Abstract
The chrysanthemum is one of the most economically important ornamental plants in the Asteraceae family. Unfortunately, the efficacy of breeding through the traditional crossing in this species is highly limited due to inefficient seed setting. Therefore, this study aimed to investigate the effect of parental components and crossing frequency on the set and germination of chrysanthemum seeds. For this purpose, seven chrysanthemum cultivars were used as parental components in 10 crossing combinations. The crossing was performed either once or twice a week, for three successive weeks, starting from November. Next, the obtained chrysanthemum seeds were collected, sown in pots in a greenhouse, and seedling growth was observed. The efficacy of the seed set, germination rate, and plant development was evaluated. The plants of the F1 generation were brought to flowering and evaluated phenotypically in the three successive vegetative propagation cycles. Both the arrangement of parental components and pollination frequency affected the production of seeds. More seeds were obtained if pollination was performed twice a week and if the ‘Wda’ cultivar was used as the maternal component. Approximately 50–100% of the seeds were able to germinate in the greenhouse, depending on the parental components, which also affected the developmental pace of the seedlings. Nearly all of the seedlings (80–100%) developed into properly growing plants. Out of 10 parental combinations tested, 7 produced the F1 offspring. The obtained plants varied in the shape, size, and color of their flowers. A total of eight new phenotypes were found, among which six new cultivars granted plant breeders’ rights, so far. The present research expands knowledge on how effective crossing should be performed.
1. Introduction
The chrysanthemum (Chrysanthemum × morifolium/Ramat./Hemsl., syn. Dendranthema grandiflorum (Ramat.) Kitamura) was already used as an ornamental garden plant in ancient China. Currently, it is one of the four major cut flowers in the world, with a high economic value [1]. Depending on the cultivar, it is suitable for bedding, rock gardens, or as a pot and cut flower. Inflorescences of chrysanthemum are compound, i.e., consist of centrally arranged, evolutionarily older, bisexual tubular/disc florets, surrounded by female ligulate/ray florets, placed on a plate-shaped extension of the stem axis, known as the settler. The inflorescences exhibit various colors and shapes, which makes them so successful in the market [2].
Breeders constantly introduce new cultivars to meet the requirements of customers and the increasing popularity of the chrysanthemum [3]. The primary goal in chrysanthemum breeding is the creation of cultivars with varied shapes and colors of inflorescences, as well as cultivars with a fast-growing capacity and increased resistance. Currently, mutation breeding using physical and chemical mutagens is often used with chrysanthemums [4,5]. However, access to physical mutagens is limited for plant breeders, while chemical ones are user- and environmentally dangerous. Genetic transformation and protoplast fusion are modern biotechnological tools used in the creation of new cultivars, but their application is technically difficult and expensive [6,7]. Classic breeding based on cross-breeding is relatively cheap and easy to perform in numerous ornamental plant species. Cross-breeding done between individuals representing desired traits of distant values can be a source of new variability, being of high value in the world of ornamentals [8]. However, in chrysanthemums, it is ineffective due to the plants’ sporophytic self-incompatibility (SI) and lack of viable pollen or inability of pollen to germinate in the style [9,10,11]. Most of the modern cultivated chrysanthemums are self-incompatible and their trait inheritance and genetic background are extremely complex [12,13]. Therefore, it is very difficult to create pure lines [13]. Self-incompatibility, also called self-infertility, means the inability of a fully fertile hermaphroditic plant to produce zygotes when self-pollinated [14]. Moreover, vastly applied mutation breeding resulted in inbreeding depression and a negative genetic load in modern greenhouse cultivars, which reduces the overall fertility of the species [15]. The development of female and male reproductive organs is well explored, as is the role of their mutual interaction in effective fertilization [16]. Nonetheless, little research has been conducted to gain better seed production in the common greenhouse chrysanthemum.
The aim of this research was to study the effect of pollination frequency and the arrangement of parental genotypes on the setting and germination of chrysanthemum seeds. Following the experiment, the in vivo seed set efficiency was examined and several new cultivars of chrysanthemum were identified and registered. The scientific hypothesis assumed that by increasing the frequency of pollination and choosing the parental genotypes correctly, it will be possible to obtain more seeds and, consequently, more new phenotypes in chrysanthemum cross-breeding.
2. Materials and Methods
2.1. Plant Material
The plant material included paternal and mother plants of seven chrysanthemum cultivars (Chrysanthemum × morifolium/Ramat./Hemsl.). Five of them were bred in the Laboratory of Ornamental Plants and Vegetable Crops of the Bydgoszcz University of Science and Technology, i.e., ‘Polka’ (P), ‘Bydgoszczanka’ (BG), ‘Wda’ (W), ‘Brda’ (B), and ‘Łuczniczka’ (Ł), and were granted plant breeder’s rights (PBR). The other two cultivars, namely UTP4 and JTY, were selected from the Laboratory’s perennial chrysanthemums collection. All of the cultivars (except JTY) used in the experiment have medium-size semi-full inflorescences with a diameter of 5–10 cm and yellow to green disc florets. Chrysanthemums ‘Polka’, UTP4, and ‘Brda’ have white ray florets of flat and tubular (‘Polka’) shape, ‘Bydgoszczanka’ and ‘Łuczniczka’ have light- and dark-pink, curved ray florets, while ‘Wda’ has short, red, flat ray florets. Chrysanthemum JTY is characterized by yellow, flat ray florets and a full type of inflorescence (Figure 1).

Figure 1.
Parental components used for crossing in the experiment. Names of cultivars and abbreviations (in brackets) are indicated in the photographs.
2.2. Cultivation in the Greenhouse and Crossing
The plants were grown in a greenhouse of the Laboratory of Ornamental Plants and Vegetable Crops (53°07′12.0″ N 18°00′29.4″ E) in pots filled with peat substrate (Hartman, Poznań, Poland) in a natural photoperiod. The dates of pollination were adjusted to the dates of maturation of selected parental components, the first crossing was performed on 10 November (Table 1). The starting point for crossing was: for the paternal component—when most of the disc florets matured and produced an abundance of bright yellow pollen; for the maternal component—when the outer whorls of disc florets of the mother plants were mature (started to open and produce pollen). The paternal components that were the pollen source for the selected maternal plants were not used in other crosses. All of the cultivars used in the experiment were previously confirmed as self-incompatible.

Table 1.
Dates of chrysanthemum pollination.
Pollination was performed once (1×) or twice (2×) a week. In each set of parental components, pollen was transferred to a total of 12 inflorescences of the mother plants (6 inflorescences were pollinated once a week and 6 inflorescences twice a week). Each inflorescence was considered a single repetition. Pollination was carried out for a total of three weeks for each parental combination.
Pollen from the paternal components was collected on the pollination day in the morning hours (9:00–11:00 a.m.) with a brush and applied to the stigmas of the pistils in the ligulate and tubular florets of the mother plants. During the crossing, the air temperature in the greenhouse was maintained at 14 ± 2 °C. The parental components and pollination dates are given in Table 1
From 2 December to 13 December, the shoots with maternal inflorescences were cut, placed in water in a light, airy room, under natural light and photoperiod, at 16 ± 2 °C, and left for 10 weeks to set seeds. Next, the seeds were collected and counted (Figure 2A,B). Based on the number of seeds obtained and the number of ligulate and tubular flowers present in the mother inflorescences, the efficiency of seeds set in individual parental systems was calculated as:
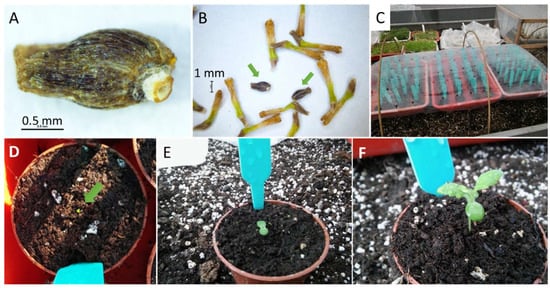
Figure 2.
Fruit (achenes, considered as seed) of chrysanthemum (A); fruits (seeds) of chrysanthemums (pointed with an arrow) among dried tubular florets (B); seeds planted in pots covered with perforated film (C); plant growth phase I—beginning of germination (D); phase II—fully developed cotyledons (E); phase III—the first fully developed leaf (F).
Sowing was performed in the second week of March in a greenhouse. Each seed was sown in a separate pot (Ø 6 cm), filled with Gramoflor Culvito substrate (Vechta, Germany), pH 4.5–6.5, mixed with perlite (2:1). The pots were covered with transparent perforated film and mesh, to protect the seedlings from excessive sunlight and loss of humidity (Figure 2C). The seeds were grown for 30 days. During that period, they were sprayed with water, aired, and watered. The seedling growth phases were recorded every two days according to the key:
Next, the F1 plants were grown and brought to full flowering, as described earlier, and the quality of their inflorescences was studied in search of new potential cultivars. To confirm the distinctness, uniformity, and stability (DUS) of the novel traits, chrysanthemums were propagated vegetatively through stem cuttings and cultivated in the following three years.
2.3. Statistical Analysis
The experiment was set in a completely randomized design and repeated six times. Nine parental combinations and two crossing frequencies were included. Data were statistically verified using Statistica 12.0 (StatSoft Polska, Cracow, Poland) software. The analysis of variance (ANOVA) was performed and means were compared with a post-hoc test (Newman-Keuls’ or Fisher’s exact test) at the significance level of p ≤ 0.05. To obtain the normal distribution of the data expressed as a percentage, the Freeman-Tukey double-arcsine transformation was used. Tables with results provide real, untransformed numerical data, with the alphabet indicating the homogeneous groups.
3. Results
A total of 15,507 ligulate and tubular florets were counted in 120 maternal inflorescences, including 93 seeds, which gives an average of 0.8 seeds per inflorescence (Table 2). The highest number of florets (178.7–182.5) was produced by the ‘Brda’ and ‘Wda’ cultivars, while the lowest (79.2–112.2) was by the JTY chrysanthemum. The highest number of seeds (13–22) was produced by crossing plants twice a week, especially in the experimental objects where the ‘Wda’ cultivar was used as the maternal component (W × P, W × UTP4, W × Ł). In contrast, no seeds were found in three parental combinations: JTY × UTP4, JTY × P, and JTY × W, which suggests that JTY does not work well as a mother plant (Table 2 and Figure 2).

Table 2.
Total and mean numbers of flowers in inflorescences, produced seeds, and percentage efficiency of seed set.
The seeds started germinating five days after sowing. Among the 93 seeds obtained in total, 67 seeds germinated (72%), however, three plants did not develop properly. The share of germinating seeds ranged from 50 to 100%, depending on the parental components (Table 3). The highest share of sprouted seeds was obtained from the following parental components: Ł × B, W × Ł, W × P, and JTY × Ł, while the lowest was in the experimental object W × BG.

Table 3.
Number and share of germinating seeds and growing plants depending on the parental components.
The beginning of germination (phase I) was the shortest in seedlings from the W × BG parental object (4 days), and the longest in seedlings obtained from the crossing of Ł × B (10.5 days; Figure 2). Phase II (fully developed cotyledons) was achieved first by the seedlings which were the offspring of the W × BG components (in less than 7 days). On the other hand, in the seedlings derived from the Ł × B crossing, phase II was only achieved after an average of 3 weeks. The seedlings that were the B × Ł offspring reached their full maturity (phase III) the earliest, at 10 days after sowing, whereas for the seedlings from the Ł × B crossing, it was after 30 days. The mean results show that the experimental object Ł × B needed the most time to achieve all of the recorded stages of seedling development. As for chrysanthemum seedlings derived from B × Ł and W × BG parent components, the successive growing phases followed quickly one after another, at 2–3 days intervals (Figure 3).

Figure 3.
The number of days needed to obtain the three following stages of development in F1 seedlings, depending on the arrangement of the parental components.
Out of 10 parental combinations tested, 7 produced the F1 offspring (Table 4).

Table 4.
Number of F1 plants obtained in the experiment from different parental combinations; number of promising phenotypes preliminarily selected for further observations after vegetative propagation; current number of phenotypes which granted PBRs and number of phenotypes being submitted to grant PBRs (DUS tests under proceedings).
The obtained plants varied in the shape, size, and color of their flowers. A total of 64 new phenotypes were found, from which 20 were selected for further observations due to their promising traits. Most of the selected phenotypes showed single or semi-full inflorescences, except one full inflorescence coming from the JTY × Ł crossing. The most abundant in new interesting phenotypes were crosses W × UTP4 and W × P. They gave six and five promising phenotypes, respectively. The crosses Ł × B and JTY × Ł produced only one and two interesting phenotypes, respectively. No new phenotype was found from W × BG. The remaining two crosses both resulted in three selected phenotypes. The distinctness, uniformity, and stability of the new phenotypes of chrysanthemums were confirmed in the successive three years of greenhouse cultivation and annual vegetative propagation of all the obtained phenotypes. Eight of them were selected based on the inflorescences’ attractiveness, novelty, type of growth, and resistance, and submitted to the Community Plant Variety Office (CPVO) in Poland for official distinctness, uniformity, and stability (DUS) testing. As a result, six new, valuable cultivars were granted PBRs in the years 2019–2022, while with the two other submissions, the procedure is ongoing (Figure 4).
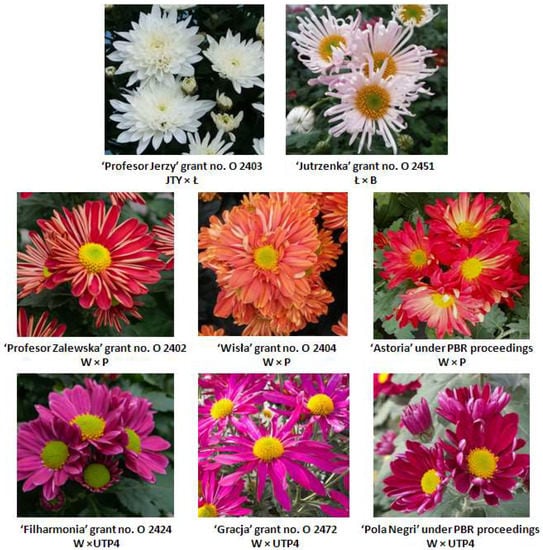
Figure 4.
New chrysanthemum cultivars obtained as a result of the experiment and submitted for granting PBRs. Denomination and grant number are followed by the parental composition from which the genotype originates.
4. Discussion
A number of factors determine the possibility of obtaining new cultivars through the crossing. In the common greenhouse chrysanthemums, one of the bottle-necks is seed establishment, since they perform a low seed-setting capability, which limits the breeding efficiency [10]. In the present study, the influence of selected factors (pollination frequency and parental genotypes) on the setting and germination of chrysanthemum seeds was analyzed.
Our research has shown that, despite a large number of florets, inflorescences of chrysanthemums contain a relatively small amount of seeds, sometimes even none. Likewise, no seeds were obtained in the crossing between Chrysanthemum × morifolium ‘Yuhuaxingchen’ and Chrysanthemum × nankingense Hand.-Mazz. [16]. On the other hand, in the cross between C. grandiflorum and C. indicum, the seed set level reached 59%. In the cross between C. grandiflorum and C. zawadskii Herbich, the seed set was only 9% [16]. In chrysanthemums, the incubation of pollen at −80 °C could significantly increase the pollen germinability and seed set efficiency [17]. On the other hand, according to Deng et al. [18], pollen viability has no significant effects on seed set in chrysanthemums.
Nonetheless, very few germinated pollen grains on stigmas and the abnormal growth of most pollen tubes before fertilization, as well as embryo abortion, are the main factors which cause the failure of cross-breeding [16]. This could be explained by the fact that the chrysanthemum is a hexaploid hybrid. Moreover, modern cultivars are commonly a result of mutation breeding, which decreases their reproductive capacities [15]. Therefore, embryo rescue under in vitro conditions appears to be a promising solution to this problem and could be considered in future studies [19].
The highest number of seeds was produced in those crossings where the ‘Wda’ cultivar was used as the maternal component (W × P, W × UTP4, W × Ł). This could be explained by the fact that it originates from garden chrysanthemums, which establish the seeds more easily [9]. On the other hand, no seeds were found in the following three experimental objects: JTY × Ł, JTY × P, and JTY × W, which indicates that the JTY genotype is not suitable as a mother plant, possibly due to low fertility. Such a low seed setting efficiency may be related to the fact that most modern greenhouse cultivars of chrysanthemums are created through induced mutagenesis, which results in the loss of their natural reproductive abilities [20].
Various seed setting efficiency in the investigated combinations of parental plants may result from the genetic background related to sporophytic self-incompatibility, which is ubiquitous in chrysanthemums [21]. It prevents inbreeding, but S alleles are widely distributed in the genomes of the greenhouse cultivars, which lead to the decrease in successful fertilization and poor seed production [21,22]. Matching genetically distinct individuals, such as ‘Wda’ (possessing garden chrysanthemums’ genetic load), for crosses with the typical greenhouse cultivars could increase the seed set in our experiment. More seeds were obtained by crossing plants twice a week than once a week. Thus, it can be concluded that the frequency of crossing has a significant influence on seed establishment in chrysanthemums. The more often the crosses are repeated, the greater the likelihood of obtaining more seeds, which is related to the structure of the chrysanthemum inflorescence. In Asteraceae, numerous single florets are collected within a head, which was developed in the span of the evolutionary process as the most complex, yet effective inflorescence type [9]. During pollination, the pollen is transferred to the pistil stigma in the florets of a mother plant, which mature gradually from the outer whorls to the center of the inflorescence [23]. The maturation of all the florets within the head from the edges to the center takes about two to three weeks [24]. The developed flowers stay receptive (retain the ability to absorb pollen) for several days, therefore, the inflorescence can be pollinated recurrently, every two or three days to the next developing whorls. Consequently, the chance of pollinating more receptive flowers is higher, resulting in a better seed setting.
Despite several obstacles, it is worth investing in chrysanthemum crossbreeding, as it is a good way to create a pool of novel interesting cultivars, useful in further breeding programs, for example, through mutagenesis [25]. It is worth mentioning that a complex chrysanthemum genome consisting of six sets of chromosomes, as well as high heterozygosity, are the source of great F1 progeny variation [9]. In the present study, it was possible to obtain eight new phenotypes of inflorescences that varied in shape, size, and color, and each one was different from another. Among these new phenotypes, six received PBRs, so far, and the remaining two are under DUS tests. These new cultivars received great interest, were awarded several prestigious awards during international innovation shows, and are currently being introduced to the Polish market. Chrysanthemums produced in our Laboratory are the sole Polish cultivars available in the market.
5. Conclusions
Greenhouse cultivars of chrysanthemums produce a relatively small number of seeds concerning the number of flowers in the inflorescence. Therefore, classical cross-breeding in this species is a considerable challenge. The frequency of chrysanthemum pollination has a significant effect on the outcome of the breeding process. Pollination performed twice a week increases the chance of obtaining more seeds. The number of seeds produced also depends on the genotype of the parental components. Chrysanthemum ‘Wda’ was the most effective mother plant, as it produced the highest number of seeds, regardless of the paternal component used. The present research confirmed the usefulness of cross-breeding in the creation of novel attractive chrysanthemum cultivars. Future studies should focus on the verification of other chemical and physical factors that could improve the effectiveness of chrysanthemum pollination.
Author Contributions
Conceptualization, N.M.; methodology, N.M.; software, N.M. and D.K.; validation, N.M. and D.K.; formal analysis, N.M. and D.K.; investigation, N.M.; resources, N.M.; data curation, N.M.; writing—original draft preparation, D.K.; writing—review and editing, N.M. and D.K.; visualization, N.M. and D.K.; supervision, N.M. and D.K.; project administration, N.M.; funding acquisition, D.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available by e-mail on reasonable request.
Acknowledgments
The authors wish to acknowledge Jagoda Dorota Gołach (student of PBS) for the technical support in performing the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, K.; Li, S.; Jia, D.; Xing, X.; Wang, H.; Song, A.; Jiang, J.; Chen, S.; Chen, F.; Ding, L. Characterization of the MADS-Box Gene CmFL3 in chrysanthemum. Agronomy 2022, 12, 1716. [Google Scholar] [CrossRef]
- Ohmiya, A. Molecular mechanisms underlying the diverse array of petal colors in chrysanthemum flowers. Breed. Sci. 2018, 68, 17075. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huo, B.; Lin, S.; Zhang, S.; Mao, C.; Jiang, J.; Chen, S.; Fang, W.; Guan, Z.; Liao, Y.; et al. Germplasm innovation and establishment of comprehensive evaluation system for hedgerow garden chrysanthemum. Agronomy 2022, 12, 1736. [Google Scholar] [CrossRef]
- Latado, R.R.; Adames, A.H.; Neto, A.T. In vitro mutation of chrysanthemum (Dendranthema grandiflora Tzvelev) with ethylmethanesulphonate (EMS) in immature floral pedicels. Plant Cell Tiss. Organ Cult. 2004, 77, 103–106. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Shimizu, A.; Hase, Y.; Tanaka, A.; Shikazono, N.; Degi, K.; Morishita, T. Effects of ion beam irradiation on mutation induction and nuclear DNA content in chrysanthemum. Breed. Sci. 2010, 60, 398–404. [Google Scholar] [CrossRef]
- Haider, S.; Gao, Y.; Gao, Y. Standardized genetic transformation protocol for chrysanthemum cv. ‘Jinba’ with TERMINAL FLOWER 1 homolog CmTFL1a. Genes 2020, 11, 860. [Google Scholar] [CrossRef]
- Adedeji, O.S.; Naing, A.H.; Kim, C.K. Protoplast isolation and shoot regeneration from protoplast-derived calli of chrysanthemum cv. White ND. Plant Cell Tiss. Organ Cult. 2020, 141, 571–581. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Kulus, D. Chrysanthemum biotechnology: Discoveries from the recent literature. Folia Hortic. 2014, 26, 67–77. [Google Scholar] [CrossRef]
- Anderson, N.O. Chrysanthemum. In Flower Breeding and Genetics; Anderson, N.O., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 389–437. [Google Scholar]
- Wang, F.; Zhang, F.J.; Chen, F.D.; Fang, W.M.; Teng, N.J. Identification of chrysanthemum (Chrysanthemum morifolium) self-incompatibility. Sci. World J. 2014, 2014, 625658. [Google Scholar] [CrossRef]
- Yang, J.S.; Endo, M. In vitro germination and viability of Dendranthema pollen. Asian J. Plant Sci. 2005, 4, 673–677. [Google Scholar] [CrossRef][Green Version]
- Spaargaren, J.; Van Geest, G. Chrysanthemum. In Ornamental Crops, Handbook of Plant Breeding; Van Huylenbroeck, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; Volume 11, pp. 319–348. [Google Scholar]
- Wang, F.; Zhong, X.; Wang, H.; Song, A.; Chen, F.; Fang, W.; Jiang, J.; Teng, N. Investigation of differences in fertility among progenies from self-pollinated chrysanthemum. Int. J. Mol. Sci. 2018, 19, 832. [Google Scholar] [CrossRef] [PubMed]
- Saumitou-Laprade, P.; Vernet, P.; Vekemans, X.; Billiard, S.; Gallina, S.; Essalouh, L.; Mhaïs, A.; Moukhli, A.; Bakkali, A.E.; Barcaccia, G.; et al. Elucidation of the genetic architecture of self-incompatibility in olive: Evolutionary consequences and perspectives for orchard management. Evol. Appl. 2017, 10, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.K. Induced mutations: Technological advancement for development of new ornamental varieties. Nucleus 2020, 63, 119–129. [Google Scholar] [CrossRef]
- Sun, C.Q.; Chen, F.D.; Teng, N.J.; Liu, Z.L.; Fang, W.M.; Hou, X.L. Factors affecting seed set in the crosses between Dendranthema grandiflorum (Ramat.) Kitamura and its wild species. Euphytica 2010, 171, 181–192. [Google Scholar] [CrossRef]
- Miler, N.; Wozny, A. Effect of pollen genotype, temperature and period of storage on in vitro germinability and in vivo seed set in chrysanthemum—Preliminary study. Agronomy 2021, 11, 2395. [Google Scholar] [CrossRef]
- Deng, Y.; Teng, N.; Chen, S.; Chen, F.; Guan, Z.; Song, A.; Chang, Q. Reproductive barriers in the intergeneric hybridization between Chrysanthemum grandiflorum (Ramat.) Kitam. and Ajania przewalskii Poljak. (Asteraceae). Euphytica 2010, 174, 41–50. [Google Scholar] [CrossRef]
- Puglisi, D.; Las Casas, G.; Ferlito, F.; Nicolosi, E.; Di Guardo, M.; Scollo, F.; Saitta, G.; La Malfa, S.; Gentile, A.; Distefano, G. Parents’ selection affects embryo rescue, seed regeneration and the heredity of seedless trait in table grape breeding programs. Agriculture 2022, 12, 1096. [Google Scholar] [CrossRef]
- Zalewska, M.; Tymoszuk, A.; Miler, N. New chrysanthemum cultivars as a result of in vitro mutagenesis with the application of different explant types. Acta Sci. Pol. Hort. Cult. 2011, 10, 109–123. [Google Scholar]
- Anderson, N.O.; Ascher, P.D. Inheritance of seed set, germination, and day neutrality/heat delay insensitivity of garden chrysanthemums (Dendranthema × grandiflora) under glasshouse and field conditions. J. Am. Soc. Hort. Sci. 2004, 129, 509–516. [Google Scholar] [CrossRef]
- Pu, Y.; Huo, R.; Lin, Q.; Wang, F.; Chun, X.; Huang, H.; Dai, S. Investigation and screening of chrysanthemum resources to identify self-compatible mutants. Sci. Hortic. 2021, 281, 2021. [Google Scholar] [CrossRef]
- Mekapogu, M.; Vasamsetti, B.M.K.; Kwon, O.-K.; Ahn, M.-S.; Lim, S.-H.; Jung, J.-A. Anthocyanins in floral colors: Biosynthesis and regulation in chrysanthemum flowers. Int. J. Mol. Sci. 2020, 21, 6537. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, C.L.; Wang, H.D.; Chen, K.L. Study on the pollen viability and stigma receptivity of Chrysanthemum morifolium ‘Fubaiju’. Zhong Yao Cai 2012, 35, 1546–1550. [Google Scholar] [PubMed]
- Din, A.; Qadri, Z.A.; Wani, M.A.; Rather, Z.A.; Iqbal, S.; Malik, S.A.; Hussain, P.R.; Rafiq, S.; Nazki, I.T. Congenial in vitro γ-ray-induced mutagenesis underlying the diverse array of petal colours in chrysanthemum (Dendranthema grandiflorum Kitam.) cv. ‘Candid’. Biol. Life Sci. Forum 2021, 4, 21. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).