | 1.95 ± 0.22 * | 2.55 ± 0.23 * | |
| 1.5 | 2.85 ± 0.34 | 1.95 ± 0.22 * | 2.10 ± 0.22 | |
| 2.0 | 3.05 ± 0.38 | 2.40 ± 0.26 * | 3.35 ± 0.39 * | |
| BAP | 0.5 | 1.00 * | 2.90 ± 0.45 * | 1.40 ± 0.18 * |
| 1.0 | 1.00 * | 2.40 ± 0.37 * | 1.75 ± 0.22 * | |
| 1.5 | 1.00 * | 2.35 ± 0.27 * | 1.75 ± 0.22 * | |
| 2.0 | 1.00 * | 2.30 ± 0.33 * | 1.85 ± 0.24 * | |
| KT | 0.5 | 1.85 ± 0.20 * | 1.65 ± 0.20 * | 2.65 ± 0.25 * |
| 1.0 | 1.90 ± 0.14 * | 3.75 ± 0.40 * | 1.90 ± 0.27 * | |
| 1.5 | 1.80 ± 0.29 * | 2.30 ± 0.24 * | 2.65 ± 0.30 * | |
| 2.0 | 2.60 ± 0.36 * | 2.75 ± 0.33 * | 2.40 ± 0.24 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chokheli, V.A.; Bakulin, S.D.; Ermolaeva, O.Y.; Kozlovsky, B.L.; Dmitriev, P.A.; Stepanenko, V.V.; Kornienko, I.V.; Bushkova, A.A.; Rajput, V.D.; Varduny, T.V. Investigation of Growth Factors and Mathematical Modeling of Nutrient Media for the Shoots Multiplication In Vitro of Rare Plants of the Rostov Region. Horticulturae 2023, 9, 60. https://doi.org/10.3390/horticulturae9010060
Chokheli VA, Bakulin SD, Ermolaeva OY, Kozlovsky BL, Dmitriev PA, Stepanenko VV, Kornienko IV, Bushkova AA, Rajput VD, Varduny TV. Investigation of Growth Factors and Mathematical Modeling of Nutrient Media for the Shoots Multiplication In Vitro of Rare Plants of the Rostov Region. Horticulturae. 2023; 9(1):60. https://doi.org/10.3390/horticulturae9010060
Chicago/Turabian StyleChokheli, Vasiliy A., Semyon D. Bakulin, Olga Yu. Ermolaeva, Boris L. Kozlovsky, Pavel A. Dmitriev, Victoriya V. Stepanenko, Igor V. Kornienko, Anastasia A. Bushkova, Vishnu D. Rajput, and Tatiana V. Varduny. 2023. "Investigation of Growth Factors and Mathematical Modeling of Nutrient Media for the Shoots Multiplication In Vitro of Rare Plants of the Rostov Region" Horticulturae 9, no. 1: 60. https://doi.org/10.3390/horticulturae9010060
APA StyleChokheli, V. A., Bakulin, S. D., Ermolaeva, O. Y., Kozlovsky, B. L., Dmitriev, P. A., Stepanenko, V. V., Kornienko, I. V., Bushkova, A. A., Rajput, V. D., & Varduny, T. V. (2023). Investigation of Growth Factors and Mathematical Modeling of Nutrient Media for the Shoots Multiplication In Vitro of Rare Plants of the Rostov Region. Horticulturae, 9(1), 60. https://doi.org/10.3390/horticulturae9010060









