Effects of Plasticulture and Conservation Tillage on Nematode Assemblage and Their Relationships with Nitrous Oxide Emission following a Winter Cover Cropping and Vegetable Production System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Design
2.2. Greenhouse Gas Emission from the Soil (N2O-N)
2.3. Nematode Community Analysis
2.4. Statistical Analysis
3. Results
3.1. General Edaphic Data
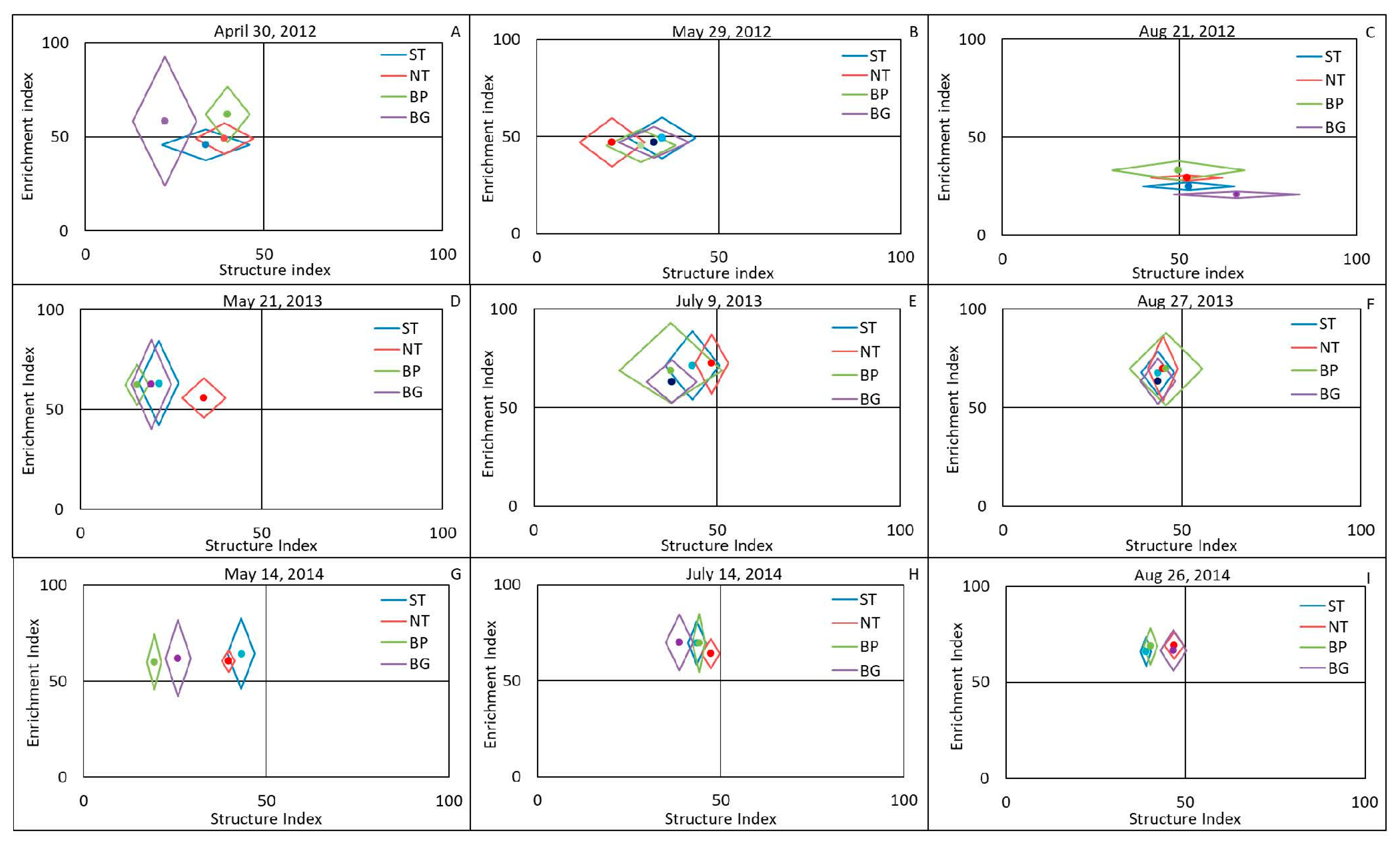
3.2. Effects of Plasticulture and Conservation Tillage on Nematode Community Assemblage
3.3. Effects of Cover Crop Treatments on Nematode Functional Metabolic Footprint over Time
3.4. N2O Emission Corresponding to Nematode Sampling Events
3.5. Relationships between Nematode Assemblage with N2O Emissions
4. Discussion
4.1. Effects of Plasticulture and Conservation Tillage on Nematode Community Assemblage
4.2. Relationships between Nematode Assemblage with N2O Emissions
4.2.1. Effects of Soil Temperature
4.2.2. Effects of Different Nematode Trophic Groups and C: N of Cover Crops
4.2.3. Soil Aeration
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2014: Synthesis Report; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Scheehle, E.A.; Kruger, D. Global Anthropogenic Methane and Nitrous Oxide Emissions. Energy J. 2006, 27, 33–44. [Google Scholar] [CrossRef]
- US EPA. Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2014. EPA 430-R-16-002. 2016. Available online: https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-andsinks-1990-2014 (accessed on 22 January 2022).
- Cole, C.V.; Duxbury, J.; Freney, J.; Heinemeyer, O.; Minami, K.; Mosier, A.; Paustian, K.; Rosenberg, N.; Sampson, N.; Sauerbeck, D.; et al. Global Estimates of Potential Mitigation of Greenhouse Gas Emissions by Agriculture. Nutr. Cycl. Agroecosyst. 1997, 49, 221–228. [Google Scholar] [CrossRef]
- van Groenigen, J.W.; Velthof, G.L.; Oenema, O.; Van Groenigen, K.J.; Van Kessel, C. Towards an Agronomic Assessment of N2O Emissions: A Case Study for Arable Crops. Eur. J. Soil Sci. 2010, 61, 903–913. [Google Scholar] [CrossRef]
- Verhoeven, E.; Decock, C.; Barthel, M.; Bertora, C.; Sacco, D.; Romani, M.; Sleutel, S.; Six, J. Nitrification and Coupled Nitrification-Denitrification at Shallow Depths Are Responsible for Early Season N2O Emissions under Alternate Wetting and Drying Management in an Italian Rice Paddy System. Soil Biol. Biochem. 2018, 120, 58–69. [Google Scholar] [CrossRef]
- Freney, J.R. Emission of Nitrous Oxide from Soils Used for Agriculture. Nutr. Cycl. Agroecosyst. 1997, 49, 1–6. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Xiu, W.; Tan, B.; Li, G.; Zhao, J.; Yang, D.; Zhang, G.; Zhang, Y. Responses of Soil Microbial and Nematode Communities to Various Cover Crop Patterns in a Tea Garden of China. Int. J. Environ. Res. Public Health 2022, 19, 2695. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, I.; de Deyn, G.B.; Thakur, M.P.; Van Groenigen, J.W. Soil Invertebrate Fauna Affect N2O Emissions from Soil. Glob. Chang. Biol. 2013, 19, 2814–2825. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.W.; Chen, D.; He, J.Z. Microbial Regulation of Terrestrial Nitrous Oxide Formation: Understanding the Biological Pathways for Prediction of Emission Rates. FEMS Microbiol. Rev. 2015, 39, 729–749. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, M.; Wang, Y.; Shen, R.; Gou, J.; Li, J.; Jin, J.; Li, L. Impacts of Soil Moisture on Nitrous Oxide Emission from Croplands: A Case Study on the Rice-Based Agro-Ecosystem in Southeast China. Chemosphere-Global Chang. Sci. 2000, 2, 207–224. [Google Scholar] [CrossRef]
- Garland, G.M.; Suddick, E.; Burger, M.; Horwath, W.R.; Six, J. Direct N2O Emissions from a Mediterranean Vineyard: Event-Related Baseline Measurements. Agric. Ecosyst. Environ. 2014, 195, 44–52. [Google Scholar] [CrossRef]
- Garland, G.M.; Suddick, E.; Burger, M.; Horwath, W.R.; Six, J. Direct N2O Emissions Following Transition from Conventional till to No-till in a Cover Cropped Mediterranean Vineyard (Vitis Vinifera). Agric. Ecosyst. Environ. 2011, 141, 234–239. [Google Scholar] [CrossRef]
- van Kessel, C.; Venterea, R.; Six, J.; Adviento-Borbe, M.A.; Linquist, B.; van Groenigen, K.J. Climate, Duration, and N Placement Determine N2O Emissions in Reduced Tillage Systems: A Meta-Analysis. Glob. Chang. Biol. 2013, 19, 33–44. [Google Scholar] [CrossRef]
- Li, Z.; Di Gioia, F.; Paudel, B.; Zhao, X.; Hong, J.; Pisani, C.; Rosskopf, E.; Wilson, P. Quantifying the Effects of Anaerobic Soil Disinfestation and Other Biological Soil Management Strategies on Nitrous Oxide Emissions from Raised Bed Plasticulture Tomato Production. J. Environ. Qual. 2022, 51, 162–180. [Google Scholar] [CrossRef]
- Chen, G.; Kolb, L.; Cavigelli, M.A.; Weil, R.R.; Hooks, C.R.R. Can Conservation Tillage Reduce N2O Emissions on Cropland Transitioning to Organic Vegetable Production? Sci. Total Environ. 2018, 618, 927–940. [Google Scholar] [CrossRef]
- Leslie, A.W.; Wang, K.-H.; Meyer, S.L.F.; Marahatta, S.; Hooks, C.R.R. Influence of Cover Crops on Arthropods, Free-Living Nematodes, and Yield in a Succeeding No-till Soybean Crop. Appl. Soil Ecol. Soil Ecol. 2017, 117–118, 21–31. [Google Scholar] [CrossRef]
- Ferris, H.; Griffiths, B.S.; Porazinska, D.L.; Powers, T.O.; Wang, K.H.; Tenuta, M. Reflections on Plant and Soil Nematode Ecology: Past, Present and Future. J. Nematol. 2012, 44, 115–126. [Google Scholar]
- Bongers, T. The Maturity Index: An Ecological Measure of Environmental Disturbance Based on Nematode Species Composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liang, W.; Jiang, Y.; Shi, Y.; Zhu, J.; Neher, D.A. Effect of Elevated CO2 and N Fertilisation on Soil Nematode Abundance and Diversity in a Wheat Field. Appl. Soil Ecol. 2007, 36, 63–69. [Google Scholar] [CrossRef]
- Ferris, H. Form and Function: Metabolic Footprints of Nematodes in the Soil Food Web. Eur. J. Soil Biol. 2010, 46, 97–104. [Google Scholar] [CrossRef]
- Tianxiang, L.; Huixin, L.; Tong, W.; Feng, H. Influence of Nematodes and Earthworms on the Emissions of Soil Trace Gases (CO2, N2O). Acta Ecol. Sin. 2008, 28, 993–999. [Google Scholar] [CrossRef]
- Chen, G.; Kolb, L.; Leslie, A.; Hooks, C.R.R. Using Reduced Tillage and Cover Crop Residue to Manage Weeds in Organic Vegetable Production. Weed Technol. 2017, 31, 557–573. [Google Scholar] [CrossRef]
- NCSS. Anapolis Series; National Cooperative Soil Survey: National Academic Soil Survey, Lincoln, NE, USA. 2015. Available online: https://soilseries.sc.egov.usda.gov/OSD_Docs/A/ANNAPOLIS.html (accessed on 28 March 2022).
- Poffenbarger, H.J.; Mirsky, S.B.; Weil, R.R.; Kramer, M.; Spargo, J.T.; Cavigelli, M.A. Legume Proportion, Poultry Litter, and Tillage Effects on Cover Crop Decomposition. Agron. J. 2015, 107, 2083–2096. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture–Agricultural Marketing Service. United States Standards for Grades of Eggplant. 2013. Available online: https://www.ams.usda.gov/sites/default/files/media/Eggplant_Standard%5B1%5D.pdf (accessed on 25 June 2022).
- USDA. United States Standards for Grades of Sweet Corn for Processing; USDA: Washington, DC, USA, 1997. Available online: https://www.ams.usda.gov/sites/default/files/media/Corn%2C_Sweet_For_Processing_Standard%5B1%5D.pdf (accessed on 25 June 2022).
- Parkin, T.B.; Venterea, R.T. Sampling Protocols. Chapter 3. Chamber-Based Trace Gas Flux Measurements. In Sampling Protocols; Follett, R.F., Ed.; USDA ARS: Washington, DC, USA, 2010. [Google Scholar]
- Rochette, P. Measurement Strategy to Quantify the Effect of Management Practices on Soil N2O Emissions. Presented at American Society of Agronomy Measuring Nitrous Oxide from the Soil Workshop. American Society of Agronomy Measuring Nitrous Oxide from the Soil Workshop. 6 November 2014. Available online: https://www.youtube.com/watch?v=gfpO8SAucNc (accessed on 5 May 2022).
- Jenkins, W.R. A Rapid Centrifugal-Flotation Technique for Separating Nematodes from Soil. Plant Dis. Rep. 1964, 48, 692. [Google Scholar]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding Habits in Soil Nematode Families and Genera-an Outline for Soil Ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar] [PubMed]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Freckman, D.W.; Ettema, C.H. Assessing Nematode Communities in Agroecosystems of Varying Human Intervention. Agric. Ecosyst. Environ. 1993, 45, 239–261. [Google Scholar] [CrossRef]
- Bongers, T.; Bongers, M. Functional Diversity of Nematodes. Appl. Soil Ecol. 1998, 10, 239–251. [Google Scholar] [CrossRef]
- Yeates, G.W. Modification and Qualification of the Nematode Maturity Index. Pedobiologia 1994, 38, 97–101. [Google Scholar]
- Ferris, H.; Bongers, T.; De Goede, R.G.M. A Framework for Soil Food Web Diagnostics: Extension of the Nematode Faunal Analysis Concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Ferris, H. NEMAPLEX: The Nematode-Plant Expert Information System. 2001. Available online: http//plpnemweb.ucdavis.edu/nemaplex/Nemaplex.htm (accessed on 25 June 2022).
- Khan, A.R.; Chandra, D.; Quraishi, S.; Sinha, R.K. Soil Aeration under Different Soil Surface Conditions. J. Agron. Crop Sci. 2000, 185, 105–112. [Google Scholar] [CrossRef]
- Coale, F.J.; Costa, J.M.; Bollero, G.A.; Schlosnagle, S.P. Small Grain Winter Cover Crops for Conservation of Residual Soil Nitrogen in the Mid-Atlantic Coastal Plain. Renew. Agric. Food Syst. 2001, 16, 66–72. [Google Scholar] [CrossRef]
- Chen, G.; Weil, R.R. Penetration of Cover Crop Roots through Compacted Soils. Plant Soil 2010, 331, 31–43. [Google Scholar] [CrossRef]
- Lawley, Y.E.; Weil, R.R.; Teasdale, J.R. Cover Crops Forage Radish Cover Crop Suppresses Winter Annual Weeds in Fall and before Corn Planting. Agron. J. 2011, 103, 137–144. [Google Scholar] [CrossRef]
- Parr, M.; Grossman, J.M.; Reberg-Horton, S.C.; Brinton, C.; Crozier, C. Nitrogen Delivery from Legume Cover Crops in No-till Organic Corn Production. Agron. J. 2011, 103, 1578–1590. [Google Scholar] [CrossRef]
- Wang, K.-H.; Waisen, P.; Leslie, A.; Paudel, R.; Meyer, S.; Hooks, C. Relationships between Soil Tillage Systems, Nematode Communities and Weed Seed Predation. Horticulturae 2022, 8, 425. [Google Scholar] [CrossRef]
- Davidson, E.A.; Keller, M.; Erickson, H.E.; Verchot, L.V.; Veldkamp, E. Testing a Conceptual Model of Soil Emissions of Nitrous and Nitric Oxides: Using Two Functions Based on Soil Nitrogen Availability and Soil Water Content, the Hole-in-the-Pipe Model Characterizes a Large Fraction of the Observed Variation of Nitric Oxide. Bioscience 2000, 50, 667–680. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous Oxide Emissions from Soils: How Well Do We Understand the Processes and Their Controls? Philos. Trans. R. Soc. Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef]
- Robertson, G.P.; Groffman, P.M. Nitrogen Transformations, 4th ed.; Paul, E.A., Ed.; Academic Press: Burlington, MA, USA, 2015; ISBN 9780124159556. [Google Scholar]
- Marquez, J.M.K. Evaluating Effects of No-Till Cover Cropping Systems on Indigenous Entomopathogenic Nematodes and Fungi. Master’s Thesis, University of Hawaii at Manoa, Honolulu, HI, USA, 2017. [Google Scholar]
- Zhu, T.; Yang, C.; Wang, J.; Zeng, S.; Liu, M.; Yang, J.; Bai, B.; Cao, J.; Chen, X.; Müller, C. Bacterivore Nematodes Stimulate Soil Gross N Transformation Rates Depending on Their Species. Biol. Fertil. Soils 2018, 54, 107–118. [Google Scholar] [CrossRef]
- Wang, K.H.; McSorley, R.; Marshall, A.; Gallaher, R.N. Influence of Organic Crotalaria Juncea Hay and Ammonium Nitrate Fertilizers on Soil Nematode Communities. Appl. Soil Ecol. 2006, 31, 186–198. [Google Scholar] [CrossRef]
- McSorley, R.; Wang, K.H.; Church, G. Suppression of Root-Knot Nematodes in Natural and Agricultural Soils. Appl. Soil Ecol. 2008, 39, 291–298. [Google Scholar] [CrossRef]
- Ferris, H.; Venette, R.C.; Van Der Meulen, H.R.; Lau, S.S. Nitrogen Mineralization by Bacterial-Feeding Nematodes: Verification and Measurement. Plant Soil 1998, 203, 159–171. [Google Scholar] [CrossRef]
- Dubeux, J.C.B.; Sollenberger, L.E. Chapter 4—Nutrient Cycling in Grazed Pastures. In Management Strategies for Sustainable Cattle Production in Southern Pastures; Rouquette, M., Aiken, G.E., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 59–75. ISBN 978-0-12-814474-9. [Google Scholar]
- Butler, D.M.; Kokalis-Burelle, N.; Albano, J.P.; McCollum, T.G.; Muramoto, J.; Shennan, C.; Rosskopf, E.N. Anaerobic Soil Disinfestation (ASD) Combined with Soil Solarization as a Methyl Bromide Alternative: Vegetable Crop Performance and Soil Nutrient Dynamics. Plant Soil 2014, 378, 365–381. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, X.; Rosskopf, E.N.; Di Gioia, F.; Hong, J.C.; McNear, D.H. Impacts of Anaerobic Soil Disinfestation and Chemical Fumigation on Soil Microbial Communities in Field Tomato Production System. Appl. Soil Ecol. 2018, 126, 165–173. [Google Scholar] [CrossRef]
- Shcherbak, I.; Millar, N.; Robertson, G.P. Global Metaanalysis of the Nonlinear Response of Soil Nitrous Oxide (N2O) Emissions to Fertilizer Nitrogen. Proc. Natl. Acad. Sci. USA 2014, 111, 9199–9204. [Google Scholar] [CrossRef]

| Nematode | Guildz | Nematode | Guild | Nematode | Guild |
|---|---|---|---|---|---|
| Achromadora | z A-3 | Zeldia | B-2 | Rotylenchulus | H-3 |
| Prochromadora | A-3 | Prismatolaimus | B-3 | Tylenchorhynchus | H-3 |
| Monochromadora | A-3 | Plectus | B-2 | Paratrichodorus | H-4 |
| Alirhabditis | B-1 | Rhabdolaimus | B-3 | Trichodorus | H-4 |
| Anguilloides | B-1 | Teratocephalus | B-3 | Longidorus | H-5 |
| Bunonema | B-1 | Alaimus | B-4 | Xiphinema | H-5 |
| Diplogasteridae | B-1 | Aphelenchoides | F-2 | Californicus | O-4 |
| Diploscapter | B-1 | Aphelenchus | F-2 | Dorylaimoides | O-4 |
| Halicephalobus | B-1 | Deladenus | F-2 | Ecumenicus | O-4 |
| Panagrobelium | B-1 | Ditylenchus | F-2 | Enchodelus | O-4 |
| Panagrolaimus | B-1 | Ecphyadophora | F-2 | Epidorylaimus | O-4 |
| Plectonchus | B-1 | Filenchus | F-2 | Eudorylaimus | O-4 |
| Rhabditidae | B-1 | Neotylenchus | F-2 | Labronema | O-4 |
| Tricephalobus | B-1 | Nothotylenchus | F-2 | Mesodorylaimus | O-4 |
| Acrobeles | B-2 | Paraphelenchus | F-2 | Pachydorylaimus | O-4 |
| Acrobeloides | B-2 | Paurodontidae | F-2 | Pungentus | O-4 |
| Cephalobus | B-2 | Pseudohalenchus | F-2 | Aporcelaimellus | O-5 |
| Cervidellus | B-2 | Tylenchus | F-2 | Aporcelaimus | O-5 |
| Chronogaster | B-2 | Psilenchus | F-2 | Belondira | O-5 |
| Drilocephalobus | B-2 | Diphtherophora | F-3 | Indodorylaimus | O-5 |
| Eucephalobus | B-2 | Triplonchium | F-3 | Paraxonchium | O-5 |
| Heterocephalobus | B-2 | Leptonchus | F-4 | Seinura | P-2 |
| Monhystera | B-2 | Tylencholaimellus | F-4 | Tobrillus | P-3 |
| Monhysterella | B-2 | Tylencholaimus | F-4 | Tripyla | P-3 |
| Panagrocephalus | B-2 | Psilenchus | H-2 | Cryptonchus | P-4 |
| Paracrobeles | B-2 | Helicotylenchus | H-3 | Mononchus | P-4 |
| Paraplectonema | B-2 | Heterodera | H-3 | Mylonchulus | P-4 |
| Plectus | B-2 | Hoplolaimus | H-3 | Prionchulus | P-4 |
| Pseudoacrobeles | B-2 | Meloidogyne | H-3 | Discolaimus | P-5 |
| Stegelletina | B-2 | Mesocriconema | H-3 | Nygolaimus | P-5 |
| Tylocephalus | B-2 | Paratylenchus | H-3 | Paravulvus | P-5 |
| Wilsonema | B-2 | Pratylenchus | H-3 |
| Parameter | Treatments | Treatments | Treatment × Date | |||||
|---|---|---|---|---|---|---|---|---|
| z BG | BP | NT | ST | F-Value | Pr > F | F-Value | Pr > F | |
| 2012 | ||||||||
| Abundance | nematodes/100 cm3 soil | |||||||
| Bacterivore | 798 ± 184 a y | 529 ± 57 ab | 408 ± 68 c | 463 ± 53 bc | 5.11 | 0.0052 | 8.47 | <0.0001 |
| Fungivore | 1003 ± 230 a | 835 ± 190 a | 1169 ± 207 a | 1071 ± 209 a | 1.06 | 0.38 | 1.19 | 0.338 |
| Herbivore | 505 ± 82 a | 733 ± 420 a | 520 ± 161 a | 469 ± 176 a | 0.71 | 0.5556 | 0.32 | 0.9209 |
| Omnivore | 120 ± 13 a | 114 ± 10 a | 120 ± 21 a | 131 ± 17 a | 0.41 | 0.7438 | 3.08 | 0.0167 |
| Predatory | 41 ± 8 a | 24 ± 5 b | 33 ± 7 ab | 34 ± 9 ab | 3.85 | 0.0182 | 5.47 | 0.0005 |
| Indices x | ||||||||
| F/F+B | 0.50 ± 0.22 b | 0.53 ± 0.24 b | 0.68 ± 0.19 a | 0.63 ± 0.2 ab | 3.72 | 0.0209 | 1.95 | 0.1025 |
| Richness | 24 ± 1 a | 24 ± 1 a | 24 ± 1 a | 24 ± 1 a | 0.31 | 0.8177 | 2.49 | 0.0424 |
| MI | 2.26 ± 0.09 ab | 2.16 ± 0.04 b | 2.28 ± 0.07 ab | 2.28 ± 0.04 a | 2.41 | 0.0842 | 5.09 | 0.0009 |
| EI | 42.01 ± 4.93 a | 46.76 ± 4.77 a | 41.78 ± 3.24 a | 40.07 ± 3.59 a | 1.54 | 0.2222 | 2 | 0.0937 |
| SI | 40.19 ± 6.1 a | 39.37 ± 3.51 a | 37.24 ± 5.26 a | 40.26 ± 3.51 a | 0.27 | 0.8642 | 3.43 | 0.0097 |
| CI | 70.65 ± 8.68 b | 61.41 ± 8.63 b | 82.46 ± 4.63 a | 82.89 ± 4 a | 6.35 | 0.0016 | 5.49 | 0.0005 |
| 2013 | ||||||||
| Abundance | nematodes/100 cm3 soil | |||||||
| Bacterivore | 664 ± 115 ab | 766 ± 79 a | 478 ± 80 b | 630 ± 131 ab | 2.84 | 0.0526 | 2.16 | 0.073 |
| Fungivore | 604 ± 98 a | 447 ± 85 b | 710 ± 127 a | 570 ± 77 ab | 3.29 | 0.0326 | 2.36 | 0.0527 |
| Herbivore | 261 ± 71 b | 770 ± 253 a | 339 ± 72 ab | 622 ± 160 a | 3.9 | 0.0172 | 1.1 | 0.3809 |
| Omnivore | 46 ± 8 a | 94 ± 21 a | 44 ± 8 a | 53 ±9 a | 1.19 | 0.3285 | 0.92 | 0.4902 |
| Predatory | 16 ± 6 b | 8 ± 2 b | 29 ± 5 a | 17 ± 2 ab | 5.25 | 0.0045 | 1.18 | 0.339 |
| Indices | ||||||||
| F/F+B | 0.48 ± 0.04 a | 0.35 ± 0.04 a | 0.58 0.06 a | 0.49 ± 0.04 a | 6.11 | 0.002 | 1.73 | 0.1451 |
| Richness | 25 ± 2 a | 26 ± 1 a | 28 ± 1 a | 28 ± 1 a | 1.28 | 0.2958 | 0.39 | 0.8830 |
| MI | 1.85 ± 0.06 a | 1.69 ± 0.03 b | 1.90 ± 0.04 a | 1.79 ± 0.02 ab | 5.11 | 0.0052 | 0.27 | 0.9487 |
| EI | 63.15 ± 1.89 a | 66.85 ± 1.75 a | 66.12 ± 3.12 a | 67.47 ± 2.25 a | 0.83 | 0.4893 | 0.99 | 0.4476 |
| SI | 33.49 ± 5 a | 32.84 ± 5.05 a | 42.47 ± 3.44 a | 36.05 ± 4.58 a | 1.8 | 0.1667 | 0.55 | 0.7643 |
| CI | 37.63 ± 3.55 a | 24.1 ± 2.99 b | 44.13 ± 7.55 a | 33.9 ± 4.5 ab | 3.73 | 0.0206 | 1.56 | 0.1906 |
| 2014 | ||||||||
| Abundance | nematodes/100 cm3 soil | |||||||
| Bacterivore | 486 ± 154 a | 418 ± 66 a | 292 ± 32 a | 387 ± 70 a | 0.28 | 0.8418 | 0.2 | 0.9933 |
| Fungivore | 384 ± 39 a | 415 ± 102 a | 364 ± 64 a | 485 ± 135 a | 0.45 | 0.7211 | 0.93 | 0.5059 |
| Herbivore | 135 ± 46 b | 234 ± 136 b | 239 ± 99 ab | 209 ± 57 a | 2.88 | 0.0461 | 0.63 | 0.7621 |
| Omnivore | 40 ± 6 a | 31 ± 4 ab | 25 ± 4 b | 27 ± 6 b | 3.67 | 0.0189 | 2.36 | 0.0579 |
| Predatory | 12 ± 3 a | 6 ± 2 b | 12 ± 3 a | 18 ± 4 a | 3.48 | 0.0233 | 0.94 | 0.4976 |
| Indices | ||||||||
| F/F+B | 0.53 ± 0.05 a | 0.49 ± 0.04 a | 0.53 ± 0.05 a | 0.52 ± 0.05 a | 0.51 | 0.6786 | 0.97 | 0.4735 |
| Richness | 22 ± 2 a | 20 ± 1 a | 23 ± 1 a | 23 ± 1 a | 1.41 | 0.2514 | 0.78 | 0.6349 |
| MI | 1.72 ± 0.07 a | 1.66 ± 0.11 a | 1.6 ± 0.1 a | 1.57 ± 0.07 a | 0.73 | 0.5375 | 0.32 | 0.9647 |
| EI | 65.55 ± 1.96 a | 68.02 ± 3.15 a | 66.87 ± 2.46 a | 68.16 ± 2.55 a | 0.31 | 0.8179 | 0.56 | 0.8211 |
| SI | 33.47 ± 2.99 a | 33.27 ± 3.74 a | 39.83 ± 3.27 a | 35.9 ± 3.62 a | 1.54 | 0.2172 | 1.98 | 0.0648 |
| CI | 39.64 ± 4.94 a | 37.38 ± 6.53 a | 38.71 ± 5.33 a | 37.17 ± 5.27 a | 0.07 | 0.9752 | 0.59 | 0.7975 |
| Parameter | Treatments | |||||
|---|---|---|---|---|---|---|
| z BG | BP | NT | ST | F-Value | Pr > F | |
| 04/30/2012 | ||||||
| Abundance | nematodes/100 cm3 soil | |||||
| Bacterivore | 1640 ± 98 a y | 563 ± 118 b | 363 ± 98 b | 464 ± 113 b | 16.18 | 0.0006 |
| Omnivore | 89 ± 16 b | 102 ± 17 ab | 183 ± 46 a | 157 ± 43 ab | 3.61 | 0.0587 |
| Predatory | 58 ± 13 a | 24 ± 5 b | 62 ± 2 a | 70 ± 16 a | 4.26 | 0.0395 |
| CI | 34 ± 7 b | 38 ± 8 b | 74 ± 8 a | 80 ± 1 a | 16.10 | 0.0006 |
| 05/29/2012 | ||||||
| Abundance | nematodes/100 cm3 soil | |||||
| Bacterivore | 424 ± 59 a | 484 ± 66 a | 654 ± 79 a | 566 ± 98 a | 1.46 | 0.2896 |
| Omnivore | 132 ± 17 a | 111 ± 14 ab | 99 ± 11 b | 141 ± 22 a | 4.08 | 0.0438 |
| Predatory | 51 ± 11 a | 42 ± 6 a | 20 ± 4 b | 15 ± 3 b | 10.92 | 0.0023 |
| CI | 78 ± 6 a | 80 ± 5 a | 73 ± 3 a | 68 ± 2 a | 1.28 | 0.3381 |
| 08/31/2012 | ||||||
| Abundance | nematodes/100 cm3 soil | |||||
| Bacterivore | 329 ± 49 ab | 540 ± 127 a | 207 ± 23 b | 360 ± 42 ab | 4.58 | 0.0329 |
| Omnivore | 140 ± 27 a | 131 ± 20 a | 79 ± 23 a | 97 ± 9 a | 1.94 | 0.1943 |
| Predatory | 16 ± 3 a | 7 ± 2 b | 17 ± 5 ab | 18 ± 5 a | 3.12 | 0.0810 |
| CI | 100 ± 0 a | 66 ± 21 a | 100 ± 0 a | 100 ± 0 a | 2.62 | 0.1153 |
| Treatments | Contrast (p Values) x | ||||||
|---|---|---|---|---|---|---|---|
| Year | z BG | BP | NT | ST | BP vs. no BP | BG vs. CT | NT vs. ST |
| N2O g ha−1 | |||||||
| 2012 | 1.97 y | 5.60 | 3.80 | 2.94 | 0.040 * | NS | NS |
| 2013 | 3.28 | 7.10 | 1.33 | 4.21 | 0.019 * | NS | NS |
| 2014 | 24.58 | 84.25 | 28.80 | 16.01 | 0.005 ** | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.-H.; Waisen, P.; Paudel, R.; Chen, G.; Meyer, S.L.F.; Hooks, C.R.R. Effects of Plasticulture and Conservation Tillage on Nematode Assemblage and Their Relationships with Nitrous Oxide Emission following a Winter Cover Cropping and Vegetable Production System. Horticulturae 2022, 8, 728. https://doi.org/10.3390/horticulturae8080728
Wang K-H, Waisen P, Paudel R, Chen G, Meyer SLF, Hooks CRR. Effects of Plasticulture and Conservation Tillage on Nematode Assemblage and Their Relationships with Nitrous Oxide Emission following a Winter Cover Cropping and Vegetable Production System. Horticulturae. 2022; 8(8):728. https://doi.org/10.3390/horticulturae8080728
Chicago/Turabian StyleWang, Koon-Hui, Philip Waisen, Roshan Paudel, Guihua Chen, Susan Lynn Fricke Meyer, and Cerruti R. R. Hooks. 2022. "Effects of Plasticulture and Conservation Tillage on Nematode Assemblage and Their Relationships with Nitrous Oxide Emission following a Winter Cover Cropping and Vegetable Production System" Horticulturae 8, no. 8: 728. https://doi.org/10.3390/horticulturae8080728
APA StyleWang, K.-H., Waisen, P., Paudel, R., Chen, G., Meyer, S. L. F., & Hooks, C. R. R. (2022). Effects of Plasticulture and Conservation Tillage on Nematode Assemblage and Their Relationships with Nitrous Oxide Emission following a Winter Cover Cropping and Vegetable Production System. Horticulturae, 8(8), 728. https://doi.org/10.3390/horticulturae8080728








