From Laboratory to Field: The Effect of Controlling Oscillations in Temperature on the Growth of Crops
Abstract
:1. Introduction
2. Materials and Methods
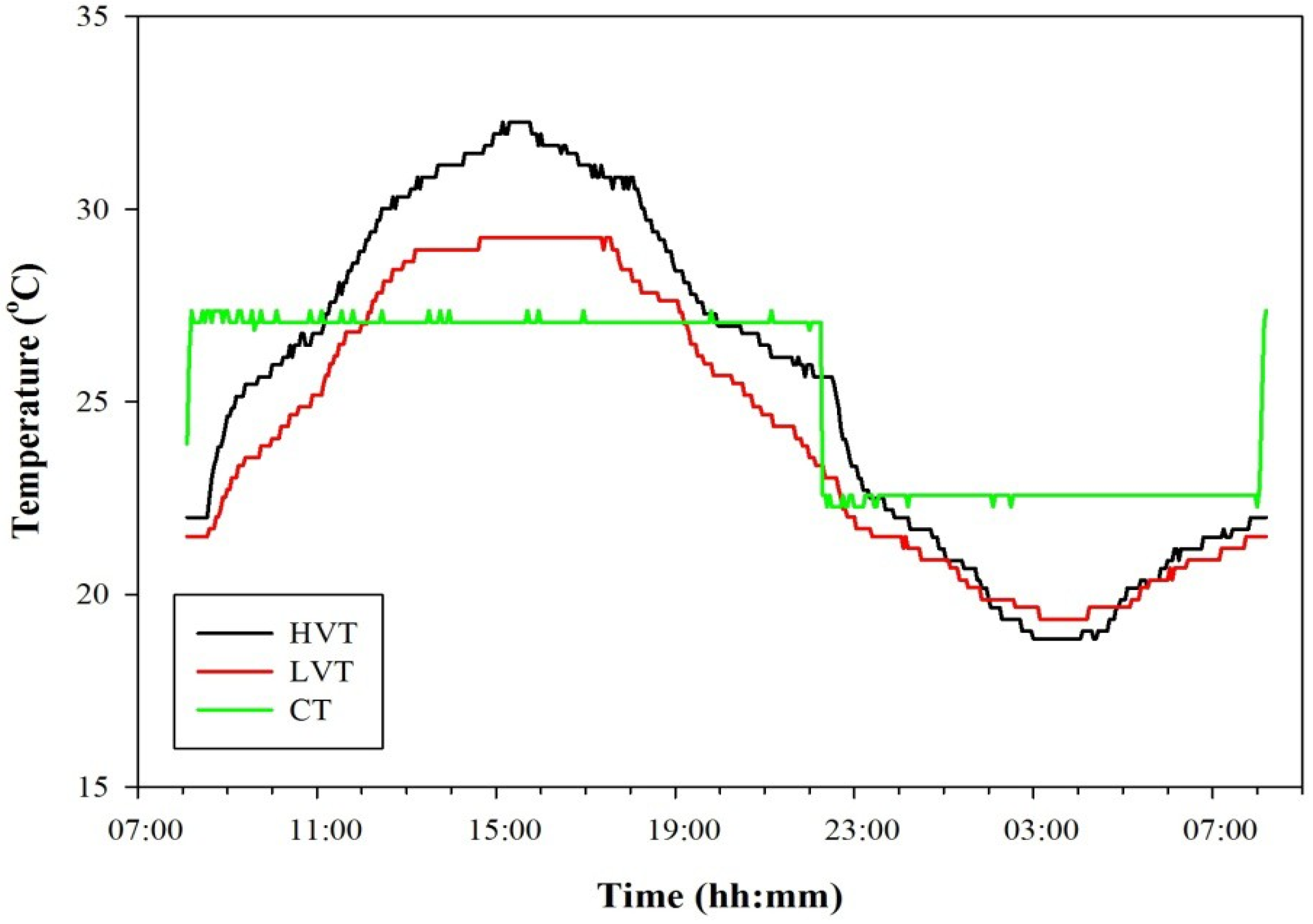
2.1. Growth Chamber with Oscillating Temperature
2.2. Testing Procedure
2.3. Specifications of the Equipment
2.3.1. Electronic Balance (BX 320H, SHIMADZU, Kyoto, Japan)
2.3.2. Leaf Area Meter (LI-3000A, LI-COR Inc., Lincoln, NE, USA)
2.3.3. Chlorophyll Meter (SPAD-502, MINOLTA, Osaka, Japan)
2.3.4. Digital Vernier Caliper (CARMA, Taipei, Taiwan)
2.3.5. Brix Meter (Pocket Refractometer PAL-1, ATAGO, Tokyo, Japan)
2.3.6. Soil Water Content Meter (WET 150 Meter (Delta-T Devices, UK)
2.4. Statistical Methods
3. Results
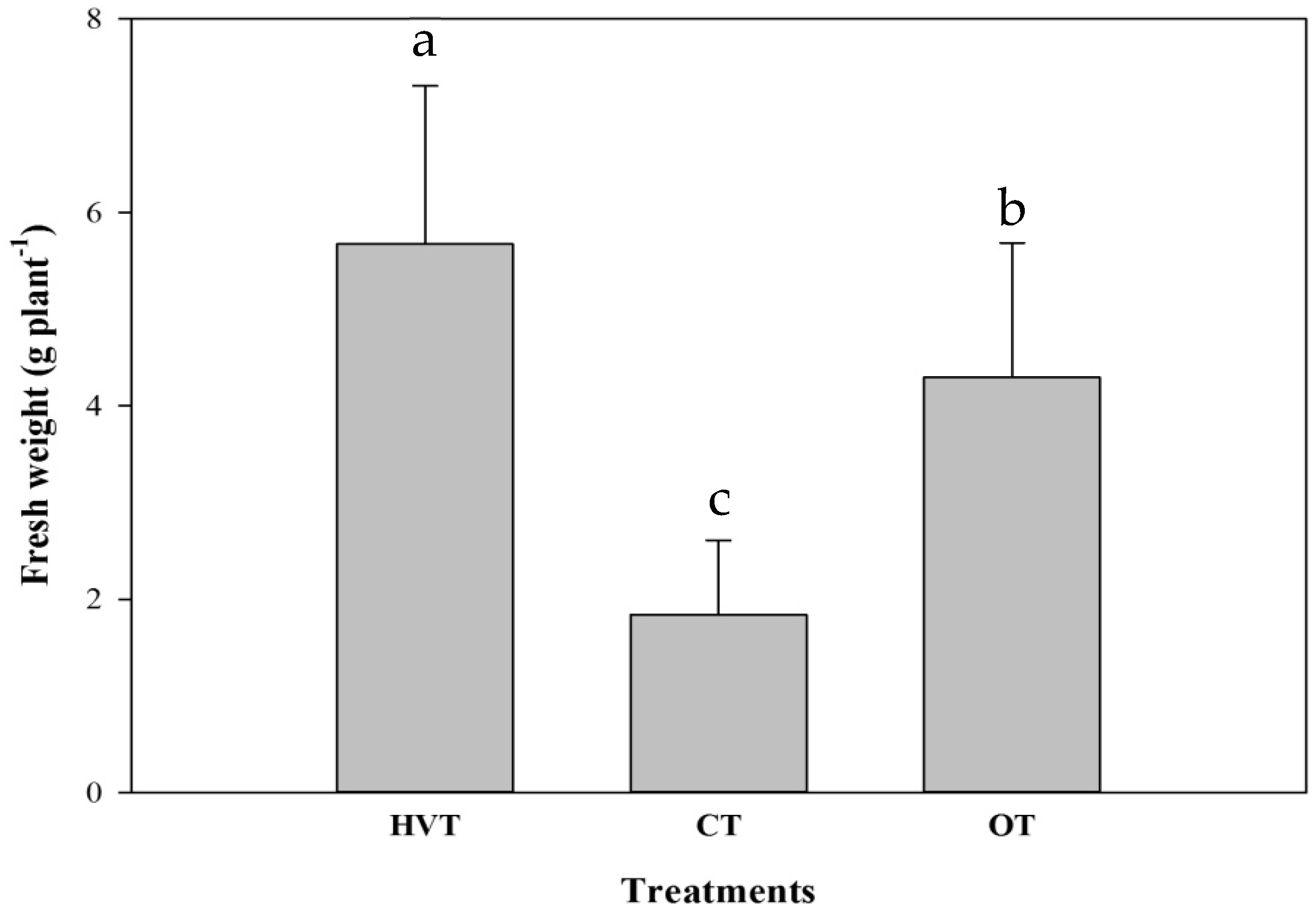
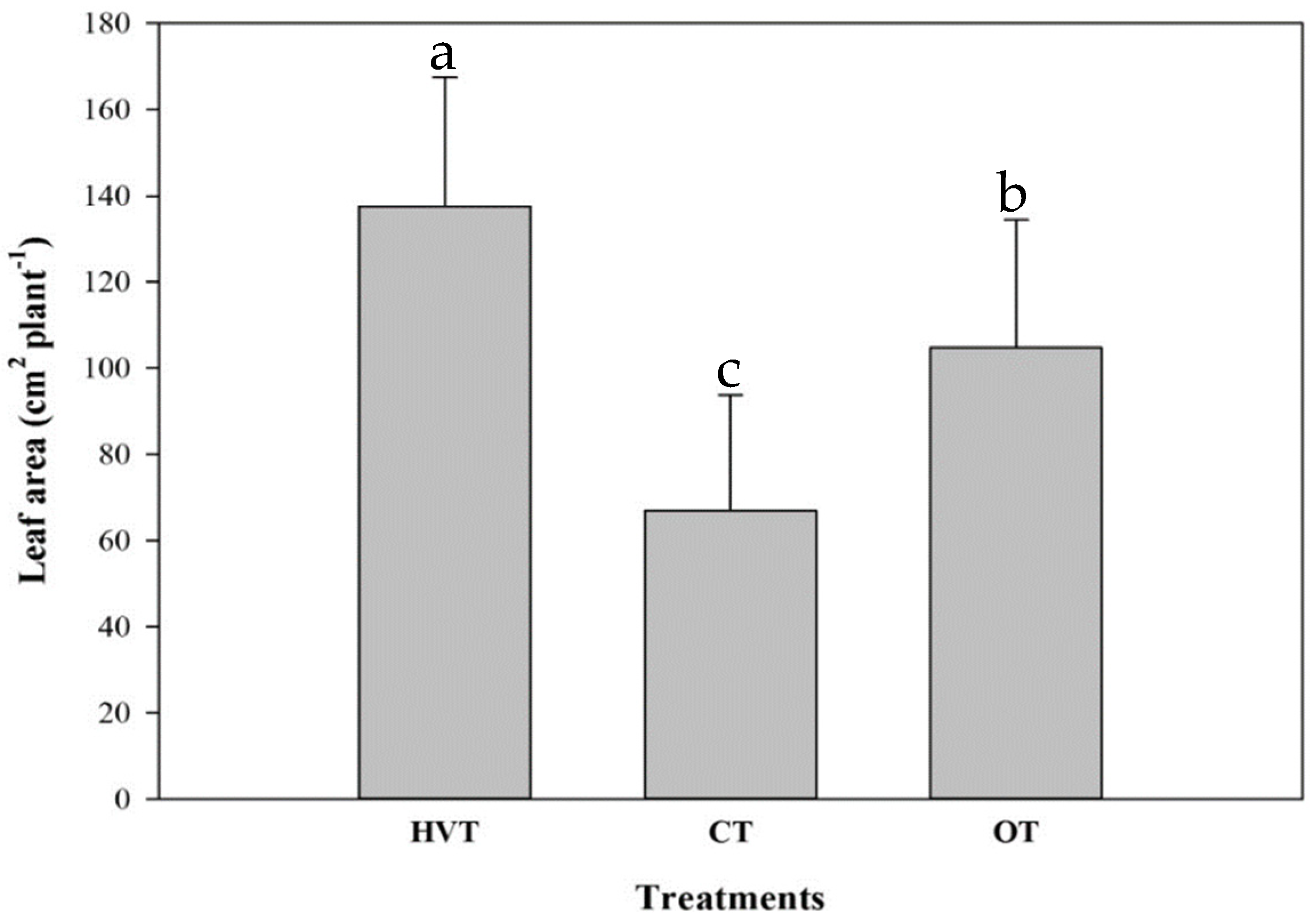
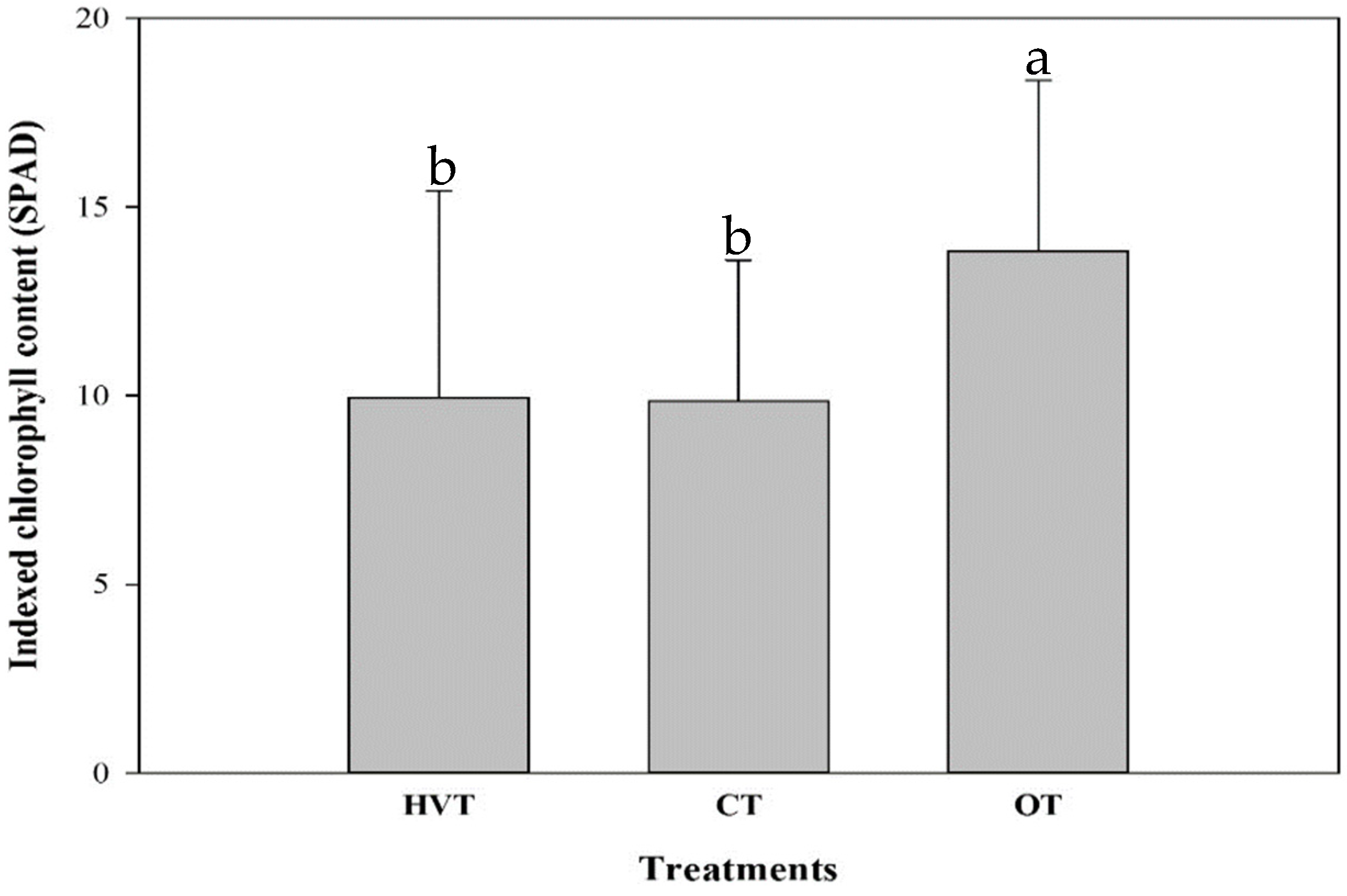
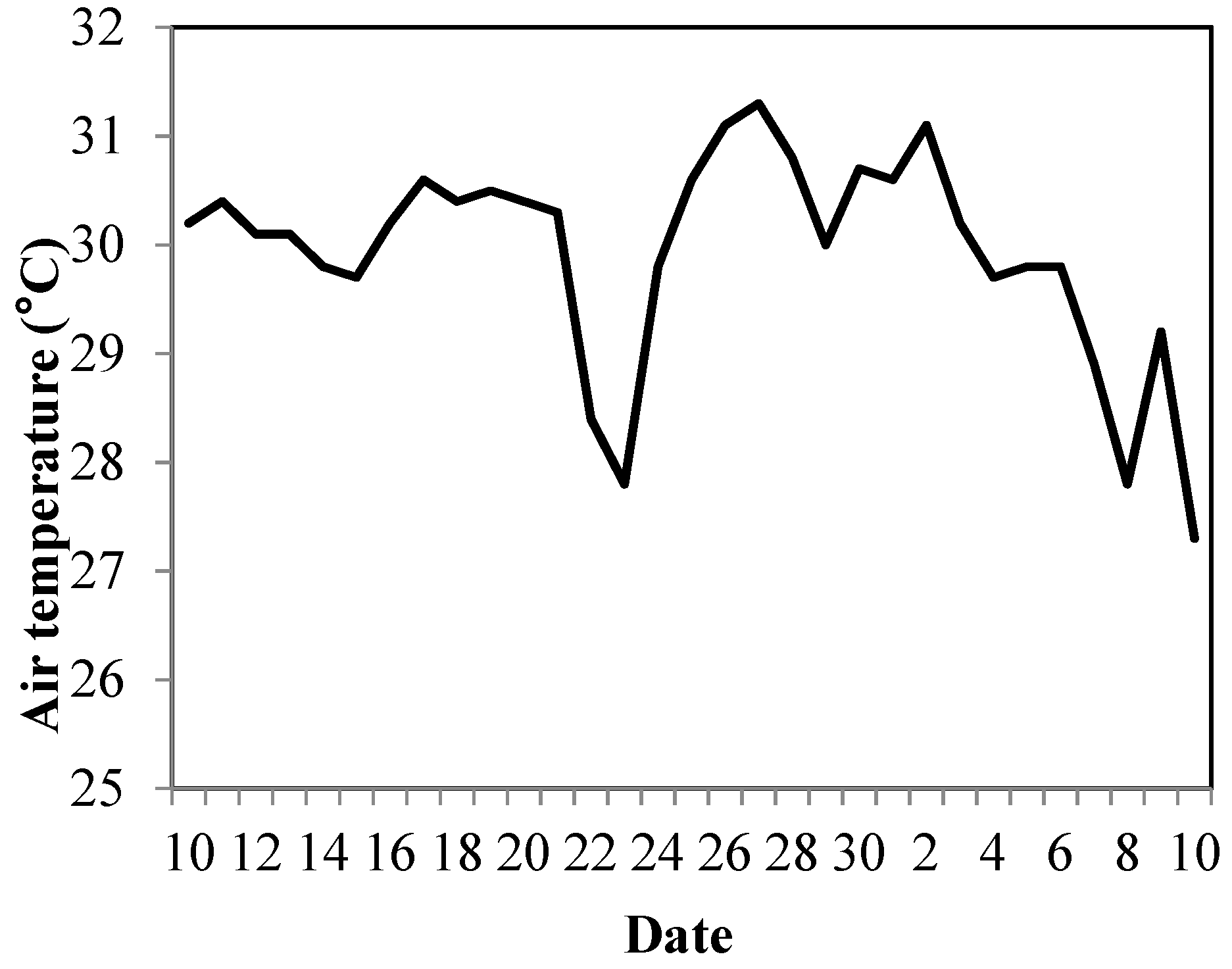
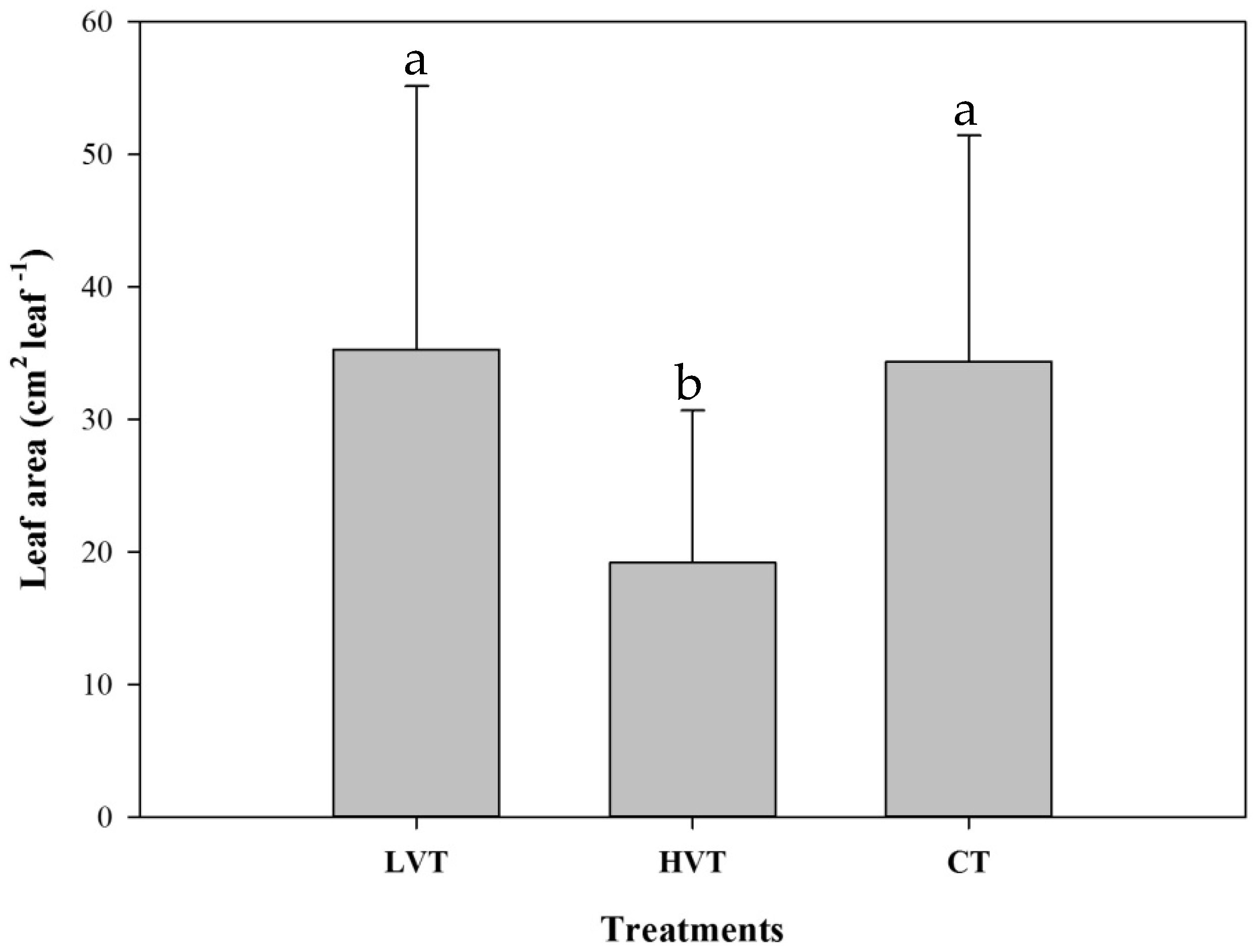
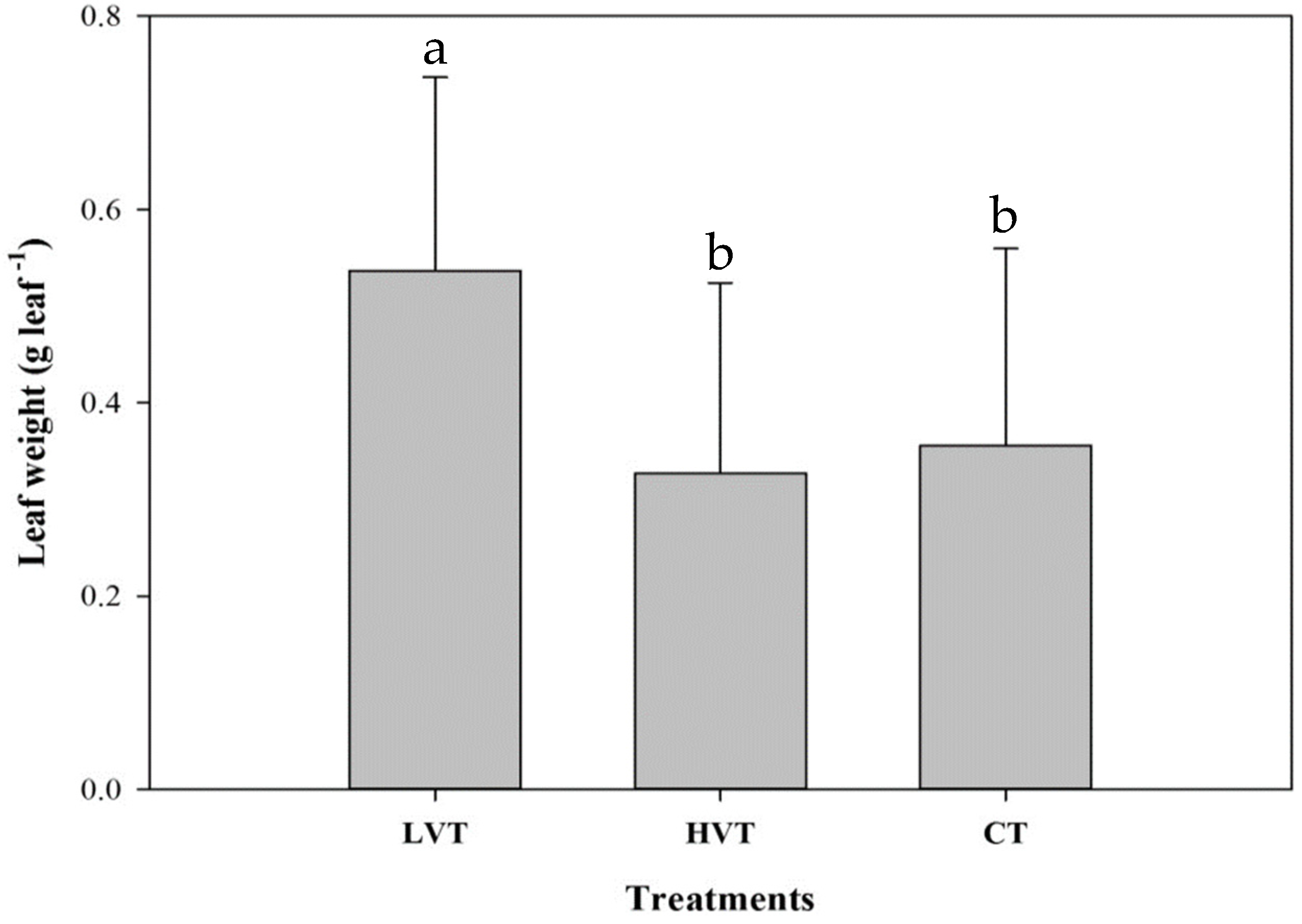
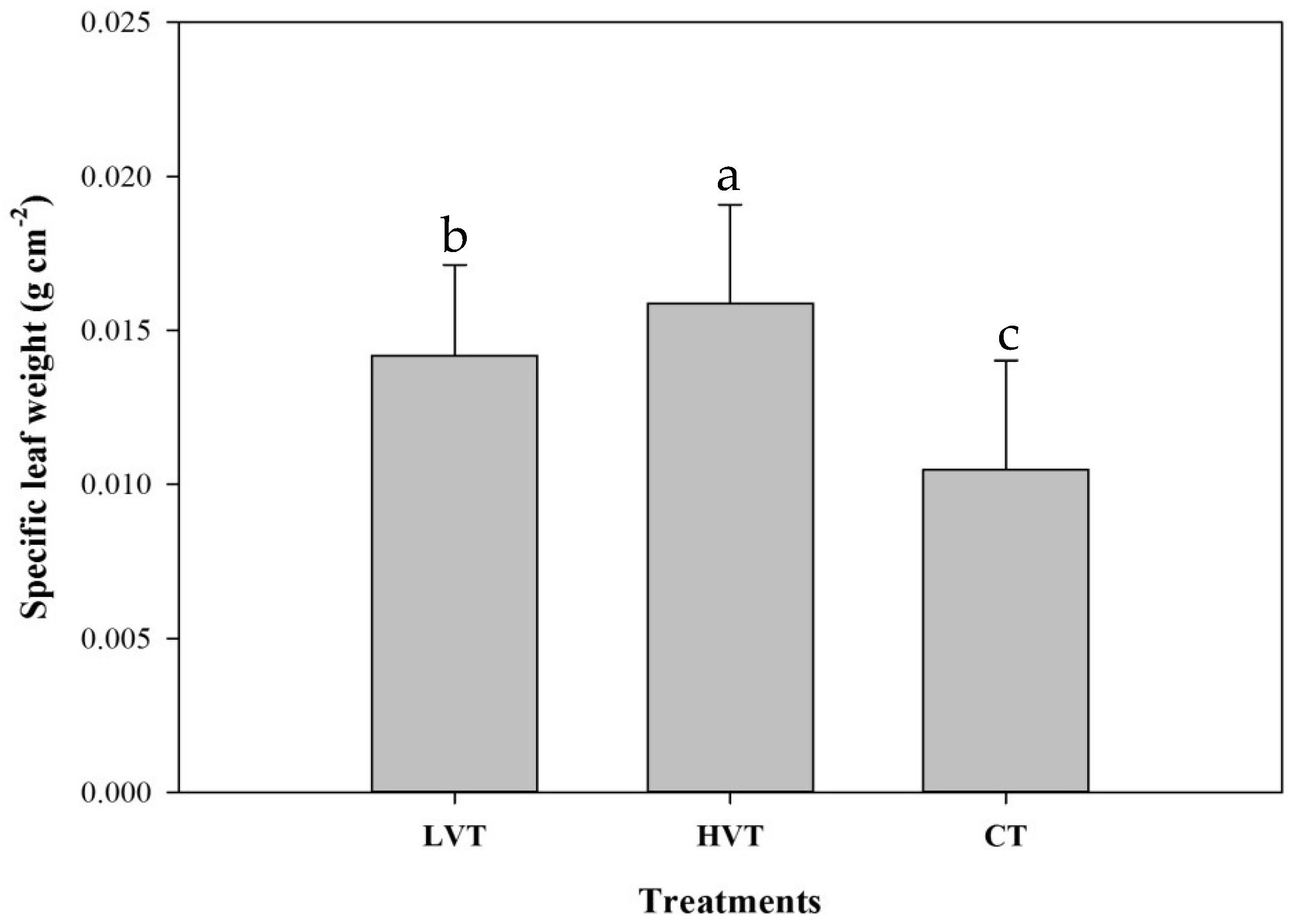
3.1. Pak Choi
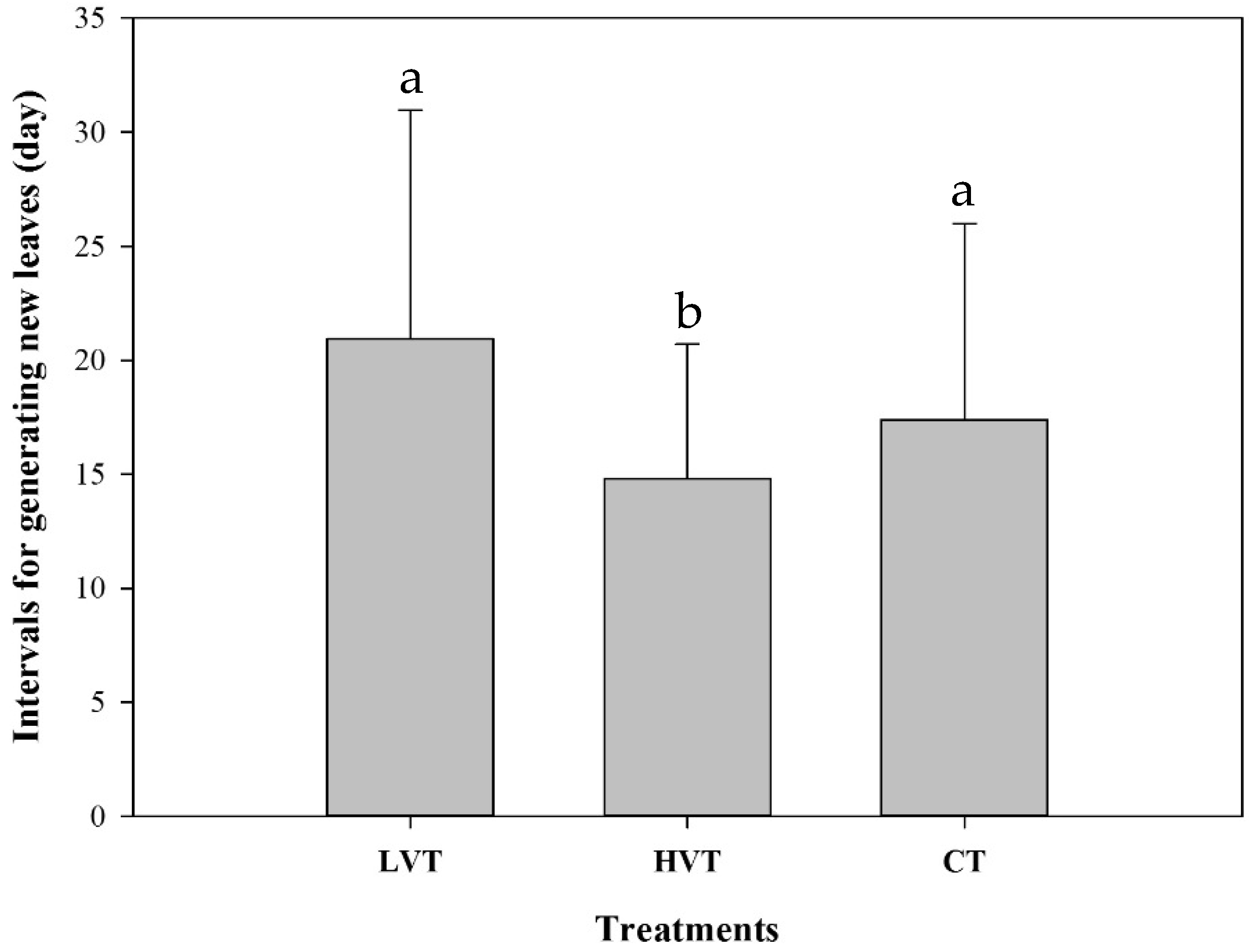
3.2. Strawberry
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall, D.O.; Scurlock, J.M.O.; Bolhar-Nordenkampf, H.R.; Leegood, R.C.; Long, S.P. Photosynthesis and Production. In A Changing Environment: A Field and Laboratory Manual, 1st ed.; Chapman & Hall, Inc.: London, UK, 1993. [Google Scholar]
- Field, C.; Berry, J.A.; Mooney, H.A. A portable system for measuring carbon dioxide and water vapour exchange of leaves. Plant Cell Environ. 1982, 5, 179–186. [Google Scholar] [CrossRef]
- Refinetti, R. Circadian Physiology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Associates, Inc.: Sunderland, UK, 2010. [Google Scholar]
- Lawlor, D.W.; Fock, D.H. Photosynthesis, respiration, and carbon assimilation in water-stressed maize at two oxygen concentrations. J. Exp. Bot. 1978, 29, 579–593. [Google Scholar] [CrossRef]
- Turpin, D.H.; Elrifi, I.R.; Birch, D.G.; Weger, H.G.; Holmes, J.J. Interactions between photosynthesis, respiration, and nitrogen assimilation in microalgae. Can. J. Bot. 1988, 66, 2083–2097. [Google Scholar] [CrossRef]
- Hennessey, T.L.; Field, C.B. Circadian rhythms in photosynthesis: Oscillations in carbon assimilation and stomatal conductance under constant Conditions. Plant Physio. 1991, 96, 831–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernacchi, C.J.; Pimentel, C.; Long, S.P. In vivo temperature response functions of parameters required to model RuBP-limited photosynthesis. Plant Cell Environ. 2003, 26, 1419–1430. [Google Scholar] [CrossRef]
- Inayama, M.; Murakami, T. Physiological studies on optimal environment in growing of the vegetable crops under the g1ass- and plastic-houses. l. Temperature analysis for growing cucumber. Bull. Chiba-Ken Agric. Exp. Stn. 1970, 10, 62–72. [Google Scholar]
- Inayama, M.; Murakami, T. Physiological studies on optimal environment in growing of the vegetable crops under the g1ass- and plastic-houses II. Effect of light experience on the management of day and night temperature in fruit vegetables. Bull. Chiba-Ken Agric. Exp. Stn. 1975, 16, 31–42. [Google Scholar]
- Gagnon, S.; Dansereau, B. Temperature and duration of pretreatment effects on growth and development of Geraniums. HortScience 1991, 27, 216–217. [Google Scholar] [CrossRef]
- Nkansah, G.O.; Ito, T. Effect of air and root-zone temperatures on physiological characteristics and yield of heat-tolerant and non heat-tolerant tomato cultivars. J. Jpn. Soc. Hortic. Sci. 1995, 64, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Dodd, A.; Salathia, N.; Hall, A.; Kévei, E.; Tóth, R.; Nagy, F.; Hibberd, J.M.; Millar, A.J.; Webb, A.A.R. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 2005, 309, 630–633. [Google Scholar] [CrossRef] [Green Version]
- Fredeen, A.L.; Hennessey, T.L.; Field, C.B. Biochemical correlates of the circadian rhythm in photosynthesis in Phaseolus vulgaris. Plant Physiol. 1991, 97, 415–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkins, M.B. Circadian rhythms: Their origin and control. New Phytol. 1992, 121, 347–375. [Google Scholar] [CrossRef]
- Webb, A.A.R. The physiology of circadian rhythms in plants. New Phytol. 2003, 160, 281–303. [Google Scholar] [CrossRef] [Green Version]
- McClung, C.R. Circadian rhythms in plant: A millennial view. Physiol. Plant. 2000, 109, 359–371. [Google Scholar] [CrossRef] [Green Version]
- Jones, H.G. Plants and Microclimate a Quantitative Approach to Environmental Plant Physiology, 3rd ed.; Cambridge University Press: Cambridge, UK, 2013; pp. 167–171. [Google Scholar]
- Yan, W.; Hunt, L.A. An equation for modelling the temperature response of plants using only the cardinal temperatures. Ann. Bot. 1999, 84, 607–614. [Google Scholar] [CrossRef] [Green Version]
- Gent, M.P.N.; Enoch, H.Z. Temperature dependence of vegetative growth and dark respiration: A mathematical model. Plant Physio. 1983, 71, 562–567. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Xue, L.; Gu, W.; Yang, C.; Wang, S.; Ling, Q.; Qin, X.; Ding, Y. Comparison of yield components and plant type characteristics of high-yield rice between Taoyuan, a ‘special eco-site’ and Nanjing, China. Field Crops Res. 2009, 112, 214–221. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Kurepin, L.V.; Reid, D.M. Effects of temperature and watering regime on growth, gas exchange and abscisic acid content of canola (Brassica napus) seedlings. Environ. Exp. Bot. 2012, 75, 107–113. [Google Scholar] [CrossRef]
- Wassner, D.F.; Ravetta, D.A. Temperature effects on leaf properties, resin content, and composition in Grindelia chiloensis (Asteraceae). Ind. Crops Prod. 2005, 21, 155–163. [Google Scholar] [CrossRef]
- Somers, D.E.; Devlin, P.F.; Kay, S.A. Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science 1998, 282, 1488–1490. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xu, B.; Wu, T.; Yang, Y.; Fan, L.; Wen, M.; Sui, J. Transcriptomic profiling of two Pak Choi varieties with contrasting anthocyanin contents provides an insight into structural and regulatory genes in anthocyanin biosynthetic pathway. BMC Genom. 2017, 18, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Xu, B.; Wu, T.; Wen, M.X.; Fan, L.X.; Feng, Z.Z.; Paoletti, E. Transcriptomic analysis of Pak Choi under acute ozone exposure revealed regulatory mechanism against ozone stress. BMC Plant Boil. 2017, 17, 236. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Zhu, Z.B.; Chen, J.H.; Huang, Y.F.; Liu, Z.L.; Zou, J.W.; Cui, J. Transcriptome analysis revealed pivotal transporters involved in the reduction of cadmium accumulation in pak choi (Brassica chinensis L.) by exogenous hydrogen-rich water. Chemosphere 2019, 216, 684–697. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Song, X.; Lyu, S.; Ren, Y.; Liu, T.; Hou, X.; Zhang, C. Integrated analysis of Hi-C and RNA-Seq reveals the molecular mechanism of autopolyploid growth advantages in Pak Choi (Brassica rapa ssp. chinensis). Front. Plant Sci. 2022, 13, 905202. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, K.; Liang, Z.; Zhu, Z.; Yang, J. Transcriptome analysis of glutathione response: RNA-Seq provides insights into balance between antioxidant response and glucosinolate metabolism. Antioxidants 2022, 11, 1322. [Google Scholar] [CrossRef]
- Dodd, A.; Kusakina, N.; Hall, J.A.; Gould, P.D.; Hanaoka, M. The circadian regulation of photosynthesis. Photosynth. Res. 2014, 119, 181–190. [Google Scholar] [CrossRef]



| Treatments | |||
|---|---|---|---|
| Parts | Fresh Weight (g) | ||
| HVT 1 | CT 2 | OT 3 | |
| Shoot | 5.35 ± 1.51 a | 1.78 ± 0.75 c | 3.62 ± 1.15 b |
| Root | 0.32 ± 0.16 b | 0.17 ± 0.03 c | 0.67 ± 0.27 a |
| |||
| |||
| |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-W.; Chen, C. From Laboratory to Field: The Effect of Controlling Oscillations in Temperature on the Growth of Crops. Horticulturae 2022, 8, 708. https://doi.org/10.3390/horticulturae8080708
Wang J-W, Chen C. From Laboratory to Field: The Effect of Controlling Oscillations in Temperature on the Growth of Crops. Horticulturae. 2022; 8(8):708. https://doi.org/10.3390/horticulturae8080708
Chicago/Turabian StyleWang, Jhih-Wei, and Chiachung Chen. 2022. "From Laboratory to Field: The Effect of Controlling Oscillations in Temperature on the Growth of Crops" Horticulturae 8, no. 8: 708. https://doi.org/10.3390/horticulturae8080708
APA StyleWang, J.-W., & Chen, C. (2022). From Laboratory to Field: The Effect of Controlling Oscillations in Temperature on the Growth of Crops. Horticulturae, 8(8), 708. https://doi.org/10.3390/horticulturae8080708






