In Vitro Germination and Propagation of Dyckia brevifolia, An Ornamental and Endangered Bromeliad
Abstract
1. Introduction
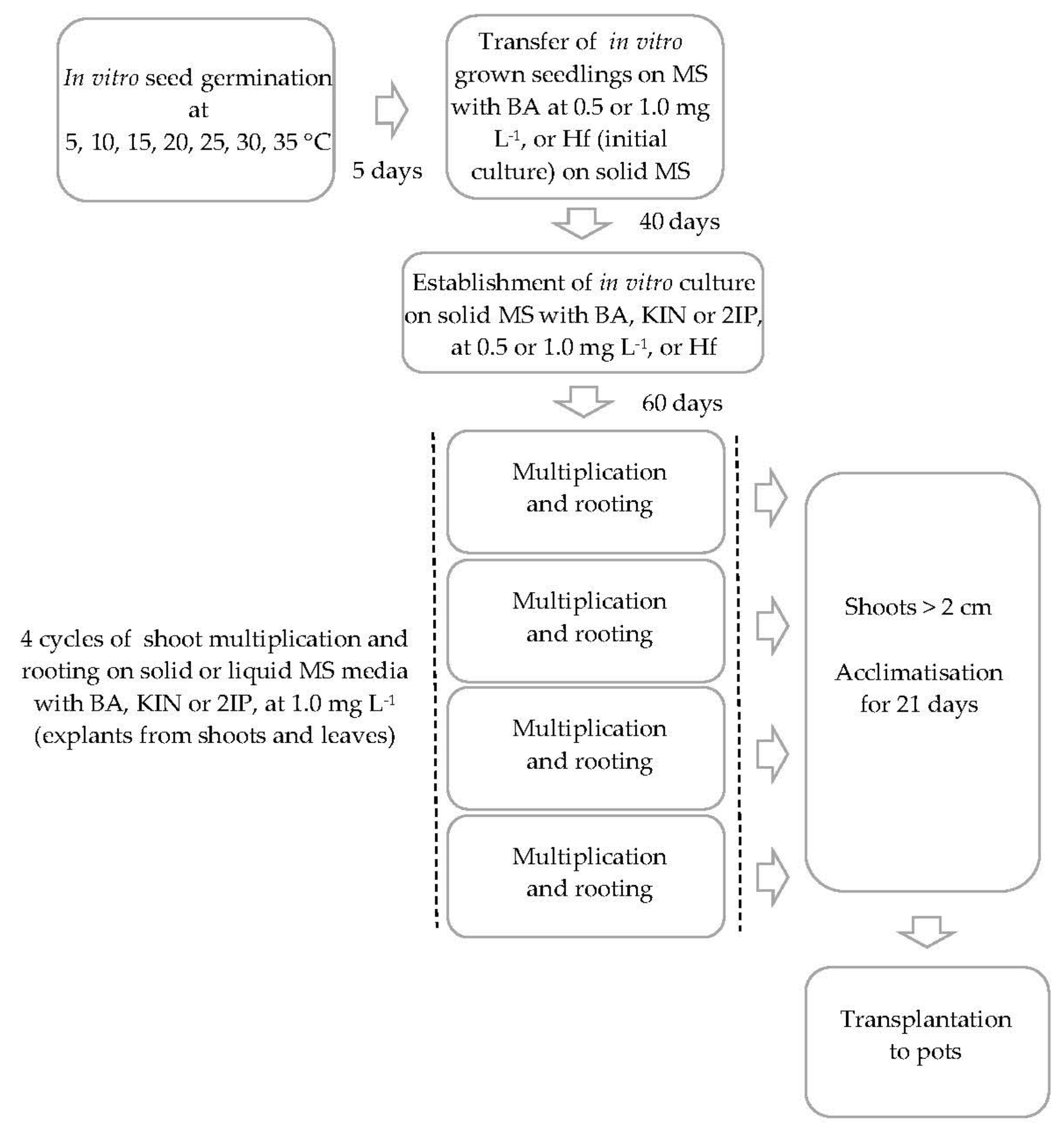
2. Materials and Methods
2.1. Plant Material
2.2. In Vitro Germination
2.3. Micropropagation
2.3.1. Establishment of Initial Cultures
2.3.2. Effect of Explant Type and Medium Type on Shoot Multiplication
2.3.3. In-Vitro Culture Conditions and Data Collection
2.3.4. Ex Vitro Acclimatisation
2.4. Statistical Analysis
3. Results
3.1. Seed Germination
3.2. Micropropagation
3.2.1. Initial Culture and Establishment
3.2.2. Multiplication Stage
Shoot Explants
Leaf Explants
3.2.3. In Vitro Rooting and Ex Vitro Acclimatisation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Martinelli, G. The Bromeliads of the Atlantic Forest. Sci. Am. 2000, 282, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Zanella, C.M.; Janke, A.; Palma-Silva, C.; Kaltchuk-Santos, E.; Pinheiro, F.G.; Paggi, G.M.; Soares, L.E.S.; Goetze, M.; Büttow, M.V.; Bered, F. Genetics, evolution and conservation of Bromeliaceae. Genet. Mol. Biol. 2012, 35, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Benzing, D.H. Bromeliaceae: Profile of an Adaptive Radiation; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar] [CrossRef]
- Givnish, T.J.; Barfuss, M.H.J.; Ee, B.V.; Riina, R.; Schulte, K.; Horres, R.; Gonsiska, P.A.; Jabaily, R.S.; Crayn, D.M.; Smith, J.A.C.; et al. Phylogeny, adaptive radiation, and historical biogeography in Bromeliaceae: Insights from an eightlocus plastid phylogeny. Am. J. Bot. 2011, 98, 872–895. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, D.; Zizka, G.; Schulte, K. Disentangling the effects of key innovations on the diversification of Bromelioideae (Bromeliaceae). Evolution 2014, 68, 163–175. [Google Scholar] [CrossRef]
- Hosoki, T.; Asahira, T. In Vitro propagation οf bromeliads in liquid culture. HortScience 1980, 15, 603–604. [Google Scholar]
- Daquinta, M.; Almeida, A.P.; Guerra, M.P. In Vitro morphogenesis of immature flower and buds of flower stalk in Dyckia distachya. J. Bromel. Soc. 1998, 49, 72–76. Available online: https://isb.emnuvens.com.br/iheringia/article/view/162 (accessed on 4 March 2022).
- Krapp, F.; de Barros Pinangé, D.S.; Benko-Iseppon, A.M.; Leme, E.M.; Weising, K. Phylogeny and evolution of Dyckia (Bromeliaceae) inferred from chloroplast and nuclear sequences. Plant Syst. Evol. 2014, 300, 1591–1614. [Google Scholar] [CrossRef]
- Mercier, H.; Kerbauy, G.B. Micropropagation of Dyckia macedoi–an endangered endemic Brazilian bromeliad. Bot. Gard. Microprop. News 1993, 1, 70–72. [Google Scholar]
- Givnish, T.J.; Millam, K.C.; Berry, P.E.; Sytsma, K.J. Phylogeny, adaptive radiation, and historical biogeography of Bromeliaceae inferred from ndhF sequence data. Aliso 2007, 23, 3–26. [Google Scholar] [CrossRef]
- Rex, M.; Schulte, K.; Zizka, G.; Peters, J.; Vásquez, R.; Ibisch, P.L.; Weising, K. Phylogenetic analysis of Fosterella L.B. Sm. (Pitcairnioideae, Bromeliaceae) based on four chloroplast DNA regions. Mol. Phylogenet. Evol. 2009, 51, 472–485. [Google Scholar] [CrossRef]
- Givnish, T.J.; Barfuss, M.H.J.; Van Ee, B.; Riina, R.; Schulte, K.; Horres, R.; Gonsiska, P.A.; Jabaily, R.S.; Crayn, D.M.; Smith, J.A.C.; et al. Adaptive radiation, correlated and contingent evolution, and net species diversification in Bromeliaceae. Mol. Phylogenetics Evol. 2014, 71, 55–78. [Google Scholar] [CrossRef]
- Santos-Silva, F.; Saraiva, D.P.; Monteiro, R.F.; Pita, P.; Mantovani, A.; Forzza, R.C. Invasion of the South American dry diagonal: What can the leaf anatomy of Pitcairnioideae (Bromeliaceae) tell us about it? Flora 2013, 208, 508–521. [Google Scholar] [CrossRef]
- Synge, P.M. Dictionary of Gardening; Clarendon Press: Oxford, UK; Cumberlege: London, UK, 1981; p. 725. [Google Scholar]
- Reitz, R. Bromeliaceas e a malaria—bromelia endémica. In Flora Ilustrada Catarinense. Parte I. Fasciculo Bromelia; Reitz, R., Ed.; Herbário Barbosa Rodrigues: Itajaí, Brazil, 1983; p. 518. [Google Scholar]
- Lobo, G.M.; de Souza, T.V.; Voltolini, C.H.; Reis, A.; Santos, M. Leaf epidermis of the rheophyte Dyckia brevifolia Baker (Bromeliaceae). Sci. World J. 2013, 2013, 307593. [Google Scholar] [CrossRef]
- da Silva Sousa, R.P.; Costa, W.S.; Matos, P.e.S.; Carvalho, A.S.; Martins, F.D.; Torres, K.R. Ornamental potential of species from the ferruginous Campo rupestre of the Carajás National Forest, Brazilian Amazon. Comun. Sci. 2012, 12, e3260. [Google Scholar] [CrossRef]
- Rogalski, J.M.; Reis, A.; Rogalski, M.; Montagna, T.; Dos Reis, M.S. Mating System and Genetic Structure Across All Known Populations of Dyckia brevifolia: A Clonal, Endemic, and Endangered Rheophyte Bromeliad. J. Hered. 2017, 108, 299–307. [Google Scholar] [CrossRef]
- Ibama. Reconhecer Como Espécies da Flora Brasileira Ameaçadas de Extinção Aquelas Constantes do Anexo i a Esta Instrução Normativa 2014, n. 443, de 17 de Dezembro de 2014. Available online: http://www.ibama.gov.br/sophia/cnia/legislacao/MMA/PT0443-171214.pdf (accessed on 4 March 2022).
- Strehl, T. Periodically submersed bromeliads. Bromélia 1994, 3, 19–21. [Google Scholar]
- Mercier, H.; Kerbauy, G.B. Micropropagation of ornamental bromeliads (Bromeliaceae). In Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin, Germany, 1997; pp. 43–57. [Google Scholar]
- Carneiro, L.; Araújo, R.; Brito, G.; Fonseca, M.H.P.B.; Costa, A.; Crocomo, O.J.; Mansur, E. In Vitro regeneration from leaf explants of Neoregelia cruenta (R. Graham) L.B. Smith, an endemic bromeliad from Eastern Brazil. Plant Cell Tissue Organ Cult. 1998, 55, 79–83. [Google Scholar] [CrossRef]
- Alves, G.M.; Guerra, M.P. Micropropagation for mass propagation and conservation of Vriesea friburgensis var. paludosa from microbuds. J. Bromel. Soc. 2001, 51, 202–212. [Google Scholar]
- Pompelli, M.F.; Fernandes, D.; Guerra, M.P. Somatic embryogenesis in Dyckia distachia Hassler (Bromeliaceae)—An endangered bromeliad from South Brazil. Propag. Ornam. Plants 2005, 5, 192–198. [Google Scholar]
- Murashige, T. Plant propagation through tissue culture. Annu. Rev. Plant Phys. 1974, 25, 135–165. Available online: http://dx.doi.org/10.1146/annurev.pp.25.060174.001031 (accessed on 4 March 2022). [CrossRef]
- Silva, A.L.L.; Franco, E.T.H.; Dornelles, E.B.; Gesing, J.P.A. Micropropagation of Dyckia maritima Baker-Bromeliaceae. Iheringia Sér. Botânica 2008, 63, 135–138. [Google Scholar]
- Pompelli, M.F. Morfogênese In Vitro, Métodos Demicropropagação e Conservação de Germoplasma de Dyckia distachya Hassler. Master’s Thesis, Universidade Federal de Santa Catarina, Florianópolis, Brazil, 2002; 93p. [Google Scholar]
- Pompelli, M.F.; Guerra, M.P. Micropropagation enables the mass propagation and conservation of Dyckia distachya Hassler. Crop Breed. Appl. Biotechnol. 2005, 5, 117–126. [Google Scholar] [CrossRef]
- Silva, A.L.L.; Dornelles, E.B.; Bisognin, D.A.; Franco, E.T.H.; Horbach, M.A. Micropropagation of Dyckia agudensis Irgang & Sobral—An extinction threatened bromeliad. Iheringia Sér. Botânica 2007, 62, 39–43. [Google Scholar]
- Papafotiou, M.; Bertsouklis, K.F.; Trigka, M. Micropropagation of Arbutus unedo, A. andrachne, and their natural hybrid, A. × andrachnoides from seedling explants. J. Hortic. Sci. Biotechnol. 2013, 6, 768–775. [Google Scholar] [CrossRef]
- Zotz, G. A longer story than expected: Seeds of several species (Tillandsioideae) remain viable for up to two years. J. Bromel. Soc. 2013, 63, 83–86. Available online: https://go.gale.com/ps/i.do?p=AONE&u=anon~898338be&id=GALE|A610341224&v=2.1&it=r&sid=bookmark-AONE&asid=b882c762 (accessed on 4 March 2022).
- Sarasan, V.; Kite, G.C.; Sileshi, G.W.; Stevenson, P.C. Applications of phytochemical and in vitro techniques for reducing over-harvesting of medicinal and pesticidal plants and generating income for the rural poor. Plant Cell Rep. 2011, 30, 1163–1172. [Google Scholar] [CrossRef]
- Generoso, A.L.; Carvalho, V.S.; Walter, R.; Campbell, G.; Araújo, L.S.; Santana, J.G.S.; Cunha, M. Mature-embryo culture in the cryopreservation of passion fruit (Passifora edulis Sims) seeds. Sci. Hortic. 2019, 256, 108638. [Google Scholar] [CrossRef]
- Silva, S.S.S.; Souza, E.H.; Souza, F.V.D.; Max, D.A.S.; Rossi, M.L.; Costa, M.A.P.C. Post-seminal development and cryopreservation of endemic or endangered bromeliads. An. Acad. Bras. Cienc. 2021, 93, e20191133. [Google Scholar] [CrossRef]
- Silva, L.F.; de Souza, D.C.; Resente, L.V.; Gonçalves, W.M. Manejo de recursos genéticos vegetais. An. da Acad. Pernambucana de Ciência Agronômica 2018, 15, 109–126. Available online: http://www.journals.ufrpe.br/index.php/apca/article/view/1824 (accessed on 4 March 2022).
- de Paula, J.C.B.; Men, G.B.; Biz, G.; Júnior, W.A.R.; de Faria, R.T. Cryopreservation of seeds from endangered Brazilian bromeliads-Dyckia brevifolia Baker and D. delicata Larocca & Sobral. Rev. Bras. de Ciências Agrárias 2020, 15, 1–8. [Google Scholar] [CrossRef]
- Moresco, V.P.; Omura, M.S.; de Paula, J.C.B.; Furlan, F.F.; Takahashi, L.S.A. Physiological potential of Dyckia spp. bromeliad seeds under different temperatures. Ciências Agrárias 2021, 42, 2639–2650. [Google Scholar] [CrossRef]
- Vlachou, G.; Papafotiou, M.; Bertsouklis, K.F. Studies on Seed Germination and Micropropagation of Clinopodium nepeta: A medicinal and aromatic plant. HortScience 2019, 54, 1558–1564. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- International Seed Testing Association. International rules for seed testing. Seed Sci. Technol. 1999, 27, 333. [Google Scholar]
- Soltani, A.; Galeshi, S.; Zeinali, E.; Latifi, N. Genetic variation for and interrelationships among seed vigor traits in wheat from the Caspian Sea coasts of Iran. Seed Sci. Technol. 2001, 29, 653–662. [Google Scholar]
- Maguire, J.D. Speed of germination-aid in selection and evaluation for seedling emergence and vigor. Crop. Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Kowalski, V.K.; Tardivo, R.S.; Oliveira, F.M.C.; Mourão, K.S.M. Morphology and anatomy of seedlings of Bromeliaceae from the perspective of ecophysiological types. Flora 2021, 285, 151959. [Google Scholar] [CrossRef]
- Malda, G.; Suzán, H.; Backhaus, R.A. In vitro culture as a potential method for the conservation of endangered plants possessing crassulacean acid metabolism. Sci. Hort. 1999, 81, 71–87. [Google Scholar] [CrossRef]
- Sharma, T.P.; Sen, D.N. A new report on abnormally fast germinating seeds of Haloxylon spp.: An ecological adaptation to saline habitat. Curr. Sci. 1989, 58, 382–385. [Google Scholar] [CrossRef][Green Version]
- Mahmoud, A.; El Sheikh, A.M.; Baset, S.A. Germination of two halophytes: Halopeplis perfoliata and Limonium axillare from Saudi Arabia. J. Arid. Environ. 1983, 6, 87–98. [Google Scholar] [CrossRef]
- Wiesbauer, M.B.; Hmeljevski, K.V.; Zimmermann, T.G.; dos Reis, M.S.; Reis, A.; de Souza, S.L. Reintrodução de Dyckia distachya Hassler nas áreas de Influência das Hidrelétricas de Itá e Machadinho. V Congresso de Inovação Tecnológica em Energia Elétrica. 2009, Volume 20011, p. 202006. Available online: https://www.cgti.org.br/publicacoes/wp-content/uploads/2016/03/Reintroduc%CC%A7a%CC%83o-de-Dyckia-distachya-Hassler-nas-a%CC%81reas-de- Influe%CC%82ncia-das-Hidrele%CC%81tricas-de-Ita%CC%81-e-Machadinho.pdf (accessed on 4 March 2022).
- Vieira, D.C.; Socolowski, F.; Takaki, M. Germinação de sementes de Dyckia tuberosa (Vell.) Beer (Bromeliaceae) sob diferentes temperaturas em luz e escuro. Rev. Bras. Bot. 2007, 30, 183–188. [Google Scholar] [CrossRef]
- Penfield, S.; MacGregor, D.R. Effects of environmental variation during seed production on seed dormancy and germination. J. Exp. Bot. 2017, 68, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Thanos, C.A.; Doussi, M.A. Ecophysiology of seed germination in endemic labiates of Crete. Isr. J. Plant Sci. 1995, 43, 227–237. [Google Scholar] [CrossRef]
- Kadis, C.; Georghiou, K. Seed dispersal and germination behavior of three threatened endemic labiates of Cyprus. Plant Spec. Biol. 2010, 25, 77–84. [Google Scholar] [CrossRef]
- Bertsouklis, K.; Papafotiou, M. Seed germination of Arbutus unedo, A. andrachne and their natural hybrid A. andrachnoides in relation to temperature and period of storage. HortScience 2013, 48, 347–351. [Google Scholar] [CrossRef]
- Pompelli, M. Germinação de Dyckia encholirioides var encholirioides (Bromeliaceae, Pitcairnioideae). Rev. Floresta E Ambiente 2004, 13, 1–9. [Google Scholar]
- Pompelli, M.F.; Fernandes, D.; Guerra, M.P. Germination of Dyckia encholirioides (Gaudichaud) Mez var. encholirioides under saline conditions. Seed Sci. Technol. 2006, 34, 759–763. [Google Scholar] [CrossRef]
- Adams, C.R.; Early, M.P. Principles of Horticulture; Elsevier Butterworth-Heinemann Publication: Burlington, MA, USA, 2004. [Google Scholar]
- Cruz-Cruz, C.A.; González-Arnao, M.T.; Engelmann, F. Biotechnology and Conservation of Plant Biodiversity. Resources 2013, 2, 73–95. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E.; Geneve, R.L. Hartmann & Kester’s Plant Propagation Principles and Practices; (No. 631.53 H2555p Ej.1 025385); Prentice Hall: Hoboken, NJ, USA, 2011; 2555p. [Google Scholar]
- Cabahug, R.A.M.; Nam, S.Y.; Lim, K.B.; Jeon, J.K.; Hwang, Y.J. Propagation techniques for ornamental succulents. Korean Flower Assoc. 2018, 26, 90–101. [Google Scholar] [CrossRef]
- Le Roux, J.J.; Hui, C.; Castillo, M.L.; Iriondo, J.M.; Keet, J.H.; Khapugin, A.A.; Médail, F.; Rejmánek, M.; Theron, G.; Yannelli, F.A.; et al. Recent Anthropogenic Plant Extinctions Differ in Biodiversity Hotspots and Coldspots. Curr. Biol. 2019, 29, 2912–2918. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Strid, A.; Dimopoulos, P. Extinction Risk Assessment of the Greek Endemic Flora. Biology 2021, 10, 195. [Google Scholar] [CrossRef]
- Leme, E.M.C. Considerations on a new Neoregelia from Brazil. J. Bromel. Soc. 1983, 33, 118–120. [Google Scholar]
- Tsay, H.S.; Lee, C.Y.; Agrawal, D.C.; Basker, S. Influence of ventilation closure, gelling agent and explant type on shoot bud proliferation and hyperhydricity in Scrophularia yoshimurae—A medicinal plant. Vitr. Cell. Dev. Biol. Plant 2006, 42, 445–449. Available online: https://www.jstor.org/stable/20461600 (accessed on 4 March 2022). [CrossRef]
- Guerra, M.P.; Vesco, L.L.D. Strategies for the Micropropagation of Bromeliads. In Protocols for In Vitro Propagation of Ornamental Plants. Methods in Molecular Biology (Methods and Protocols); Jain, S., Ochatt, S., Eds.; Humana Press: Totowa, NJ, USA, 2010; Volume 589. [Google Scholar] [CrossRef]
- Varadarajan, G.S.; Varadarajan, U.; Locy, R.D. Application of tissue culture techniques to maintain a rare species, Puya tuberosa. J. Bromel. Soc. 1993, 43, 112–118. [Google Scholar]




| Temperature (°C) | Germination (%) ± SD * | T50 (Days) | GSI |
|---|---|---|---|
| 5 | 0 | - | - |
| 10 | 0 | - | - |
| 15 | 86.00 ± 8.21 a | 15 | 25.24 c |
| 20 | 89.00 ± 5.47 a | 5 | 135.33 ab |
| 25 | 84.00 ± 11.81 a | 2 | 191.51 a |
| 30 | 35.00 ± 16.62 b | 4 | 84.17 b |
| 35 | 0 | - | - |
| Cytokinin | Concentration (mg L−1) | Survival (%) | Growth (cm) | Number of Leaves |
|---|---|---|---|---|
| Hf † | - | 95.0 a | 0.7 ab | 8.4 a |
| BA | 0.5 | 100.0 a | 0.5 c | 6.5 c |
| 1.0 | 97.5 a | 0.6 abc | 6.4 c | |
| KIN | 0.5 | 97.5 a | 0.8 a | 6.8 bc |
| 1.0 | 95.0 a | 0.6 abc | 7.3 ab | |
| 2IP | 0.5 | 100.0 a | 0.7 abc | 6.1 c |
| 1.0 | 100.0 a | 0.7 abc | 7.6 ab | |
| Fone-way ANOVA | NS | *** | *** | |
| Fcyt | NS | |||
| Fconc | NS | |||
| Fcyt×conc | NS | * | * | |
| MS | Cytokinin | Stem Growth (cm) | Number of Leaves/Main Shoot | Formation of Lateral Shoots (%) | Lateral Shoot Number | MI | Number of Leaves/Lateral Shoot |
|---|---|---|---|---|---|---|---|
| Solid | Hf † | 0.9 b | 8.5 cd | 0.0 c | 0.0 c | - | 0.0 c |
| BA | 0.8 b | 6.2 e | 83.0 a | 1.8 ab | 1.50 ab | 4.6 b | |
| KIN | 0.9 b | 7.7 de | 0.0 c | 0.0 c | - | 0.0 c | |
| 2IP | 0.7 b | 6.1 e | 54.0 b | 2.0 ab | 1.08 b | 4.9 b | |
| Liquid | Hf † | 1.7 ab | 12.1 a | 8.0 b | 1.5 bc | 0.11 b | 4.9 b |
| BA | 1.9 ab | 10.2 bc | 73.0 a | 2.7 a | 1.97 a | 4.9 b | |
| KIN | 2.3 a | 11.6 ab | 63.0 ab | 1.9 ab | 1.19 b | 6.1 a | |
| 2IP | 2.2 a | 10.5 bc | 84.0 a | 2.3 ab | 1.93 a | 6.0 a | |
| Fone-way ANOVA | *** | *** | ** | *** | * | *** | |
| Fmed | *** | *** | *** | ** | *** | ||
| Fcyt | NS | ** | *** | ** | *** | ||
| Fcyt×med | NS | NS | * | NS | NS | NS | |
| MS | Cytokinin | Formation of Lateral Shoots (%) | Growth (cm) | Lateral Shoot Number | MI | Number of Leaves/Shoot |
|---|---|---|---|---|---|---|
| Solid | Hf † | 20.0 b | 1.0 ab | 1.4 c | 0.28 c | 8.8 a |
| BA | 41.0 a | 0.5 c | 1.4 c | 0.57 c | 7.0 a | |
| KIN | 35.0 a | 0.8 bc | 1.5 c | 0.53 c | 8.0 a | |
| 2IP | 44.5 a | 0.9 b | 1.5 c | 0.67 bc | 8.9 a | |
| Liquid | Hf† | 17.0 b | 1.2 ab | 1.0 c | 0.17 c | 7.4 a |
| BA | 26.0 ab | 1.0 ab | 7.4 a | 1.90 a | 6.6 a | |
| KIN | 22.5 b | 1.3 a | 2.6 b | 0.59 bc | 7.6 a | |
| 2IP | 27.5 b | 1.0 ab | 3.3 b | 0.90 b | 6.3 a | |
| Fone-way ANOVA | *** | ** | *** | ** | NS | |
| Fmed | *** | * | ||||
| Fcyt | NS | NS | ||||
| Fcyt×med | NS | NS | * | * | * | |
| Cytokinin | Concentration (mg L−1) | Rooting (%) | Root Number | Root Length (cm) |
|---|---|---|---|---|
| Hf † | - | 95.0 a | 2.1 a | 1.2 a |
| BA | 0.5 | 20.0 b | 1.0 b | 0.5 c |
| 1.0 | 13.0 b | 1.0 b | 0.5 c | |
| KIN | 0.5 | 93.0 a | 2.3 a | 1.1 ab |
| 1.0 | 81.0 a | 1.6 ab | 0.9 bc | |
| 2IP | 0.5 | 100.0 a | 1.3 b | 0.9 bc |
| 1.0 | 79.0 ab | 1.6 ab | 0.8 c | |
| Fone-way ANOVA | ** | ** | ** | |
| Fcyt | NS | |||
| Fconc | ** | |||
| Fcyt×conc | * | * | NS | |
| Shoot-Origin | Leaf-Origin | ||||||
|---|---|---|---|---|---|---|---|
| MS | Cytokinin | Rooting (%) | Root Number | Root Length (cm) | Rooting (%) | Root Number | Root Length (cm) |
| Solid | Hf † | 94.0 a | 2.2 b | 1.1 c | 73.5 a | 2.0 a | 1.2 a |
| BA | 0.0 c | 0.0 c | 0.0 c | 50.0 ab | 1.0 a | 0.5 a | |
| KIN | 47.0 b | 1.4 b | 1.0 c | 18.0 b | 1.7 a | 1.0 a | |
| 2IP | 43.0 b | 1.8 b | 0.9 c | 52.5 ab | 2.0 a | 0.9 a | |
| Liquid | Hf † | 74.5 ab | 4.2 a | 1.7 b | 62.5 ab | 2.4 a | 1.4 a |
| BA | 0.0 c | 0.0 c | 0.0 c | 0.0 b | 0.0 b | 0.0 b | |
| KIN | 18.3 b | 3.9 a | 2.3 a | 0.0 b | 0.0 b | 0.0 b | |
| 2IP | 77.5 ab | 3.8 a | 2.4 a | 0.0 b | 0.0 b | 0.0 b | |
| Fone-way ANOVA | *** | *** | *** | * | NS | NS | |
| Fmed | *** | ||||||
| Fcyt | NS | ||||||
| Fcyt × med | *** | NS | * | ** | *‘ | * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertsouklis, K.; Panagaki, K.-P. In Vitro Germination and Propagation of Dyckia brevifolia, An Ornamental and Endangered Bromeliad. Horticulturae 2022, 8, 390. https://doi.org/10.3390/horticulturae8050390
Bertsouklis K, Panagaki K-P. In Vitro Germination and Propagation of Dyckia brevifolia, An Ornamental and Endangered Bromeliad. Horticulturae. 2022; 8(5):390. https://doi.org/10.3390/horticulturae8050390
Chicago/Turabian StyleBertsouklis, Konstantinos, and Konstantina-Panagiota Panagaki. 2022. "In Vitro Germination and Propagation of Dyckia brevifolia, An Ornamental and Endangered Bromeliad" Horticulturae 8, no. 5: 390. https://doi.org/10.3390/horticulturae8050390
APA StyleBertsouklis, K., & Panagaki, K.-P. (2022). In Vitro Germination and Propagation of Dyckia brevifolia, An Ornamental and Endangered Bromeliad. Horticulturae, 8(5), 390. https://doi.org/10.3390/horticulturae8050390







