Lipidomics Analysis of Tea Leaves Cultured in Hydroponics Reveals That High Nitrogen Application Decreases Tea Plant Resistance to Ultraviolet Radiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatment
2.2. Lipid Extraction
2.3. Detecting Lipid Components by UPLC/MS
3. Results
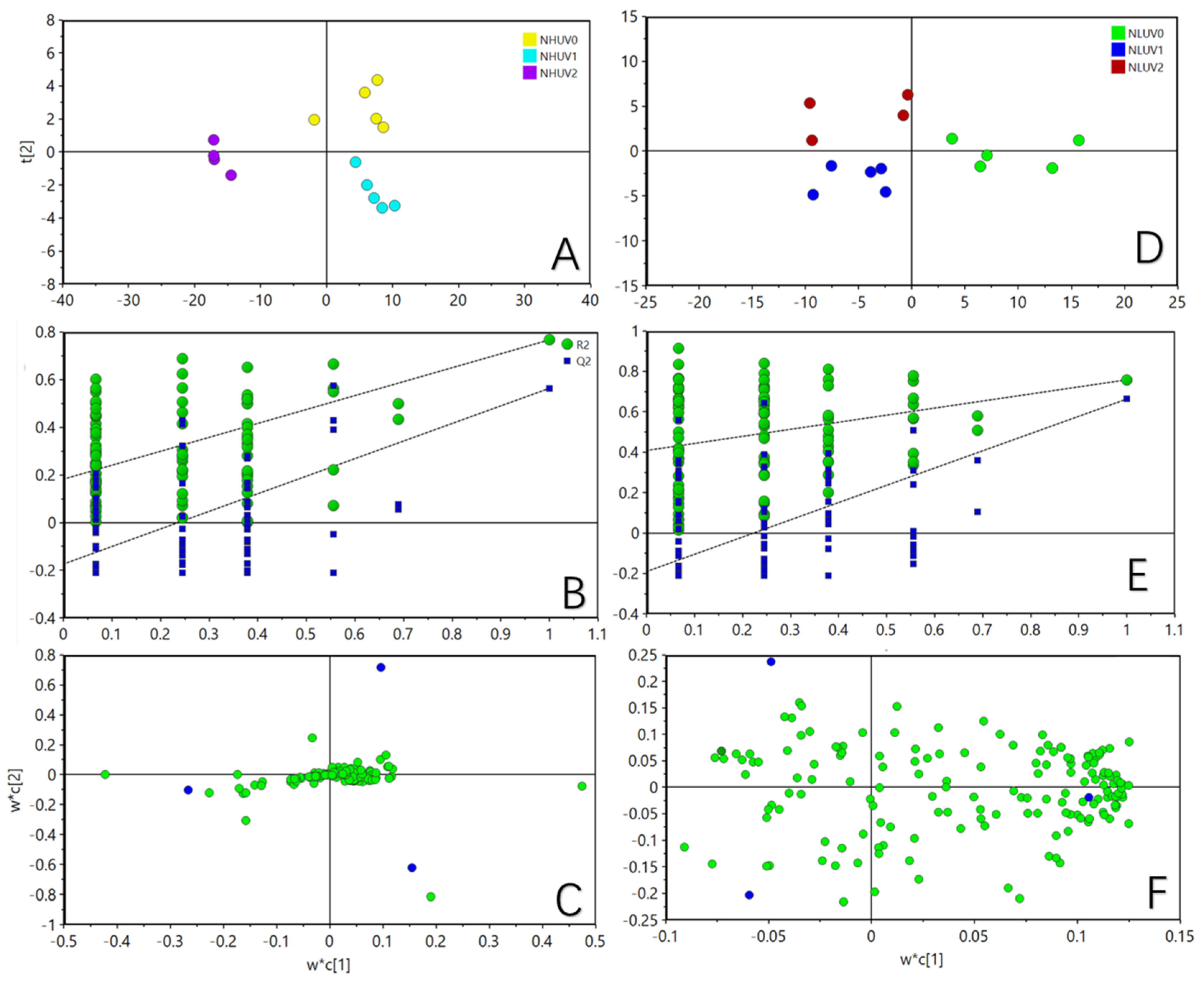
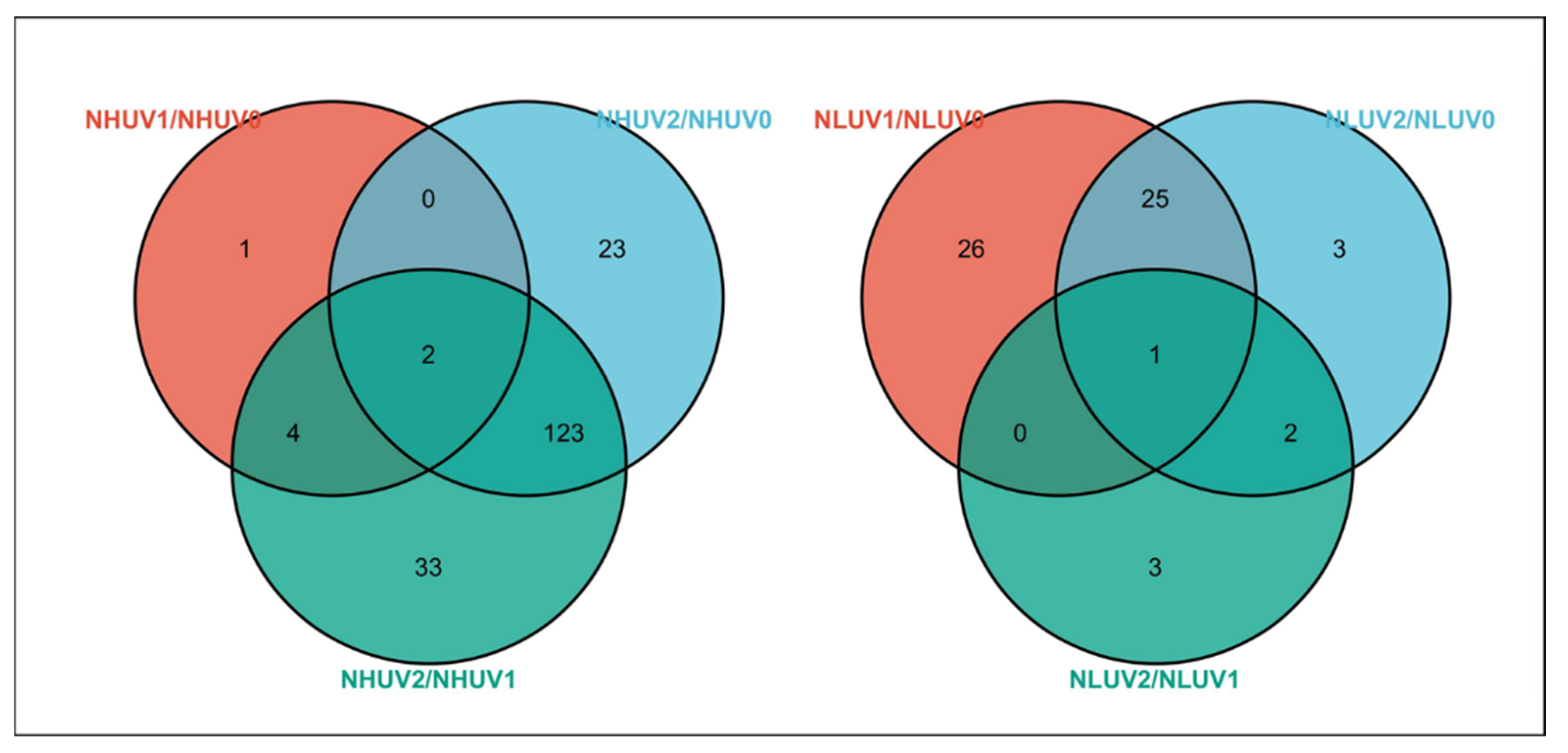
3.1. Lipid Profile Analysis after Ultraviolet Radiation Treatment under Different Nitrogen Levels
3.2. Effect of Ultraviolet Radiation on Lipids in Tea Leaves under Different Nitrogen Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TAG | Triacylglycerol |
| PC | Phosphatidylcholine |
| LysoPC | Lysophosphatidylcholine |
| PE | Phosphatidylethanolamine |
| LysoPE | Lysophosphatidylethanolamine |
| PG | Phosphatidylglycerol |
| LysoPG | Lysophosphatidylglycerol |
| PI | Phosphatidylinositol |
| PA | Phosphatidylic acid |
| PS | Phosphatidylserine |
| MGDG | Monogalactosyldiglyceride |
| DGDG | Digalactosyldiglyceride |
| DAG | Diacylglycerol |
References
- Liang, Y.; Lu, J.; Shang, S. Effect of Gibberellins on Chemical Composition and Quality of Tea (Camellia sinensis L.). J. Sci. Food Agric. 1996, 72, 411–414. [Google Scholar] [CrossRef]
- Punyasiri, P.; Abeysinghe, I.; Kumar, V.; Treutter, D.; Duy, D.; Gosch, C.; Martens, S.; Forkmann, G.; Fischer, T. Flavonoid biosynthesis in the tea plant Camellia sinensis: Properties of enzymes of the prominent epicatechin and catechin pathways. Arch. Biochem. Biophys. 2004, 431, 22–30. [Google Scholar] [CrossRef]
- Zhang, Q.; Ruan, J.T. Analysis and Tasting. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Li-Beisson, Y., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 256–267. ISBN 978-0-12-384953-3. [Google Scholar]
- Ruan, J.; Haerdter, R.; Gerendás, J. Impact of nitrogen supply on carbon/nitrogen allocation: A case study on amino acids and catechins in green tea [Camellia sinensis (L.) O. Kuntze] plants. Plant Biol. 2010, 12, 724–734. [Google Scholar] [CrossRef]
- Du, X.H.; Peng, F.R.; Jiang, J.; Tan, P.P.; Wu, Z.Z.; Liang, Y.W.; Zhong, Z.K. Inorganic nitrogen fertilizers induce changes in ammonium assimilation and gas exchange in Camellia sinensis L. Turk. J. Agric. For. 2015, 39, 28–38. [Google Scholar] [CrossRef]
- Lin, Z.-H.; Qi, Y.-P.; Chen, R.-B.; Zhang, F.-Z.; Chen, L.-S. Effects of phosphorus supply on the quality of green tea. Food Chem. 2012, 130, 908–914. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of Macronutrients. In Marschers’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 135–189. ISBN 978-0-12-384905-2. [Google Scholar]
- Krapp, A. Plant nitrogen assimilation and its regulation: A complex puzzle with missing pieces. Curr. Opin. Plant Biol. 2015, 25, 115–122. [Google Scholar] [CrossRef]
- Sharma, S.N.; Sirohi, G.S. The effect of ammonium and nitrate on CO2 assimilation, RuBP and PEP carboxylase activity and dry matter production in wheat. Photosynth. Res. 1987, 12, 265–272. [Google Scholar] [CrossRef]
- Rosen, C.J.; Allan, D.L.; Luby, J.J. Nitrogen Form and Solution pH Influence Growth and Nutrition of Two Vaccinium Clones. J. Am. Soc. Hortic. Sci. 1990, 115, 83–89. [Google Scholar] [CrossRef]
- Raab, T.K.; Terry, N. Nitrogen Source Regulation of Growth and Photosynthesis in Beta vulgaris L. Plant Physiol. 1994, 105, 1159–1166. [Google Scholar] [CrossRef] [Green Version]
- Reifenrath, K.; Müller, C. Species-specific and leaf-age dependent effects of ultraviolet radiation on two Brassicaceae. Phytochemistry 2007, 68, 875–885. [Google Scholar] [CrossRef]
- Frohnmeyer, H.; Staiger, D. Ultraviolet-B Radiation-Mediated Responses in Plants. Balancing Damage and Protection. Plant Physiol. 2003, 133, 1420–1428. [Google Scholar] [CrossRef] [Green Version]
- Mosadegh, H.; Trivellini, A.; Lucchesini, M.; Ferrante, A.; Maggini, R.; Vernieri, P.; Sodi, A.M. UV-B Physiological Changes Under Conditions of Distress and Eustress in Sweet Basil. Plants 2019, 8, 396. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Anjum, N.A.; Gill, R.; Jha, M.; Tuteja, N. DNA Damage and Repair in Plants under Ultraviolet and Ionizing Radiations. Sci. World J. 2015, 2015, 250158. [Google Scholar] [CrossRef] [Green Version]
- Predieri, S.; Norman, H.; Krizek, D.; Pillai, P.; Mirecki, R.; Zimmerman, R. Influence of UV-B radiation on membrane lipid composition and ethylene evolution in ‘Doyenne d’Hiver’ pear shoots grown in vitro under different photosynthetic photon fluxes. Environ. Exp. Bot. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Zheng, X.Q.; Jin, J.; Chen, H.; Du, Y.Y.; Ye, J.H.; Lu, J.L.; Lin, C.; Dong, J.J.; Sun, Q.L.; Wu, L.Y.; et al. Effect of Ultraviolet B Irradiation on Accumulation of Catechins in Tea (Camellia sinensis (L.) O. kuntze). Afr. J. Biotechnol. 2008, 7, 3283–3287. [Google Scholar] [CrossRef]
- Zagoskina, N.; Dubravina, G.A.; Alyavina, A.K.; Goncharuk, E.A. Effect of Ultraviolet (UV-B) Radiation on the Formation and Localization of Phenolic Compounds in Tea Plant Callus Cultures. Russ. J. Plant Physiol. 2003, 50, 270–275. [Google Scholar] [CrossRef]
- Shimojima, M. Biosynthesis and functions of the plant sulfolipid. Prog. Lipid Res. 2011, 50, 234–239. [Google Scholar] [CrossRef]
- Horn, P.J.; Chapman, K.D. Lipidomics in situ: Insights into plant lipid metabolism from high resolution spatial maps of metabolites. Prog. Lipid Res. 2014, 54, 32–52. [Google Scholar] [CrossRef]
- Lavell, A.A.; Benning, C. Cellular Organization and Regulation of Plant Glycerolipid Metabolism. Plant Cell Physiol. 2019, 60, 1176–1183. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Nakamura, Y.; Harwood, J. Lipids: From Chemical Structures, Biosynthesis, and Analyses to Industrial Applications. In Lipids in Plant and Algae Development; Nakamura, Y., Li-Beisson, Y., Eds.; Springer International Publishing: Basel, Switzerland, 2016; Volume 86, pp. 1–18. ISBN 978-3-319-25977-2. [Google Scholar]
- Gai, Z.; Wang, Y.; Ding, Y.; Qian, W.; Qiu, C.; Xie, H.; Sun, L.; Jiang, Z.; Ma, Q.; Wang, L.; et al. Exogenous abscisic acid induces the lipid and flavonoid metabolism of tea plants under drought stress. Sci. Rep. 2020, 10, 12275. [Google Scholar] [CrossRef]
- Li, J.; Hua, J.; Zhou, Q.; Dong, C.; Wang, J.; Deng, Y.; Yuan, H.; Jiang, Y. Comprehensive Lipidome-Wide Profiling Reveals Dynamic Changes of Tea Lipids during Manufacturing Process of Black Tea. J. Agric. Food Chem. 2017, 65, 10131–10140. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Burgos, A.; Ma, L.; Zhang, Q.; Tang, D.; Ruan, J. Lipidomics analysis unravels the effect of nitrogen fertilization on lipid metabolism in tea plant (Camellia sinensis L.). BMC Plant Biol. 2017, 17, 165. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zu, Y.; Gao, Z.; Gao, G. Physiological and Biochemical Effects of UV-B Radiation on Primula Malacoides. Acta Bot. Boreali-Occident. Sin. 2006, 26, 179–182. [Google Scholar]
- Pospíšil, P.; Yamamoto, Y. Damage to photosystem II by lipid peroxidation products. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 457–466. [Google Scholar] [CrossRef]
- Ruan, J.; Gerendás, J.; Härdter, R.; Sattelmacher, B. Effect of Nitrogen Form and Root-zone pH on Growth and Nitrogen Uptake of Tea (Camellia sinensis) Plants. Ann. Bot. 2007, 99, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Giavalisco, P.; Li, Y.; Matthes, A.; Eckhardt, A.; Hubberten, H.-M.; Hesse, H.; Segu, S.; Hummel, J.; Köhl, K.; Willmitzer, L. Elemental formula annotation of polar and lipophilic metabolites using 13C, 15N and 34S isotope labelling, in combination with high-resolution mass spectrometry. Plant J. 2011, 68, 364–376. [Google Scholar] [CrossRef]
- Bates, P.D.; Ohlrogge, J.B.; Pollard, M. Incorporation of Newly Synthesized Fatty Acids into Cytosolic Glycerolipids in Pea Leaves Occurs via Acyl Editing. J. Biol. Chem. 2007, 282, 31206–31216. [Google Scholar] [CrossRef] [Green Version]
- Bates, P.; Durrett, T.P.; Ohlrogge, J.B.; Pollard, M. Analysis of Acyl Fluxes through Multiple Pathways of Triacylglycerol Synthesis in Developing Soybean Embryos. Plant Physiol. 2009, 150, 55–72. [Google Scholar] [CrossRef] [Green Version]
- Quartacci, M.F.; Pinzino, C.; Sgherri, C.; Navari-Izzo, F. Lipid Composition and Protein Dynamics in Thylakoids of Two Wheat Cultivars Differently Sensitive to Drought. Plant Physiol. 1995, 108, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Gigon, A.; Matos, A.-R.; Laffray, D.; Zuily-Fodil, Y.; Pham-Thi, A.-T. Effect of Drought Stress on Lipid Metabolism in the Leaves of Arabidopsis thaliana (Ecotype Columbia). Ann. Bot. 2004, 94, 345–351. [Google Scholar] [CrossRef]
- Chapman, K.D.; Dyer, J.M.; Mullen, R.T. Commentary: Why don’t plant leaves get fat? Plant Sci. 2013, 207, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.M.; Vu, H. Photoinactivation of Superoxide Synthases of the Plasma Membrane from Rose (Rosa damascena Mill.) Cells. Photochem. Photobiol. 1996, 64, 106–109. [Google Scholar] [CrossRef]
- Mishra, R.K.; Singhal, G.S. Function of Photosynthetic Apparatus of Intact Wheat Leaves under High Light and Heat Stress and Its Relationship with Peroxidation of Thylakoid Lipids. Plant Physiol. 1992, 98, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Kou, M.; Gao, Z.; Liu, Y.; Xuan, Y.; Liu, Y.; Tang, Z.; Cao, Q.; Li, Z.; Sun, J. Involvement of Phosphatidylserine and Triacylglycerol in the Response of Sweet Potato Leaves to Salt Stress. Front. Plant Sci. 2019, 10, 1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry & Molecular Biology of Plants, 1st ed.; American Society of Plant Physiologists: Rockville, MD, USA, 2000; p. 1367. ISBN 7-03-012013-2. [Google Scholar]
- Theodoulou, F.L.; Eastmond, P.J. Seed storage oil catabolism: A story of give and take. Curr. Opin. Plant Biol. 2012, 15, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Testerink, C.; Munnik, T. Phosphatidic acid: A multifunctional stress signaling lipid in plants. Trends Plant Sci. 2005, 10, 368–375. [Google Scholar] [CrossRef]
- Welti, R.; Li, W.; Li, M.; Sang, Y.; Biesiada, H.; Zhou, H.E.; Rajashekar, C.B.; Williams, T.D.; Wang, X. Profiling membrane lipids in plant stress responses: Role of phospholipase Dα in freezing-induced lipid changes in arabidopsis. J. Biol. Chem. 2002, 277, 31994–32002. [Google Scholar] [CrossRef] [Green Version]
- Zien, C.A.; Wang, C.; Wang, X.; Welti, R. In vivo substrates and the contribution of the common phospholipase D, PLDα, to wound-induced metabolism of lipids in Arabidopsis. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2001, 1530, 236–248. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, Z.; Li, J.; Wang, B.; Huang, J. Effect of Water Stress on Membrane Lipid Peroxidation of Chloroplast from Wheat. J. Beijing Agric. Coll. 2003, 02, 86–88. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Huang, G.; Feng, F.; Liu, X.; Guo, R.; Gu, F.; Zhong, X.; Mei, X. Dynamic changes in membrane lipid composition of leaves of winter wheat seedlings in response to PEG-induced water stress. BMC Plant Biol. 2020, 20, 84. [Google Scholar] [CrossRef]
- Hölzl, G.; Witt, S.; Gaude, N.; Melzer, M.; Schöttler, M.A.; Dörmann, P. The Role of Diglycosyl Lipids in Photosynthesis and Membrane Lipid Homeostasis in Arabidopsis. Plant Physiol. 2009, 150, 1147–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorne, A.J.; Joyard, J.; Douce, R. Do thylakoids really contain phosphatidylcholine? Proc. Natl. Acad. Sci. USA 1990, 87, 71–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarazona, P.; Feussner, K.; Feussner, I. An enhanced plant lipidomics method based on multiplexed liquid chromatography-mass spectrometry reveals additional insights into cold- and drought-induced membrane remodeling. Plant J. 2015, 84, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Boudière, L.; Michaud, M.; Petroutsos, D.; Rébeillé, F.; Falconet, D.; Bastien, O.; Roy, S.; Finazzi, G.; Rolland, N.; Jouhet, J.; et al. Glycerolipids in photosynthesis: Composition, synthesis and trafficking. Biochim. Biophys. Acta 2014, 1837, 470–480. [Google Scholar] [CrossRef]
- Moellering, E.R.; Benning, C. Galactoglycerolipid metabolism under stress: A time for remodeling. Trends Plant Sci. 2011, 16, 98–107. [Google Scholar] [CrossRef]
- Bencze, S.; Keresztényi, E.; Veisz, O. Change in heat stress resistance in wheat due to soil nitrogen and atmospheric CO2 levels. Cereal Res. Commun. 2007, 35, 229–232. [Google Scholar] [CrossRef]
- Xu, C.; Shanklin, J. Triacylglycerol Metabolism, Function, and Accumulation in Plant Vegetative Tissues. Annu. Rev. Plant Biol. 2016, 67, 179–206. [Google Scholar] [CrossRef]




| NL | NH | |||||
|---|---|---|---|---|---|---|
| UV1/UV0 | UV2/UV0 | UV2/UV1 | UV1/UV0 | UV2/UV0 | UV2/UV1 | |
| PC | 1.01 | 0.85 | 0.84 | 0.86 | 1.31 | 1.53 * |
| LysoPC | 1.10 | 1.68 | 1.52 | 0.86 | 0.87 | 1.01 |
| PE | 1.00 | 0.82 | 0.82 | 0.73 | 2.87 * | 3.93 * |
| LysoPE | 1.11 | 1.63 | 1.46 | 0.83 | 4.08 * | 4.91 * |
| PG | 1.44 | 1.19 | 0.83 | 0.95 | 3.91 * | 4.10 * |
| LysoPG | 1.44 * | 1.14 | 0.79 | 0.83 | 0.44 * | 0.53 * |
| PI | 1.51 | 1.46 | 0.97 | 1.56 | 0.17 * | 0.11 * |
| PA | 1.11 | 2.86 | 2.59 | 0.88 | 1.25 | 1.42 |
| PS | 1.04 | 0.93 | 0.89 | 0.94 | 1.42 | 1.51 |
| MGDG | 1.41 | 1.25 | 0.89 | 1.23 | 4.42 | 3.60 |
| DGDG | 1.34 | 1.18 | 0.89 | 0.86 | 1.37 | 1.58 |
| DAG | 0.91 | 0.75 * | 0.82 * | 1.06 | 0.78 | 0.74 |
| TAG | 0.64 * | 0.65 * | 1.02 | 1.12 | 0.39 * | 0.35 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, S.; Liu, M.; Dong, F.; Yue, C.; Ruan, J.; Cao, H.; Zhang, Q. Lipidomics Analysis of Tea Leaves Cultured in Hydroponics Reveals That High Nitrogen Application Decreases Tea Plant Resistance to Ultraviolet Radiation. Horticulturae 2022, 8, 724. https://doi.org/10.3390/horticulturae8080724
Du S, Liu M, Dong F, Yue C, Ruan J, Cao H, Zhang Q. Lipidomics Analysis of Tea Leaves Cultured in Hydroponics Reveals That High Nitrogen Application Decreases Tea Plant Resistance to Ultraviolet Radiation. Horticulturae. 2022; 8(8):724. https://doi.org/10.3390/horticulturae8080724
Chicago/Turabian StyleDu, Sijia, Meiya Liu, Fang Dong, Chuan Yue, Jianyun Ruan, Hongli Cao, and Qunfeng Zhang. 2022. "Lipidomics Analysis of Tea Leaves Cultured in Hydroponics Reveals That High Nitrogen Application Decreases Tea Plant Resistance to Ultraviolet Radiation" Horticulturae 8, no. 8: 724. https://doi.org/10.3390/horticulturae8080724
APA StyleDu, S., Liu, M., Dong, F., Yue, C., Ruan, J., Cao, H., & Zhang, Q. (2022). Lipidomics Analysis of Tea Leaves Cultured in Hydroponics Reveals That High Nitrogen Application Decreases Tea Plant Resistance to Ultraviolet Radiation. Horticulturae, 8(8), 724. https://doi.org/10.3390/horticulturae8080724








