Changes in Fruit Quality and Sugar Components of Wild Actinidia eriantha of Different Varieties (Lines) at the Ripening Stage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Collection
2.2. Determination of Fruit Quality
2.3. Determination of Soluble Sugar and Sugar Components
2.4. Determination of Sucrose Metabolism-Related Enzyme Activities
2.5. Expression Analysis of Genes Related to Sucrose Metabolism
2.6. Data Processing and Analysis
3. Results
3.1. Analysis of Fruit Appearance Quality of Wild A. eriantha with Different Varieties (Lines)
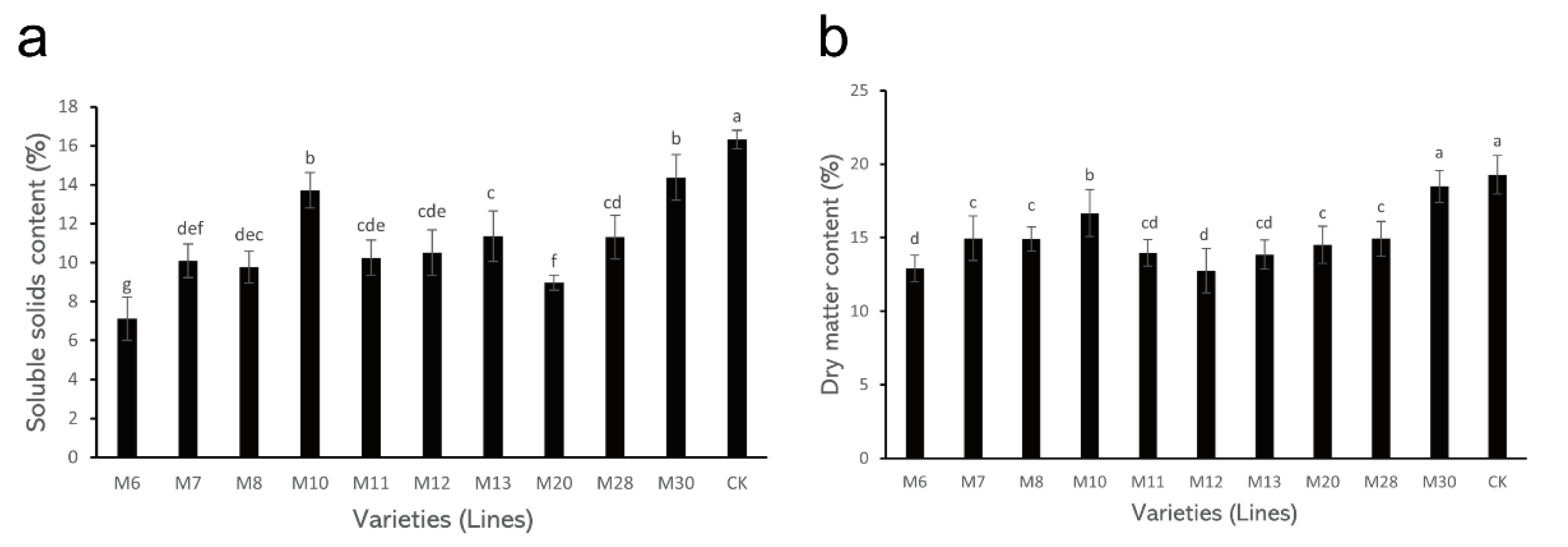
3.2. Changes in SSC and DM Content in Different Wild A. eriantha Varieties (Lines)
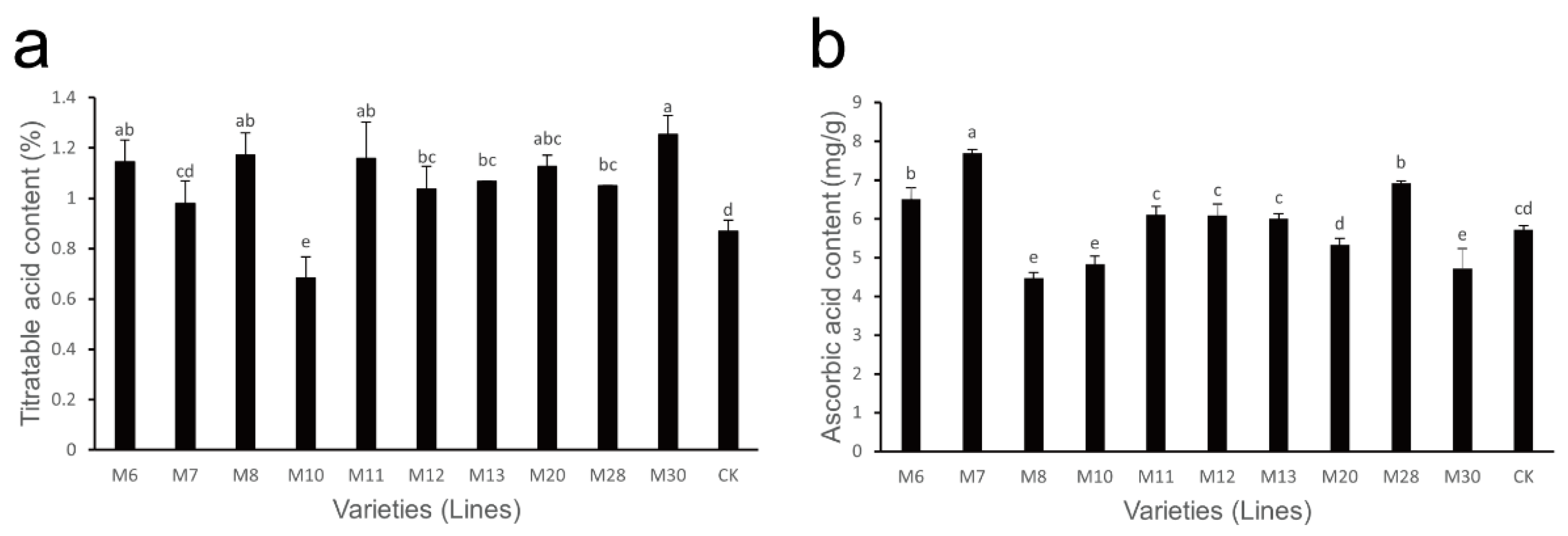
3.3. Changes in Titratable Acid and AsA Content in Wild A. eriantha Fruit of Different Varieties (Lines)
3.4. Changes in Sugar Components in Wild A. eriantha of Different Varieties (Lines)
3.5. Principal Component Analysis and Comprehensive Evaluation of Different Varieties (Lines) of Wild A. eriantha at the Soft Ripening Stage
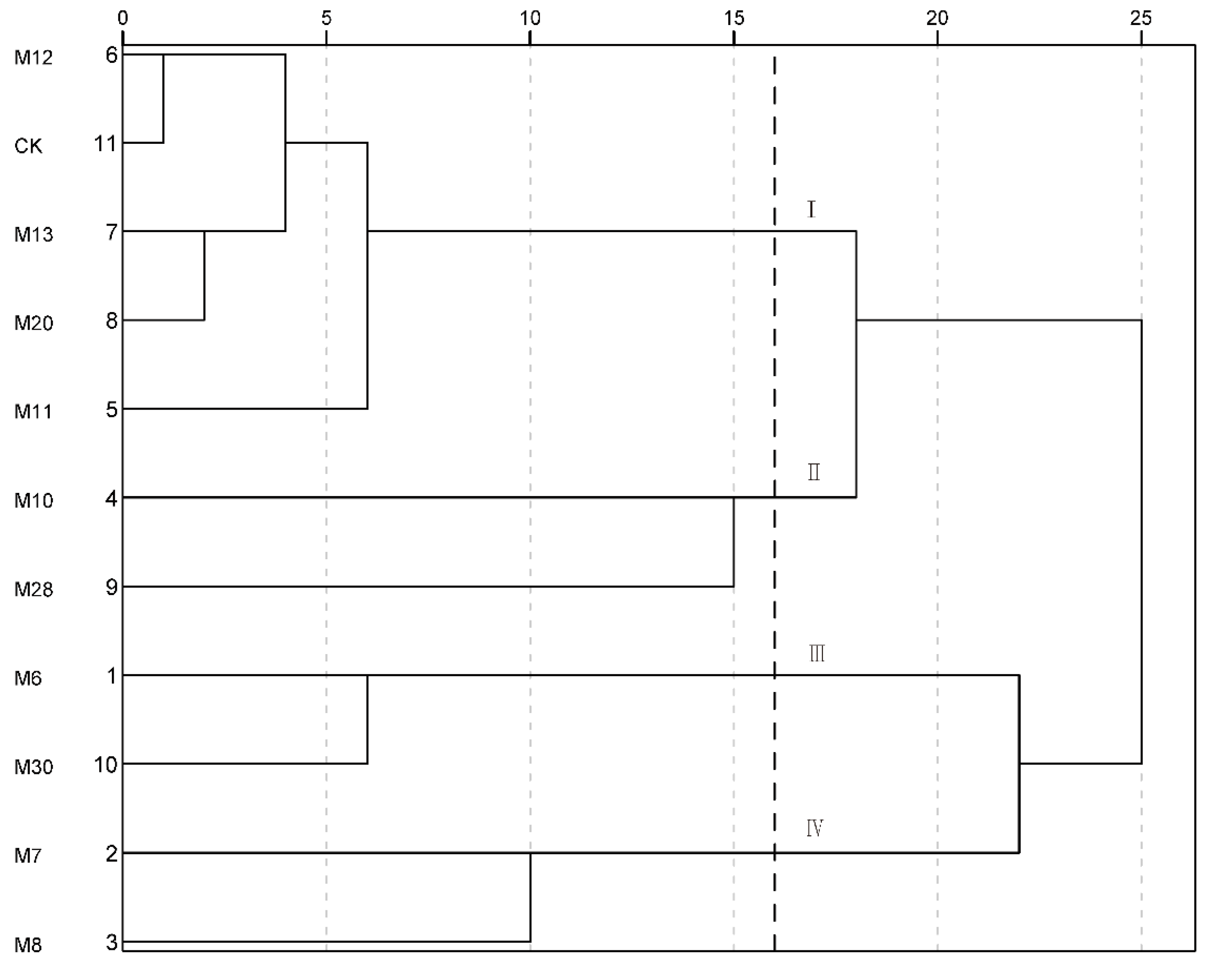
3.6. Systematic Cluster Analysis of Fruit Quality of Wild A. eriantha Samples
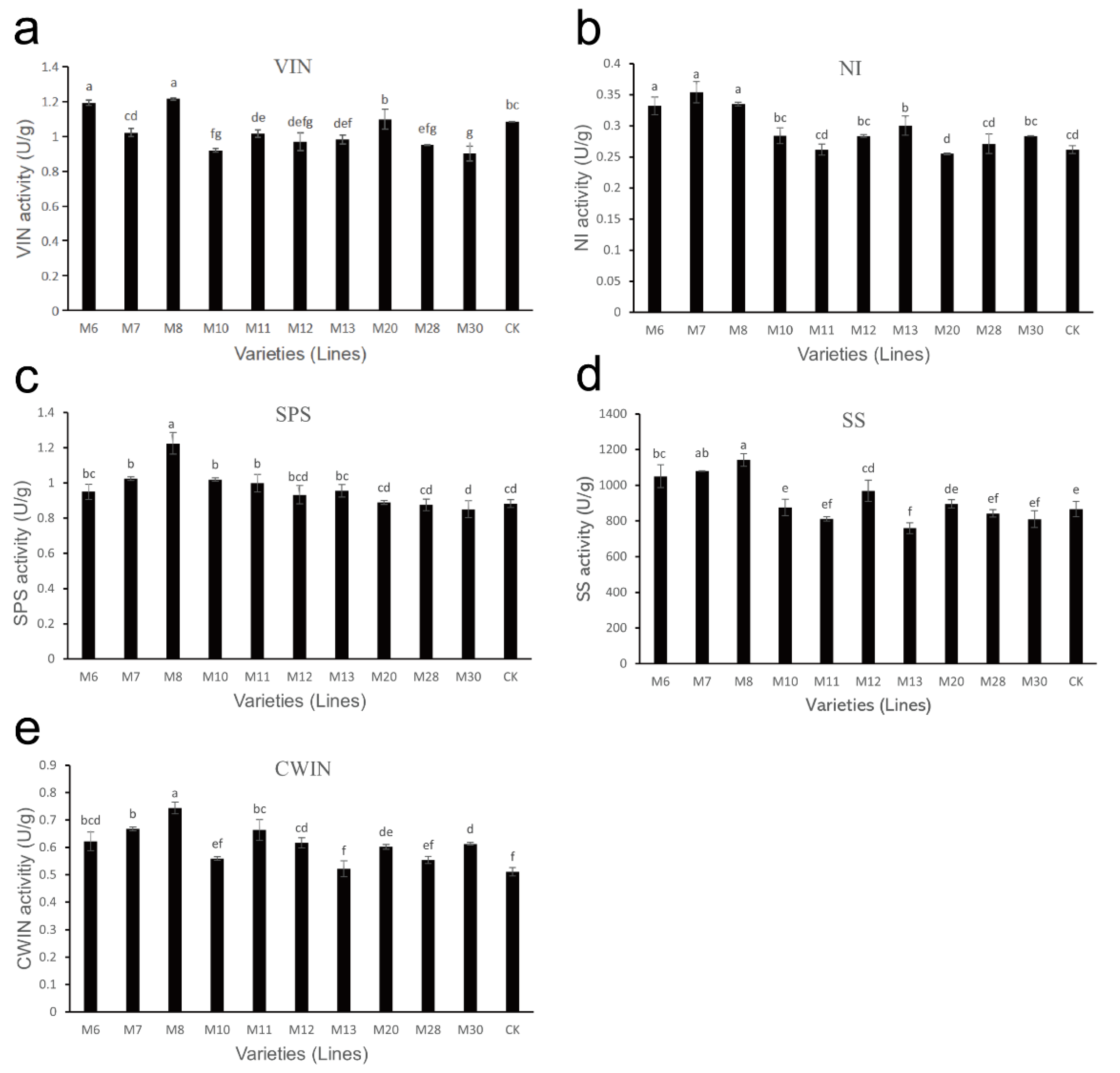
3.7. Analysis of Sucrose Metabolism-Related Enzyme Activities in Wild A. eriantha of Different Varieties (Lines)
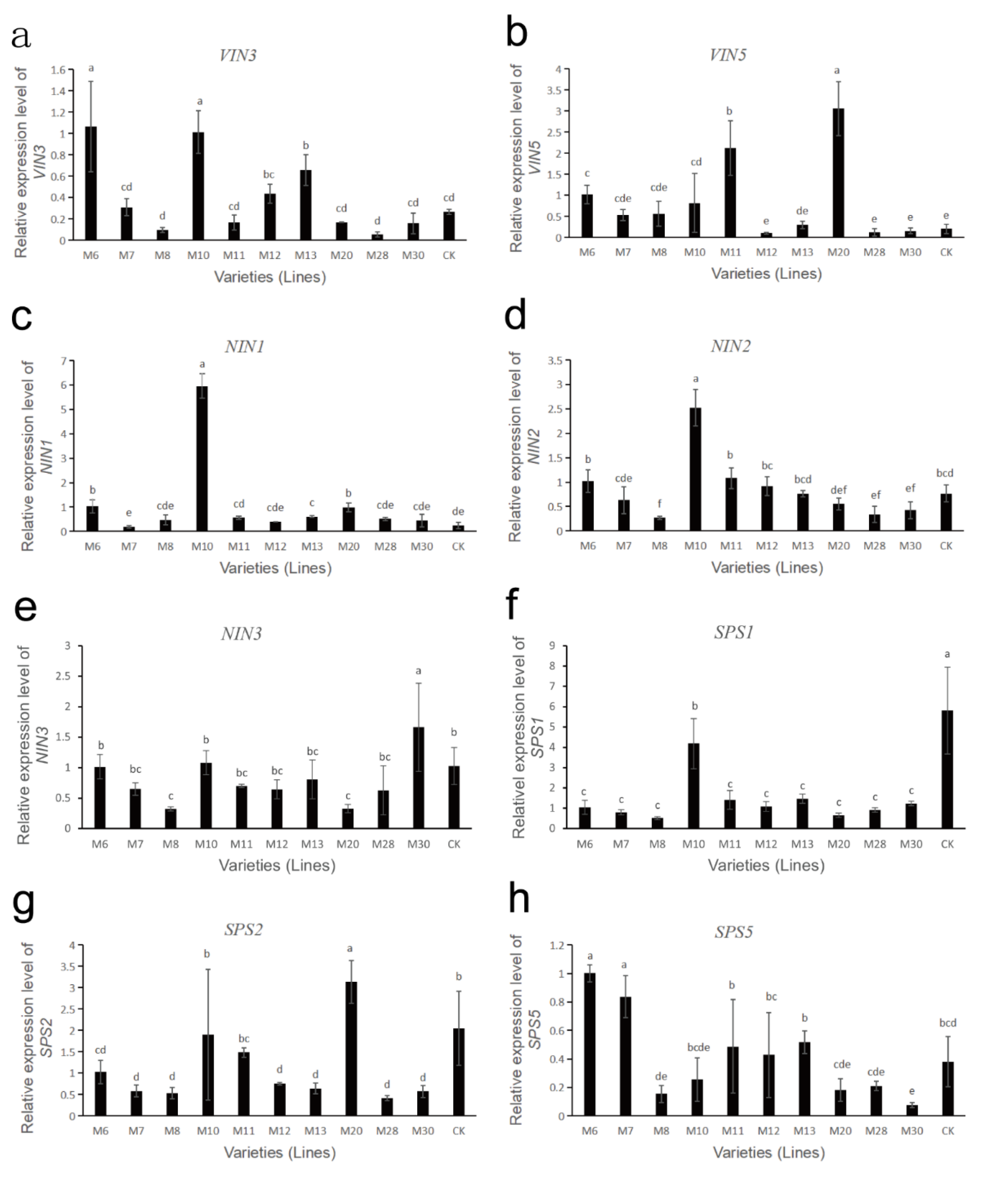
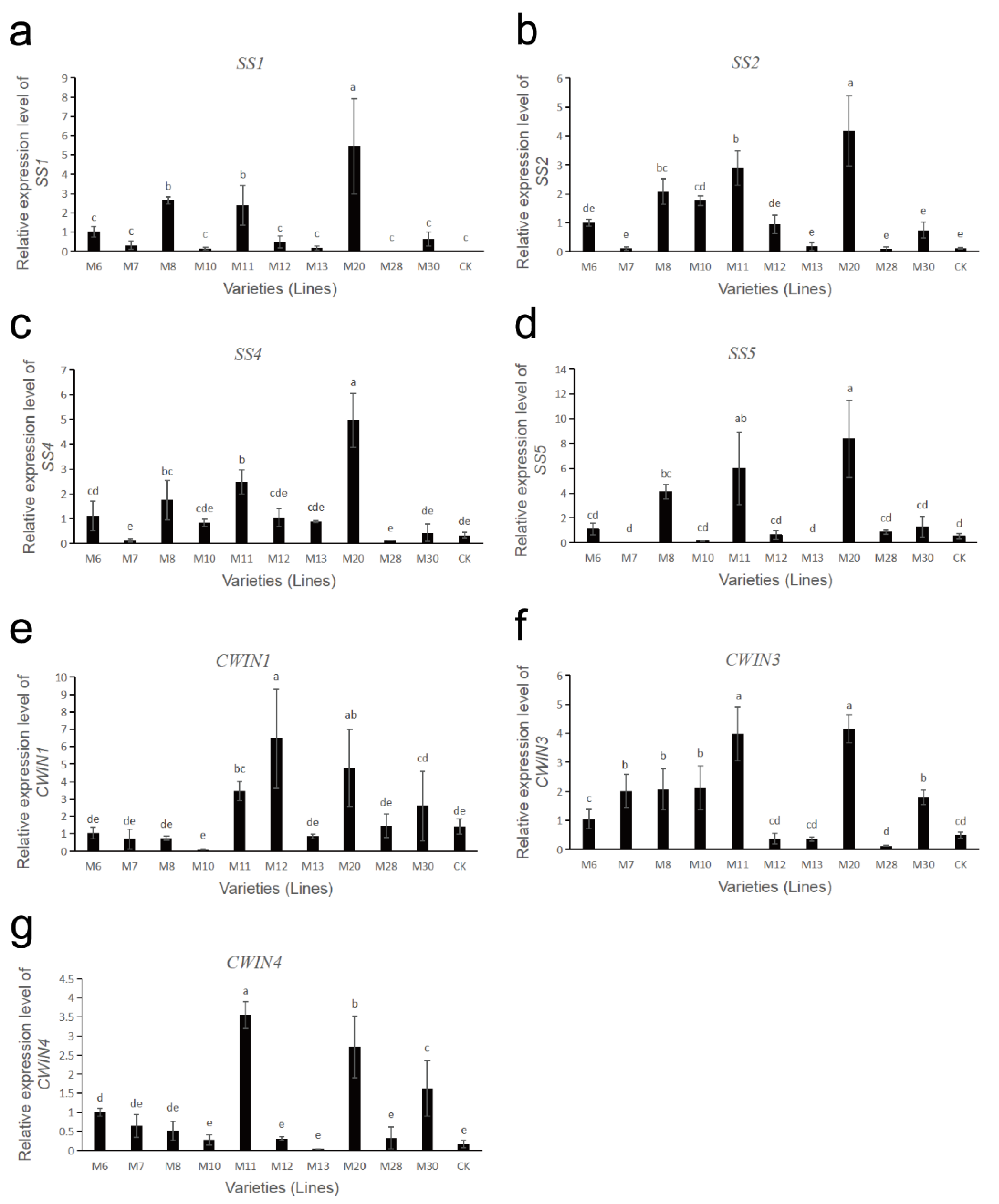
3.8. Expression Analysis of Sucrose Metabolism-Related Enzyme Genes in Wild A. eriantha of Different Varieties (Lines)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, H.W. Actinidia Germplasm Resources in China; China Forestry Publishing House: Beijing, China, 2013. [Google Scholar]
- Huang, H.W. The Genus Actinidia, a World Monograph; Science Press: Beijing, China, 2014. [Google Scholar]
- Atkinson, R.G.; Sharma, N.N.; Hallett, I.C.; Johnston, S.L.; Schröder, R. Actinidia eriantha: A parental species for breeding kiwifruit with novel peelability and health attributes. N. Z. J. For. Sci. 2009, 39, 207–216. Available online: https://www.researchgate.net/publication/285686139 (accessed on 20 June 2022).
- Zhong, C.H.; Zhang, P.; Jiang, Z.W.; Wang, S.M.; Han, F.; Xu, L.Y.; Huang, H.W. Dynamic changes of carbohydrate and vitamin C in fruit of Actinidia chinensis and A. eriantha during growing season. Plant Sci. J. 2011, 29, 370–376. [Google Scholar] [CrossRef]
- Xu, H.S.; Wu, Y.W.; Xu, S.F.; Sun, H.X.; Chen, F.Y.; Yao, L. Antitumor and immunomodulatory activity of polysaccharides from the roots of Actinidia eriantha. J. Ethnopharmacol. 2009, 125, 310–317. [Google Scholar] [CrossRef]
- Du, J.; Chen, X.F.; Wang, C.Y.; Sun, H.X. Pathway analysis of global gene expression change in dendritic cells induced by the polysaccharide from the roots of Actinidia eriantha. J. Ethnopharmacol. 2018, 214, 141–152. [Google Scholar] [CrossRef]
- Wang, Z.P.; Liu, Y.F.; Li, L.; Li, D.W.; Zhang, Q.; Guo, Y.T.; Wang, S.B.; Zhong, C.H.; Huang, H.W. Whole transcriptome sequencing of Pseudomonas syringae pv. actinidiae-infected kiwifruit plants reveals species-specific interaction between long non-coding RNA and coding genes. Sci. Rep. 2017, 7, 491. [Google Scholar] [CrossRef]
- Wang, Z.M.; Zhang, Q.Y.; Liu, X.M. Observed the adaptability and resistibility of Actinidia chinensis and A. eriantha. J. Fruit Sci. 1996, 13, 29–30. [Google Scholar] [CrossRef]
- Zhong, M.; Zhang, W.B.; Zou, L.F.; Huang, Q.; Chen, L.; Huang, C.H.; Tao, J.J.; Xu, X.B. Diurnal variation of photosynthesis and chlorophyll fluore-scence characteristics in kiwifruit under high temperature condition. Acta Agric. Univ. Jiangxiensis 2018, 40, 472–478. [Google Scholar] [CrossRef]
- Wang, F.M.; Mo, Q.H.; Ye, K.Y.; Gong, H.J.; Qi, B.B.; Liu, P.P.; Jiang, Q.S.; Li, J.W. Evaluation of the wild Actinidia germplasm for resistance to Pseudomonas syringae pv. actinidiae. Plant Pathol. 2020, 69, 979–989. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, Y.H.; Zhang, H.J.; Landis, J.B.; Zhang, X.; Wang, H.C.; Yao, X.H. Molecular phylogeography and species distribution modelling evidence of ‘oceanic’ adaptation for Actinidia eriantha with a refugium along the oceanic-continental gradient in a biodiversity hotspot. BMC Plant Biol. 2022, 22, 89. [Google Scholar] [CrossRef]
- Liu, Y.F.; Liu, Y.L.; Huang, H.W. Genetic variation and natural hybridization among sympatric Actinidia species and the implications for introgression breeding of kiwifruit. Tree Genet. Genomes 2010, 6, 801–813. [Google Scholar] [CrossRef]
- Guo, R.; Landis, J.B.; Moore, M.J.; Meng, A.; Jian, S.; Yao, X.; Wang, H. Development and application of transcriptome-derived microsatellites in Actinidia eriantha (Actinidiaceae). Front. Plant Sci. 2017, 8, 1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.L.; Huang, C.H.; Wu, H.; Lang, B.B.; Qu, X.Y.; Xu, X.B. Genetic diversity of wild Actinidia eriantha germplasm based on fruit traits and SSR markers. Acta Hortic. Sin. 2014, 41, 1198–1206. [Google Scholar] [CrossRef]
- Qu, X.Y.; Lang, B.B.; Zhong, M.; Zhu, B.; Tao, J.J.; Huang, C.H.; Xu, X.B. Principal component analysis and comprehensive evaluation of fruit quality of Actinidia eriantha. Chin. Agric. Sci. Bull. 2016, 32, 92–96. [Google Scholar] [CrossRef]
- Lang, B.B.; Zhu, B.; Xie, M.; Zhang, W.B.; Seyrek, U.A.; Huang, C.H.; Xu, X.B. Variation and probability grading of the main quantitative characteristics of wild Actinidia eriantha germplasm resources. J. Fruit Sci. 2016, 33, 8–15. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Pan, Z.M. Comprehensive evaluation of 22 Actinidia eriantha germplasm resources based on principal components analysis. Acta Agric. Zhejiangensis 2021, 33, 825–830. [Google Scholar] [CrossRef]
- He, Y.; Li, Z.Y.; Liao, G.L.; Chen, L.; Zhong, M.; Huang, C.H.; Jia, D.F.; Qu, X.Y.; Xu, X.B. Variation in fruit quality within wild Actinidia eriantha germplasm. N. Z. J. Crop Hortic. Sci. 2020, 48, 153–163. [Google Scholar] [CrossRef]
- Jo, Y.S.; Cho, H.S.; Park, M.Y.; Bang, G.P. Selection of a sweet Actinidia eriantha, “Bidan”. Acta Hortic. 2007, 753, 253–258. [Google Scholar] [CrossRef]
- Xie, M.; Wu, Y.J.; Jiang, G.H.; Zhang, Q.Z.; Zhang, H.Q.; Peng, S.L.; Liu, K.M. A new big fruit Actinidia eriantha Benth. cultivar “White”. Acta Hortic. Sin. 2008, 35, 1555. [Google Scholar] [CrossRef]
- Xu, X.B.; Huang, C.H.; Qu, X.Y.; Chen, M.; Zhong, M.; Lang, B.B.; Chen, C.J.; Xie, M.; Zhang, W.B. A new easy peeling Actinidia eriantha cultivar “Ganmi 6”. Acta Hortic. Sin. 2015, 42, 2539–2540. [Google Scholar] [CrossRef]
- Xu, X.B.; Liao, G.L.; Huang, C.H.; Zhong, M.; Jia, D.F.; Qu, X.Y.; Liu, Q.; He, Y.Q.; Li, Y.Q. Differences of sucrose accumulation concentration and related genes expression between two sucrose accumulation types of Actinidia eriantha. Sci. Rep. 2020, 10, 20474. [Google Scholar] [CrossRef]
- MacRae, E.; Quick, W.P.; Benker, C.; Stitt, M. Carbohydrate metabolism during postharvest ripening in kiwifruit. Planta 1992, 188, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.Y.; Gonzales-Vigil, E.; Mansfield, S.D. Arabidopsis sucrose synthase localization indicates a primary role in sucrose translocation in phloem. J. Exp. Bot. 2020, 71, 1858–1869. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yuan, Y.L.; Zhang, C.; Li, H.X.; Ma, F.W.; Li, M.J. Sucrose phloem unloading follows an apoplastic pathway with high sucrose synthase in Actinidia fruit. Plant Sci. 2017, 255, 40–50. [Google Scholar] [CrossRef]
- Klages, K.; Donnison, H.; Boldingh, H.; Macrae, E. myo-Inositol is the major sugar in Actinidia arguta during early fruit development. Funct. Plant Biol. 1998, 25, 61–68. [Google Scholar] [CrossRef]
- Ruan, Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Yang, Y.; Yan, Y.Q. Identification of Sucrose Synthase Gene Family in Kiwifruit and Their Expression during Fruit Development. Mol. Plant Breed. 2021. Available online: http://kns.cnki.net/kcms/detail/46.1068.S.20210422.1703.010.html (accessed on 17 April 2022).
- Liao, G.L.; Li, Y.Q.; Wang, H.L.; Liu, Q.; Zhong, M.; Jia, D.F.; Huang, C.H.; Xu, X.B. Genome-wide identification and expression profiling analysis of sucrose synthase (SUS) and sucrose phosphate synthase (SPS) genes family in Actinidia chinensis and A. eriantha. BMC Plant Biol. 2022, 22, 215. [Google Scholar] [CrossRef]
- Tao, J.J.; Wu, M.T.; Jiao, X.D.; Chen, S.S.; Jia, D.F.; Xu, X.B.; Huang, C.H. Dynamic changes of fruit physiological quality and sugar components during fruit growth and development of Actinidia eriantha. Horticulturae 2022, 8, 529. [Google Scholar] [CrossRef]
- Cao, J.K.; Jiang, W.B.; Zhao, Y.M. Experiment Guidance of Postharvest Physiology and Biochemistry of Fruits and Vegetables; China Light Industry Press: Beijing, China, 2007. [Google Scholar]
- Yue, J.Y.; Liu, J.C.; Tang, W.; Wu, Y.Q.; Tang, X.F.; Li, W.; Yang, Y.; Wang, L.H.; Huang, S.X.; Fang, C.B.; et al. Kiwifruit Genome Database (KGD): A comprehensive resource for kiwifruit genomics. Hortic. Res. 2020, 7, 117. [Google Scholar] [CrossRef]
- Liu, K.P.; Huang, C.H.; Leng, J.H.; Chen, k.; Yan, Y.P.; Gu, Q.Q.; Xu, X.B. Principal component analysis and comprehensive evaluation of the fruit quality of ‘Jinkui’ kiwifruit. J. Fruit Sci. 2012, 29, 867–871. [Google Scholar] [CrossRef]
- Wu, Y.J.; Xie, M.; Zhang, Q.C.; Jiang, G.H.; Zhang, H.Q.; Long, Q.J.; Han, W.J.; Chen, J.W.; Shong, G.H. Characteristics of ‘White’: A new easy-peel cultivar of Actinidia eriantha. N. Z. J. Crop Hortic. Sci. 2009, 37, 369–373. [Google Scholar] [CrossRef]
- Cheng, B.X.; Yu, C.; Fu, H.L.; Zhou, L.J.; Luo, L.; Pan, H.T.; Zhang, Q.X. Evaluation of the morphological diversity of tea roses (Rosa x odorata) based on phenotypic traits. Plant Genet. Resour. 2020, 18, 149–158. [Google Scholar] [CrossRef]
- Tang, Q.Y. DPS Data Processing System: Experimental Design, Statistical Analysis and Data Mining; Science Press: Beijing, China, 2010. [Google Scholar]
- Melgarejo-Sánchez, P.; Martínez, J.J.; Legua, P.; Martínez, R.; Hernández, F.; Melgarejo, P. Quality, antioxidant activity and total phenols of six Spanish pomegranates clones. Sci. Hortic. 2015, 182, 65–72. [Google Scholar] [CrossRef]
- Byeon, S.E.; Lee, J. Differential responses of fruit quality and major targeted metabolites in three different cultivars of cold-stored figs (Ficus carica L.). Sci. Hortic. 2020, 260, 108877. [Google Scholar] [CrossRef]
- Qi, W.Y.; Zhou, C.H.; Song, L.J.; Zhong, Y.; Zheng, X.L. Study on sugar metabolism of Actinidia eriantha Benth ‘White’ during storage. J. Fruit Sci. 2016, 33, 744–751. [Google Scholar] [CrossRef]
- Richardson, A.C.; Boldingh, H.L.; McAtee, P.A.; Gunaseelan, K.; Luo, Z.; Atkinson, R.G.; David, K.M.; Burdon, J.N.; Schaffer, R.J. Fruit development of the diploid kiwifruit, Actinidia chinensis “Hort16A”. BMC Plant Biol. 2011, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Moscatello, S.; Famiani, F.; Proietti, S.; Farinelli, D.; Battistelli, A. Sucrose synthase dominates carbohydrate metabolism and relative growth rate in growing kiwifruit (Actinidia deliciosa, cv Hayward). Sci. Hortic. 2011, 128, 197–205. [Google Scholar] [CrossRef]
- Wang, D.F.; Yang, Z.J.; Sun, J.M.; Cao, Y.F.; Huang, X.S.; Zhang, S.L.; Wu, J. Difference in soluble sugar accumulation and related enzyme activity of fruits among different pear cultivars. J. Fruit Sci. 2014, 31, 30–38. [Google Scholar] [CrossRef]
- Vimolmangkang, S.; Zheng, H.Y.; Peng, Q.; Jiang, Q.; Wang, H.L.; Fang, T.; Liao, L.; Wang, L.; He, H.P.; Han, Y.P. Assessment of sugar components and genes involved in the regulation of sucrose accumulation in peach fruit. J. Agric. Food Chem. 2016, 64, 6723–6729. [Google Scholar] [CrossRef]
- Zheng, G.Q.; Zheng, Z.Y.; Xu, X.; Hu, Z.H. Variation in fruit sugar composition of Lycium barbarum L. and Lycium chinense Mill. of different regions and varieties. Biochem. Syst. Ecol. 2010, 38, 275–284. [Google Scholar] [CrossRef]
- Lombardo, V.A.; Osorio, S.; Borsani, J.; Lauxmann, M.A.; Bustamante, C.A.; Budde, C.O.; Andreo, C.S.; Lara, M.V.; Fernie, A.R.; Drincovich, M.F. Metabolic profiling during peach fruit development and ripening reveals the metabolic networks that underpin each developmental stage. Plant Physiol. 2011, 157, 1696–1710. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Zhang, D.P. A study on the relationships between acid invertase, sucrose synthase and sucrose metabolism in ‘Red Fuji’ apple fruit. Acta Hortic. Sin. 2001, 28, 259–261. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Xie, M.; Zhang, C.; Yang, L.Q.; Zhang, Z.; Xiao, J.P.; Zhou, L.Q. Difference in starch accumulation and characterization of sugar metabolism during fruit development of kiwifruit. Sci. Agric. Sin. 2014, 47, 3453–3464. [Google Scholar] [CrossRef]

| Variety | Longitudinal (mm) | Transverse Diameter (mm) | Lateral Diameter (mm) | Single Fruit Weight (g) | Fruit Shape Index |
|---|---|---|---|---|---|
| M6 | 36.11 g | 24.41 c | 22.11 def | 11.15 f | 1.48 g |
| M7 | 44.50 e | 25.17 c | 23.85 cd | 15.06 d | 1.77 e |
| M8 | 33.75 h | 20.2 e | 19.05 f | 7.37 g | 1.68 ef |
| M10 | 55.08 b | 26.51 b | 24.69 bcd | 23.34 b | 2.08 b |
| M11 | 49.67 c | 28.83 a | 26.66 abc | 22.50 b | 1.72 e |
| M12 | 50.38 c | 29.16 a | 26.94 ab | 21.82 b | 1.73 e |
| M13 | 46.87 d | 26.64 b | 29.01 a | 19.64 c | 1.76 e |
| M20 | 40.59 f | 25.39 bc | 22.86 de | 13.80 de | 1.6 f |
| M28 | 56.09 b | 23.09 d | 21.89 def | 17.67 c | 2.43 a |
| M30 | 40.90 f | 21.92 d | 20.70 ef | 12.04 ef | 1.87 d |
| CK | 58.37 a | 29.37 a | 28.19 a | 30.00 a | 1.99 c |
| Quality Index | Minimum Value | Maximum Value | Average Value | Standard Deviation | Coefficient of Variation (%) |
|---|---|---|---|---|---|
| Longitudinal (mm) | 33.75 | 58.37 | 46.57 | 8.20 | 17.61 |
| Transverse diameter (mm) | 20.2 | 29.37 | 25.52 | 3.00 | 11.76 |
| Lateral diameter (mm) | 19.05 | 29.01 | 24.18 | 3.21 | 13.28 |
| Single fruit weight (g) | 7.37 | 30.00 | 17.67 | 6.56 | 37.13 |
| Fruit shape index | 1.48 | 2.43 | 1.83 | 0.26 | 14.21 |
| Dry matter (%) | 12.75 | 19.27 | 15.20 | 2.12 | 13.95 |
| Soluble solids content (%) | 7.12 | 16.31 | 11.25 | 2.62 | 23.29 |
| Titratable acid content (%) | 0.68 | 1.25 | 1.05 | 0.16 | 15.24 |
| AsA (mg/g) | 4.47 | 7.70 | 5.85 | 0.98 | 16.75 |
| Fructose (mg/g) | 12.12 | 116.81 | 52.02 | 29.62 | 56.94 |
| Glucose (mg/g) | 8.70 | 63.53 | 37.69 | 14.68 | 38.95 |
| Sucrose (mg/g) | 13.13 | 71.53 | 35.27 | 19.14 | 54.27 |
| Quality Index | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Factor 6 | Factor 7 | Factor 8 | Factor 9 |
|---|---|---|---|---|---|---|---|---|---|
| Fruit weight | 0.831 | 0.226 | 0.002 | −0.09 | 0.238 | 0.376 | −0.188 | 0.068 | 0.109 |
| Fruit shape index | 0.77 | 0.301 | −0.003 | 0.359 | −0.353 | −0.137 | 0.067 | −0.135 | 0.145 |
| Dry matter | 0.674 | −0.303 | −0.464 | 0.07 | 0.313 | −0.257 | 0.125 | 0.228 | 0.042 |
| Soluble solids content | 0.741 | −0.339 | −0.43 | 0.169 | 0.102 | 0.172 | 0.083 | −0.24 | −0.133 |
| Titratable acid | −0.738 | −0.004 | −0.32 | 0.428 | −0.133 | 0.311 | 0.174 | 0.159 | 0.026 |
| AsA | −0.046 | 0.887 | 0.115 | −0.087 | 0.329 | 0.003 | 0.28 | −0.06 | −0.022 |
| Fructose | 0.695 | 0.241 | 0.528 | 0.326 | −0.103 | −0.011 | −0.055 | 0.19 | −0.156 |
| Glucose | 0.449 | −0.529 | 0.543 | −0.302 | −0.156 | 0.142 | 0.291 | 0.038 | 0.037 |
| Sucrose | −0.361 | −0.396 | 0.579 | 0.425 | 0.425 | −0.033 | −0.019 | −0.105 | 0.061 |
| Eigenvalues | 3.649 | 1.63 | 1.426 | 0.739 | 0.631 | 0.374 | 0.259 | 0.21 | 0.083 |
| Contribution rate (%) | 40.543 | 18.107 | 15.841 | 8.215 | 7.013 | 4.155 | 2.876 | 2.328 | 0.922 |
| Cumulative contribution rate (%) | 40.543 | 58.65 | 74.491 | 82.706 | 89.719 | 93.874 | 96.75 | 99.078 | 100 |
| Varieties (Lines) | Factor 1 | Factor 2 | Factor 3 | Comprehensive Score | Rank |
|---|---|---|---|---|---|
| M28 | 0.766 | 1.809 | 0.335 | 0.928 | 1 |
| M10 | 1.617 | −0.814 | 0.786 | 0.850 | 2 |
| CK | 1.659 | −0.394 | −0.831 | 0.630 | 3 |
| M8 | −0.093 | 1.009 | 0.022 | 0.199 | 4 |
| M12 | −0.131 | 0.387 | 0.460 | 0.120 | 5 |
| M13 | −0.042 | 0.019 | 0.194 | 0.023 | 6 |
| M11 | −0.183 | 0.348 | 0.061 | −0.002 | 7 |
| M20 | −0.977 | −1.127 | 1.870 | −0.408 | 8 |
| M30 | −0.095 | −0.946 | −1.976 | −0.701 | 9 |
| M6 | −1.602 | 1.005 | −0.672 | −0.771 | 10 |
| M7 | −0.920 | −1.296 | −0.249 | −0.869 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, J.; Wu, M.; Zhong, W.; Jiao, X.; Chen, S.; Jia, H.; Jia, D.; Huang, C. Changes in Fruit Quality and Sugar Components of Wild Actinidia eriantha of Different Varieties (Lines) at the Ripening Stage. Horticulturae 2022, 8, 824. https://doi.org/10.3390/horticulturae8090824
Tao J, Wu M, Zhong W, Jiao X, Chen S, Jia H, Jia D, Huang C. Changes in Fruit Quality and Sugar Components of Wild Actinidia eriantha of Different Varieties (Lines) at the Ripening Stage. Horticulturae. 2022; 8(9):824. https://doi.org/10.3390/horticulturae8090824
Chicago/Turabian StyleTao, Junjie, Mengting Wu, Wenqi Zhong, Xudong Jiao, Shuangshuang Chen, Huimin Jia, Dongfeng Jia, and Chunhui Huang. 2022. "Changes in Fruit Quality and Sugar Components of Wild Actinidia eriantha of Different Varieties (Lines) at the Ripening Stage" Horticulturae 8, no. 9: 824. https://doi.org/10.3390/horticulturae8090824
APA StyleTao, J., Wu, M., Zhong, W., Jiao, X., Chen, S., Jia, H., Jia, D., & Huang, C. (2022). Changes in Fruit Quality and Sugar Components of Wild Actinidia eriantha of Different Varieties (Lines) at the Ripening Stage. Horticulturae, 8(9), 824. https://doi.org/10.3390/horticulturae8090824







