Abstract
This work was conducted on the use of the RE (radicle emergence) test to estimate the seedling emergence (SE) and quality (seedling shoot weight (SSW), seedling root weight (SRW) and seedling height (SH)) of twelve hybrid cucumber seed lots with high germination (>95%) at low temperature conditions, high salt conditions and a combination of the two. The percentages of RE, which is a 2 mm radicle protrusion at 25 °C in the dark, were counted between 18 h and 32 h. The seeds were sown in peat moss in trays and kept at 15 °C (low temperature stress), or salty water was applied as 100 mM NaCl at 25 °C (salt stress) for three days, and both were applied in a combined-stress environment. Seedling emergence and quality parameters were reduced by both stress environments and their combination. SE, SSW, SRW and SH values ranged between: 91–100%, 614–844 mg, 102–143 mg, 6.8–8.8 cm at low temperature; 90–98%, 598–904 mg, 101–154 mg, 6.5–7.8 cm at salt stress; and 76–92%, 464–608 mg, 97–133 mg, 5.8–6.9 cm at their combination. The RE values with great differences seen among the lots (20–28 h) were regressed with seedling emergence and quality values. RE 24 h had the highest R2 and significant values in all stress environments as R2 = 0.596–0.858, p < 0.05–0.001 at low temperature; R2 = 0.620–0.827, p < 0.05–0.001 with salt; and R2 = 0.686–0.842, p < 0.05–0.001 with combined stresses. We concluded that RE as a vigour test can be used successfully to estimate the seedling quality of highly germinating hybrid cucumber seed lots. Use of the RE test in hybrid cucumber seeds and its influence on high-quality seedling production were discussed.
1. Introduction
High-quality transplant production is an important issue in vegetable crops. Most summer season vegetables such as cucumbers are produced through transplants. High-quality transplants and their size are related to high seed quality (i.e., germination rate) as faster emerging seeds produce larger-sized seedlings. This has been experimentally proven in maize [1], tomato [2], pepper [3], rocket [4], bean [5] and cauliflower [6] seed lots. The production of cucumbers under glasshouse conditions is conducted with F1 hybrid seeds, which are high-value inputs. Cucumber seeds in general have high germination percentages, but the standard germination tests may not necessarily indicate the seedling emergence potential. Therefore, seed vigour based on the differences among seed lots with high germination percentages under field or glasshouse (stressful) conditions are preferred [7,8]. Perry [9] defined seed vigour as follows: “Seed vigour is a sum of those properties that determine the activity and level of performance of seed lots of acceptable germination in a wide range of environments”. This has evolved and extended to the current International Seed Testing Association (ISTA) definition: “Seed vigour is the sum of those properties that determine the activity and performance of seed lots of acceptable germination in a wide range of environments” [10]. It is a concept that describes the rate and uniformity of seedling growth and the emergence ability of seeds under unfavourable environmental conditions. Cucumber seeds require reasonably high temperatures (i.e., 25 °C) for optimum germination [11,12]. The temperature may be sub-optimal in late winter or early spring sowings in the glasshouse. Saline conditions can reduce the rate of germination and the size of seedlings [13], particularly in the early germination stage, and this results in lower transplant quality and seedling size [14,15]. Vigour tests add to the information provided by the standard laboratory germination test by measuring the potential of seed lots with high levels of normal germination to emerge and produce high-quality transplants under stressful conditions. There are various seed vigour tests, such as accelerated ageing in soybeans, electrical conductivity in peas and controlled deterioration in brassicas, that have been validated by ISTA. However, quick, reliable and easy vigour assessments are valuable for the seed industry. The RE test (early single count of radicle emergence) has been validated by ISTA to assess seed vigour in maize, radishes, oil seed rape and wheat [16]. The RE test has been related to seedling emergence potential in various species [4,6,17,18,19]. The physiological basis of the RE test is the time (lag period) between imbibition and radicle emergence, which is longer in low-vigour (aged) seeds than in undeteriorated (high-vigour) seeds [20,21]. This is because metabolic repair of deterioration takes place during the lag period, which is longer in aged seed lots.
The optimum RE count time varies among the species in relation to the prediction of seedling emergence: for example, 68 h in watermelon, 44 h in melon and 48 h in cucumber [12], 66 h in maize [1], 48 h in viola [22], 48 h in Geranium and Gazania [23], 120 h in leek [24], 52 h in oat [25], 24 h in alfalfa and white clover [17], 48 h in French bean [5], 48 h in petunia [18], 49/66 h in marigold [19], 24h in rocket [4] and 20 h in Chinese milk vetch [26]. This shows that the optimum RE count is influenced by the germination physiology of each species as well as by the germination temperature. For example, RE at 66 h at 20 °C or 6 days at 13 °C is significantly related to field emergence in maize [1]. Higher correlation values were reported between the RE count and normal germination percentages in aubergine, even though 104 h was found to be successfully predictive [27]. Similarly, RE at 48 h was found to successfully predict normal germination at constant and variable temperatures in cucumber seed lots [28]. In the other work, 48 h at 25 °C gave the best RE number for the relationship between field emergence and salt stresses at low temperatures in open-pollinated cucumber seed lots in field sowings [12]. However, it is likely that RE can also be influenced by the production type of seeds such as hybrids, which are produced through crossing between two different parent lines (i.e., heterosis) and can have very different genetic backgrounds compared to open-pollinated types. Mavi et al. [12] tested a stress environment in the field, but hybrid cucumber transplants are produced in greenhouse conditions and seeds are sown in peat moss.
In this study, our aim was to test whether RE can estimate transplant quality as measured by seedling shoot and root fresh weight and seedling height under low temperature and salt stress conditions in cucumber lots. All cultivars were F1 hybrids and had high standard laboratory germination (>95%, normal germination). We tested RE as a vigour test to discriminate cucumber seed lots in relation to their potential to produce high-quality transplants.
2. Materials and Methods
Twelve F1 hybrid cucumber seed cultivars (Cucumis sativus L.) produced in 2020 and 2021 were obtained from different companies (Table 1). The cultivars were Nun 32357, Nun 32355, Nun 32359, Nun 32427, SC 180368, Senyal, Nobel, Samba, Princestar, Captainstar, Dimstar and Julystar. The seeds were kept at 5 °C in sealed aluminium foil packets until use (approximately two months).

Table 1.
Normal germination percentages and production year of hybrid cucumber seed cultivars. NG means with different letters are significant at the 5% level.
For germination tests, three replicates of 50 seeds per cultivar were placed between paper towels (Filtrak, Niederschlag, Germany) (200 × 200 mm) and moistened with 10 mL distilled water. The rolled paper towels were then placed in plastic bags in order to prevent water loss during the test. The bags were held at 25 °C for eight days in the dark. Radicle emergence test counts (RE) (number of seeds with a radicle ≥ 2 mm-long) were made at 18, 20, 22, 24, 26, 28, 30 and 32 h. The counting was performed by taking samples from the incubator and took less than a minute for each replicate. Normal germination percentages were determined after the 8th day of the germination test [16].
The seedling emergence tests were conducted with three replicates of 25 seeds in each seed lot at low temperature, salt stress and the two combined. The seeds were sown 3 cm deep in compost (Plantaflor, Humus Verkaufs, GmBH, Vechta, Germany) in sandwich boxes (360 × 220 × 60 mm). After sowing, the boxes were put in the growing cabinet at 15 °C and kept in the dark for three days for low temperature stress. For salt stress, the boxes were watered with 100 mM NaCl and kept at 25 °C for 3 days. For combined stresses, the boxes were watered with 100 mM NaCl and kept in the dark for 3 days at 15 °C. After that, all boxes were transferred to a growing cabinet for 14 days at 20 ± 2 °C. Light was provided by cool fluorescent lamps (Philips, Hamburg, Germany) at a rate of 78 μmol m−2 s−1 for 12 h d−1 at the seedling level. The relative humidity in the cabinet was maintained above 75% throughout the experiment to minimize water loss from the boxes. Watering was done with an equal amount of distilled water and at the same time of the day for each box. The boxes were rotated every day in the growing cabinet to obtain uniform temperatures during emergence (Figure 1). The appearance of a hypocotyl hook on the compost surface was used as an emergence criterion, and emerged seedlings were recorded daily at the same time of day. To determine seedling growth, destructive harvests were taken 14 days after sowing on 10 randomly selected and normally developed seedlings of each replicate in each cultivar and stressful environment (Figure 2). Then, 30 seedlings in each lot were taken out of the peat moss and cleaned, and seedling height (cm/plant), shoot (mg/plant above ground) and root fresh weight (mg/plant below ground) were determined.

Figure 1.
(a): cucumber seedlings in the growing cabinet, (b): seedling growth at 15 °C +salt, (c): seedling growth at salt, (d): seedling growth at 15 °C, (e): seedling growth at control.
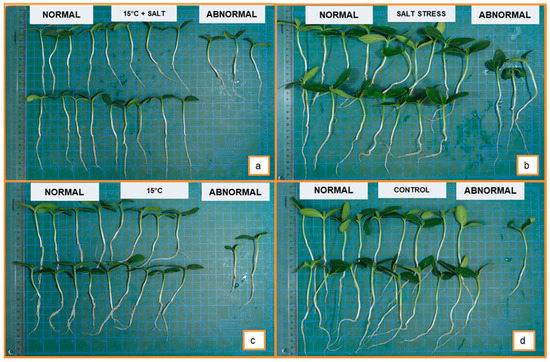
Figure 2.
(a): normal and abnormal seedlings at 15 °C +salt, (b): normal and abnormal seedlings at salt, (c): normal and abnormal seedlings at 15 °C, (d): normal and abnormal seedlings at control.
Duncan’s multiple range tests were performed using SPSS (IBM version 25) as a randomized complete block design (p < 0.05) in order to compare the means. Moreover, correlation coefficient (r) values were determined between RE counts and seedling emergence, seedling shoot and root fresh weight and seedling height. Finally, regression coefficients (R2) were also performed.
3. Results
3.1. Changes in RE Counts
Total germination (radicle emergence) percentages after 8 days were 100% in all seed lots (unpresented data). The normal germination capacity of the twelve seed lots ranged between 95 and 100%, which is well above the commercially acceptable level (Table 1). A comparison of the RE counts of the seed lots between 18 and 32 h showed that differences between the lots were particularly clear in the first 24 h. Four seed lots out of 12 had faster germination and reached 98–100% after 24 h (Figure 3), while germination in the remaining eight lots increased until 32 h. By this hour, lot 1 had 88% RE and lot 2 had 90% RE, while the others had 98–100% RE.
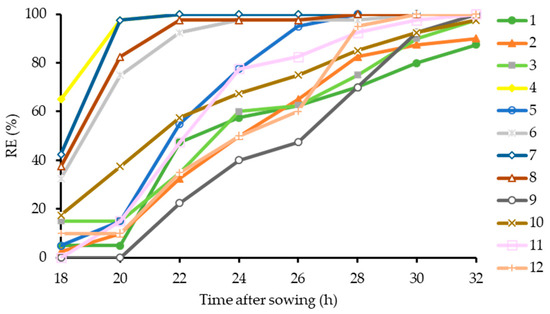
Figure 3.
Germination progress curves to radicle emergence (RE > 2 mm) between 18 and 32 h at 25 °C of 12 hybrid cucumber seed lots.
3.2. Seedling Emergence at Low Temperature and Salt Stresses
Seedling emergence percentages ranged between 91 and 100%, 90 and 98%, and 76 and 92% in low temperature, salt and the combination, respectively. Significance among the seed lots regarding emergence was greater in combined stresses than in individual ones (Table 2, Table 3 and Table 4). Combined stresses reduced emergence to as low as 76% (lot 9). There were differences in the seedling emergence of lots with similar normal germinations. For example, lots 5 and 11 had 100% normal germination percentages, but seedling emergence was 98–93% at low temperature, 97–93% with salt and 78–86% in combined stress conditions (Figure 4, Figure 5 and Figure 6). Similar cases were also seen in the different seed lots.

Table 2.
Variation in seedling emergence (SE, %), seedling shoot weight (SSW, mg), seedling root weight (SRW, mg) and seedling height (SH, cm) with low temperature stress. Means with different letters are significant at the 5% level in each column.

Table 3.
Variation in seedling emergence (SE, %), seedling shoot weight (SSW, mg), seedling root weight (SRW, mg) and seedling height (SH, cm) at salt stress. Means with different letters are significant at the 5% level in each column.

Table 4.
Variation in seedling emergence (SE, %), seedling shoot weight (SSW, mg), seedling root weight (SRW, mg) and seedling height (SH, cm) at low temperature and salt stress combination. Means with different letters are significant at the 5% level in each column.
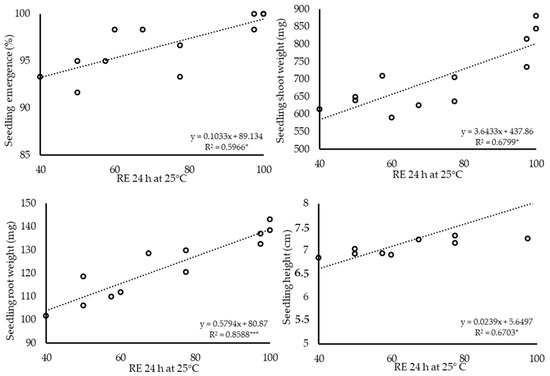
Figure 4.
The relationship between radicle emergence percentages after 24 h (RE 24 h) after germination test were set up at 25 °C and seedling emergence (%), seedling shoot weight (mg), seedling root weight (mg) and seedling height (cm) of 12 hybrid cucumber seed lots in low temperature stress. Seedling parameters were calculated after 14 days, in which seedling trays were kept at 15 °C for 3 days and 22 °C subsequently. Values were derived from Table 2. * p < 0.05, *** p < 0.001.
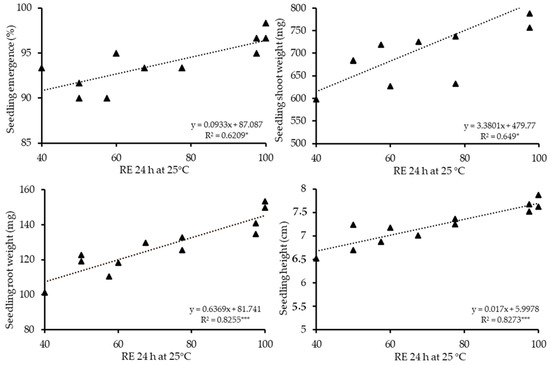
Figure 5.
The relationship between radicle emergence percentages after 24 h (RE24 h) after the germination test was set up at 25 °C and seedling emergence (%), seedling shoot weight (mg), seedling root weight (mg) and seedling height (cm) of 12 hybrid cucumber seed lots in salt stress. Seedling parameters were calculated after 14 days, in which seedling trays were watered with 100 mM of NaCl for 3 days and tap water subsequently. Values were derived from Table 3. * p < 0.05, *** p < 0.001.
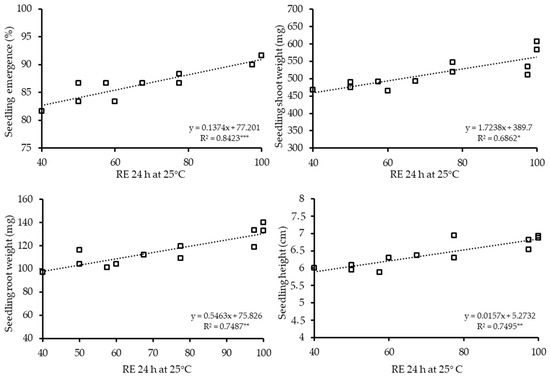
Figure 6.
The relationship between RE 24 h at 25 °C and seedling emergence (%), seedling shoot weight (mg), seedling root weight (mg) and seedling height (cm) of 12 hybrid cucumber seed lots at low temperature and salt stress combinations. Values were derived from Table 4. * p < 0.05, ** p < 0.01, *** p < 0.001.
3.3. Correlation Values in between RE and SEEDLING Parameters
The RE count values were correlated (r) with seedling quality parameters, and the r values are shown in Table 5. RE 24 h had consistently high r values in all three stress conditions. The significance of this RE count was higher than p < 0.01 in all 12 parameters measured (9: p < 0.001, 3: p < 0.01). Regression values were conducted between RE 24 h and seedling quality parameters at the two individual conditions and the combined stress condition. The regression values ranged between R2 = 0.596 and 0.858 in low temperature, R2 = 0.620 and 0.827 in salt and R2 = 0.686 and 0.842 in combined stress conditions and were significant (p < 0.05–0.001) at all twelve (3 stresses × 4 parameters) cases (Figure 4, Figure 5 and Figure 6).

Table 5.
Correlation coefficient values (r) for hybrid cucumber lots between RE after 20, 22, 24, 26 and 28 h at 25 °C, NG and their seedling emergence (SE, %), seedling shoot weight (SSW, mg), seedling root weight (SRW, mg), seedling height (SH, cm) at low temperature, salt and combination stress.
This shows that 59% and 85% of the variation in the seedling quality parameters was accounted for by the regression on RE. Thus, seed lots with low seedling emergence, shoot and root weight and seedling height can be identified by RE after 24 h. Seed lots with high seedling parameters, such as lot 4 and 7, can be identified by a high RE count after 24 h. In contrast, very low variation in the seedling parameters (11 out of 12 cases were non-significant) was accounted for by the regression on standard germination (r = 0.1954–0.582) (Table 5).
4. Discussion
The observations in this study indicated that the RE count at 24 h of seed germination can be used to predict seedling emergence and quality at low temperature, salt stress and a combined stress environment in cucumbers. The seed lots had various seedling quality parameters under low, salt and their combination (Table 2, Table 3 and Table 4). Obviously, vigorous seed lots had higher values than less vigorous ones. The correlation values between RE 24 h and seedling emergence, shoot and root weight and seedling height were significant at various levels (p < 0.05–0.001) in all three sowing environments (Figure 4, Figure 5 and Figure 6). These significant coefficients of determination between the RE counts and seedling quality show that a large proportion of the variance in seedling emergence and size can be explained by RE (Table 5). The RE count provided a more rapid test of the ability of a seed lot to produce better seedling production potential under low temperature and salt stress environments. The RE test will help to distinguish a seed lot that has better seedling production potential under stressful conditions.
The prediction of seedling emergence by a single count of RE after 24 h in germination tests at 25 °C supported previous findings that showed that RE can identify differences in seedling emergence and growing potential [1,4,5,6,17,18,19,22,23,24,25,26].
In our study, RE distinguished the seedling production potential of seed lots with normal germination of above 95% where all of the seed lots were hybrids. This indicates that RE distinguishes seed lots with high germination percentages in regard to seed vigour, which fits the definition of seed vigour as “Seed vigour is the sum of those properties that determine the activity and performance of seed lots of acceptable germination in a wide range of environments” [10]. Vigour tests are expected to add information about any lot provided by the standard laboratory germination test in relation to the potential to produce high emergence percentages and seed storage longevity [7,8]. Various seed lots had 100% normal germination percentages, but seedling emergence percentages at low temperatures, salt and combined stresses varied. For example, lots 5, 7 and 11 had various seedling emergence percentages even though they had 100% normal germination (Table 1, Table 2, Table 3 and Table 4). The differences in emergence were more evident in the combined stress environment (Table 4). It appears that vigour problems in commercial seeds may be more of a feature of species that maintain high levels of germination of normal seedlings in standard laboratory germination tests, particularly in stress sowings. This can be more important when they are carried over and sown in more than one season, due to extended seed ageing. Low coefficient values in 11 cases (p < 0.05) between normal germination, even though it is very high (between 95–100%), and seedling quality features indicate that standard laboratory germination is not sufficient to discriminate potential seedling quality (Table 5). This confirms not only the necessity of the vigour test to evaluate the seed lot quality to estimate seedling quality potential but also the sound conclusion about RE as a successful vigour test in cucumbers. RE 48 h was related to normal germination percentages [29] and seedling emergence in stressful field sowing environments [12] in cucumbers. We found that a much earlier count of RE (24 h) was more successful in predicting potential emergence and seedling quality (Table 5). In our study, the RE count was completed, and the differences among the lots were lost by 32 h of the count (Figure 3). This may originate from the differences in the quality of the seed lots that we used. Such fast germination (i.e., when hybrids are involved in the research) makes very narrowly timed counting necessary (i.e., every 2 h). One other reason for such fast RE counts was due to hybrid seed use and their very high normal germination (95%) percentages. Therefore, the optimum RE count occurred much earlier than what was suggested in open-pollinated cultivars [12,28]. This gives an indication that optimum RE counts can vary when very high-quality seed lots are used. One way, in our opinion, may be to make counts over a large range of time during germination to see the differences among the lots or use lower germination to extend RE germination time as Matthews et al. [1] did in maize seeds.
Differences in RE, as seen here, have been related to the degree of seed ageing and to the metabolic repair of the deterioration caused by ageing before RE can occur [7,20,21,30]. It appears that, even though seed lots in hybrids were produced by optimum production practices (harvesting, drying, etc.), some ageing may occur before RE, which influences the lag period between water imbibition and radicle appearance. Moreover, hybrid seeds were more likely to be stored in optimum storage conditions (cold storage) than are low-value open-pollinated ones due to their high value. Therefore, ageing is likely to occur to a lesser extent in hybrids.
In some earlier studies, RE was used to distinguish seed vigour in lots belonging to the same cultivar [4,12]. More recently, RE has successfully differentiated normal germination among lots belonging to different cultivars of lettuce [31]. It is easier to find more lots in the same cultivar in open-pollinated species. Open-pollinated cultivars are produced by many different companies in different regions. However, this is not the case with hybrid ones, unless the lots have been produced in various growing environments or processed by different methods. Therefore, each lot should be a unique cultivar. Furthermore, hybrid seeds are expensive, and so it is not easy to get a large number of lots for research purposes. Therefore, we considered each cultivar as a lot in this study.
Some abiotic stresses are predominant in cucumber growing conditions, such as low temperature and salinity. Glasshouse cucumber production is characterized by early production, and so seeds are sown in early spring or late winter in modules for seedling production. At this time of the year, the temperature in the glasshouse may decline to lower than optimum, particularly during the night, which makes germination slower and decreases seedling quality [32]. Moreover, in the Mediterranean region, the use of groundwater is common in production systems, and so salty water can be another reason for the lowering of seedling quality [14]. Individual stress environments reduced seedling emergence and quality (Table 2 and Table 3), but when both stress factors were combined, the reduction was greater. This was more prominent in some lots than in others. For example, lot 5 had 97 and 98% emergence with salt and low temperature stresses, respectively, but when the stresses were combined, the emergence was reduced to as low as 78% (Table 2, Table 3 and Table 4). Similar reductions were also observed in the seedling quality measurements. These results support the definition of seed vigour as discrimination performance of seed lots of acceptable germination in a wide range of environments [10].
Manual RE counting is not an easy task when a large number of lots are concerned. Automated systems to do this have been developed for such purposes [33]. An automated system for the calculation of RE along with the determination of germination curves and mean germination time has been successfully used by GEVES over 45 crops [34]. Comparison of an automated system with a manual one was tested and found successful in oil seed rape [35] and cauliflower [6]. In our opinion, an automated machine system is more useful for those seed lots that have high germination percentages and that germinate faster, since more frequent counts are necessary to determine the differences in such lots. The RE count timings are not easy to determine unless the germination physiology of the species is known. This is not a problem in machine counting. Moreover, opening and closing the germination paper each time may be time consuming when many samples need to be processed. We pay attention to being as gentle as possible in our work. However, this may not be easy when a large number of lots must be dealt with and finished on time.
5. Conclusions
We identified the seedling emergence and quality potential of hybrid cucumber seed lots by using an RE vigour test at low temperature and salt stress environments. This reveals the potential for a rapid assessment of seed vigour in high-germinating seeds (>95%) by the RE test. The RE 24 h count at 25 °C can give a fast and an efficient ranking of cucumber seed lots regarding seedling quality. Our results can be used for automated use of the RE test and the comparison by both manual and automated systems. This can be used by transplant-producing companies in order to determine the seed vigour potential of cucumber lots before sowing, particularly when low temperatures and salt stresses are concerned.
Author Contributions
Conceptualization: I.D.; analysis of data, production of figures: S.E. and G.Ö.; writing, review and editing: I.D., S.E. and G.Ö.; funding acquisition: C.O.K.; supervision of research, structuring the paper, writing the original and draft preparation: S.E. and I.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. We would like to thank Syngenta Seeds, Genetika Seeds, Multi Seeds, Nunhems Seeds and Rijk Zwaan Seed companies for helping us sourcing the seeds.
Conflicts of Interest
The authors declare that there are no conflicts of interest related to this article.
References
- Matthews, S.; Beltrami, E.; El-Khadem, R.; Khajeh-Hosseini, M.; Nasehzadeh, M.; Urso, G. Evidence that time for repair during early germination leads to vigour differences in maize. Seed Sci. Technol. 2011, 39, 501–509. [Google Scholar] [CrossRef]
- Mavi, K.; Powell, A.A.; Matthews, S. Rate of radicle emergence and leakage of electrolytes provide quick predictions of percentage normal seedlings in standard germination tests of radish (Raphanus sativus). Seed Sci. Technol. 2016, 44, 393–409. [Google Scholar] [CrossRef]
- Demir, I.; Ermis, S.; Mavi, K.; Matthews, S. Mean germination time of pepper seed lots (Capsicum annuum) predicts size and uniformity of seedlings in germination tests and transplant modules. Seed Sci. Technol. 2008, 36, 21–30. [Google Scholar] [CrossRef]
- Ozden, E.; Memis, N.; Gokdas, Z.; Catıkkas, E.; Demir, I. Seed vigour evaluation of rocket (Eruca sativa Mill) seed lots. J. Inst. Sci. Technol. 2020, 10, 1486–1493. [Google Scholar] [CrossRef]
- Ozden, E.; Ermis, S.; Demir, I. Evaluation of seed vigour in White coat French bean (Phaseolus vulgaris L.) seed lots under waterlogged or field capacity conditions. J. Inst. Sci. Technol. 2019, 9, 1860–1865. [Google Scholar] [CrossRef]
- Shinohara, T.; Ducournau, S.; Matthews, S.; Wagner, M.H.; Powell, A.A. Early counts of radicle emergence, counted manually and by image analysis, can reveal differences in the production of normal seedlings and the vigour of seed lots of cauliflower. Seed Sci. Technol. 2021, 49, 219–235. [Google Scholar] [CrossRef]
- Powell, A.A.; Matthews, S. Seed ageing/repair hypothesis leads to new testing methods. Seed Technol. 2012, 34, 15–25. [Google Scholar]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef]
- Perry, D.A. Report of the vigour test committee, 1974–1977. Seed Sci. Technol. 1978, 6, 159–181. [Google Scholar]
- ISTA. International Rules for Seed Testing, 2015 ed.; International Seed Testing Association: Bassersdorf, Switzerland, 2015. [Google Scholar]
- Bates, M.D.; Robinson, R.W. Cucumbers, Melons and Watermelons. Evolution of Crop Plants, 2nd ed.; Smartt, J., Simmonds, N.W., Eds.; Longman Scientific & Technical: Essex, UK, 1995; pp. 89–97. [Google Scholar]
- Mavi, K.; Demir, I.; Matthews, S. Mean germination time estimates the relative emergence of seed lots of three cucurbit crops under stress conditions. Seed Sci. Technol. 2010, 3, 14–25. [Google Scholar] [CrossRef]
- Foolad, M.; Lin, G.Y. Genetic potential for salt tolerance during germination in Lycopersicon species. HortScience 1997, 32, 296–300. [Google Scholar] [CrossRef]
- Sivritepe, H.O.; Sivritepe, N.; Eris, A.; Turhan, E. The effects of NaCl pre-treatments on salt tolerance of melons grown under long-term salinity. Sci. Hortic. 2005, 106, 568–581. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Debaeke, P.; Steinberg, C.; You, M.P.; Barbetti, M.J.; Aubertot, J.N. Abiotic and biotic factors affecting crop seed germination and seedling emergence: A conceptual framework. Plant Soil 2018, 432, 1–28. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing, 2021 ed.; International Seed Testing Association: Bassersdorf, Switzerland, 2021. [Google Scholar]
- Lv, Y.Y.; Mo, Q.; Powell, A.A.; Wang, Y.R. DNA replication during the seed germination, deterioration, and its relation to vigour in alfalfa and white clover. Crop Sci. 2018, 57, 1393–1401. [Google Scholar] [CrossRef]
- Demir, I.; Erturk, N.; Gokdas, Z. Seed vigour evaluation in petunia seed lots to predict seedling emergence and longevity. Seed Sci. Technol. 2020, 48, 391–400. [Google Scholar] [CrossRef]
- Ilbi, H.; Powell, A.A.; Alan, O. Single radicle emergence counts for predicting vigour of marigold (Tagetes spp.) seed lots. Seed Sci. Technol. 2020, 48, 381–389. [Google Scholar] [CrossRef]
- Guy, P.A.; Black, M. Germination related proteins in wheat revealed by differences in seed vigour. Seed Sci. Res. 1998, 8, 99–111. [Google Scholar] [CrossRef]
- Matthews, S.; Khajeh Hosseini, M. Length of the lag period of germination and metabolic repair explain vigour differences in seed lots of maize (Zea mays). Seed Sci. Technol. 2007, 35, 200–212. [Google Scholar] [CrossRef]
- Demir, I.; Celikkol, T.; Sarıkamıs, G.; Eksi, C. Vigor tests to estimate seedling emergence potential and longevity in viola seed lots. HortScience 2011, 46, 402–405. [Google Scholar] [CrossRef]
- Guloksuz, T.; Demir, I. Vigour tests in Geranium, Salvia, Gazania, and Impatiens seed lots to estimate seedling emergence potential in modules. Propag. Ornam. Plants 2012, 12, 133–138. [Google Scholar]
- Ermis, S.; Karslıoglu, M.; Ozden, E.; Demir, I. Use of a single radicle emergence count as a vigour test in prediction of seedling emergence potential of leek seed lots. Seed Sci. Technol. 2015, 43, 308–312. [Google Scholar] [CrossRef]
- Lv, Y.Y.; Wang, Y.R.; Powell, A.A. Frequent individual counts of radicle emergence and mean just germination time predict seed vigour of Avena sativa and Elymus nutans. Seed Sci. Technol. 2016, 44, 189–198. [Google Scholar] [CrossRef]
- Tao, Q.; Sun, J.; Zhang, Y.; Sun, X.; Li, Z.; Zhong, S.; Sun, J. Single count of radicle emergence and mean germination time estimate seed vigour of Chinese milk vetch (Astragalus sinicus). Seed Sci. Technol. 2022, 50, 47–59. [Google Scholar] [CrossRef]
- Ozden, E.; Ozdamar, C.; Demir, I. Radicle emergence test estimates predictions of percentage normal seedlings in standard germination tests of aubergine (Solanum melongena L.) seed lots. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 177–182. [Google Scholar] [CrossRef]
- Powell, A.A. The potential for new approaches to seed testing: The role of the seed science advisory group. Seed Test. Int. 2020, 159, 18–22. [Google Scholar]
- Powell, A.A. Invited Review: Seed vigour in the 21st century. Seed Sci. Technol. 2022, 50, 45–73. [Google Scholar] [CrossRef]
- Waterworth, W.M.; Footitt, S.; Bray, C.M.; Finch-Savage, W.E.; West, C.E. DNA damage checkpoint kinase ATM regulates germination and maintains genome stability in seeds. Proc. Natl. Acad. Sci. USA 2016, 113, 9647–9652. [Google Scholar] [CrossRef]
- Serpil, M.; Ermis, S.; Powell, A.A.; Demir, I. Radicle emergence (RE) test identifies differences in normal germination percentages (NG) of watermelon, lettuce and carrot seed lots. Seed Sci. Technol. 2022, 50, 257–267. [Google Scholar] [CrossRef]
- Nerson, H. Seed production and germinability of cucurbit crops. Seed Sci. Biotechnol. 2007, 1, 1–10. [Google Scholar]
- Demilly, D.; Ducournau, S.; Wagner, M.H.; Dürr, D. Digital imaging of seed germination. In Plant Image Analysis: Fundamentals and Applications; Gupta, S.D., Ibaraki, Y., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 147–162. [Google Scholar] [CrossRef]
- Wagner, M.H.; Ducournau, S.; Luciani, A.; Léchappé, J. From knowledge-based research towards accurate and rapid testing of seed quality in winter rape. Seed Sci. Res. 2012, 22, 80–85. [Google Scholar] [CrossRef]
- Matthews, S.; Wagner, M.H.; Kerr, L.; McLaren, G.; Powell, A.A. Automated determination of germination time courses by image capture and early counts of radicle emergence lead to a new vigor test for winter oilseed rape (Brassica napus). Seed Sci. Technol. 2012, 40, 413–424. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).