Effects of Passive Modified Atmosphere Packaging on Physico-Chemical Traits and Antioxidant Systems of ‘Dottato’ Fresh Fig
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Samples and Experimental Design
2.2. Physico-Chemical Traits
2.3. Sugar and Acid Analysis
2.4. Bioactive Compounds and Antioxidant Activity
2.5. Enzyme Extraction and Activity Assays
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Traits
3.2. Bioactive Compounds and Antioxidant Activity
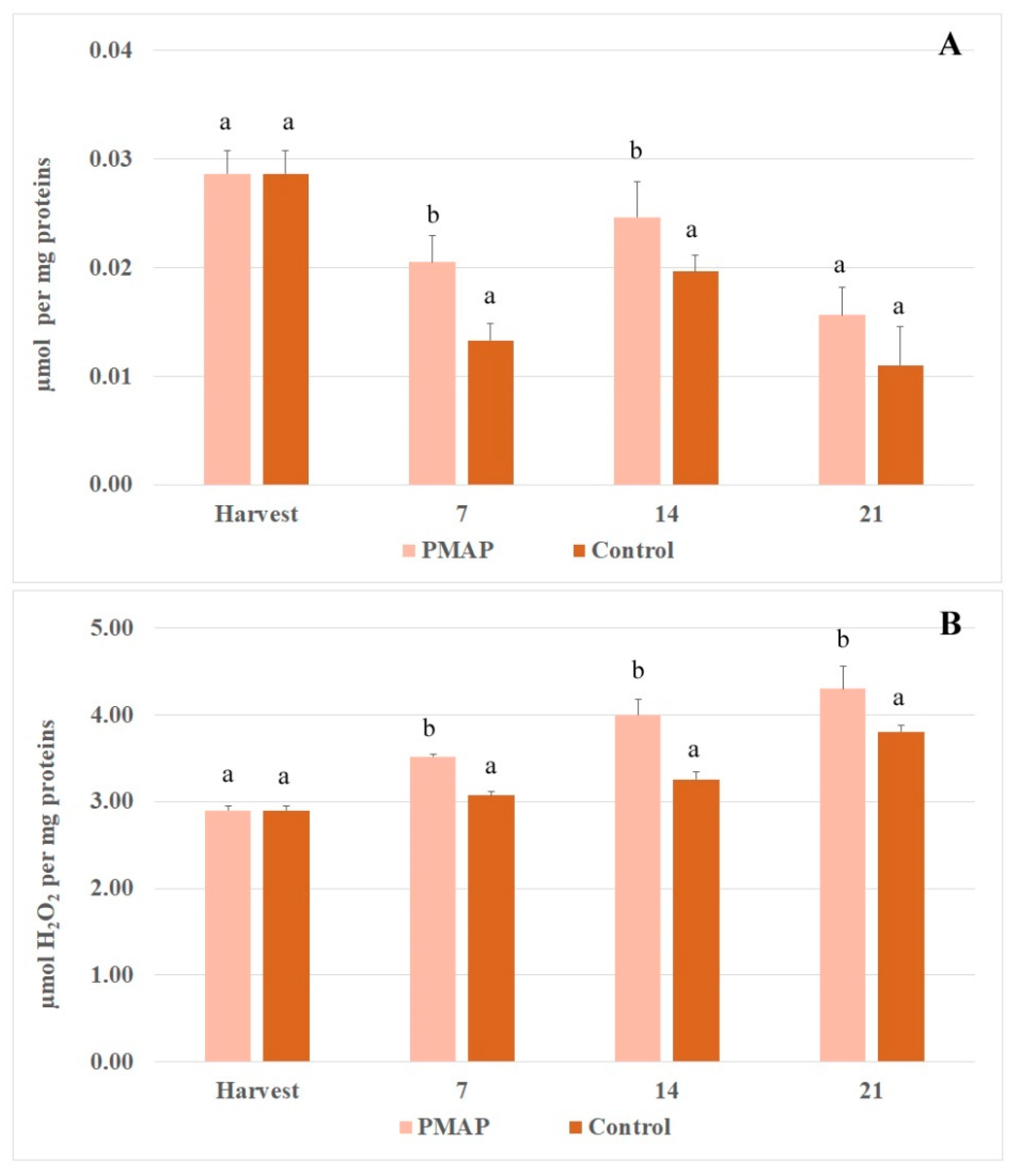
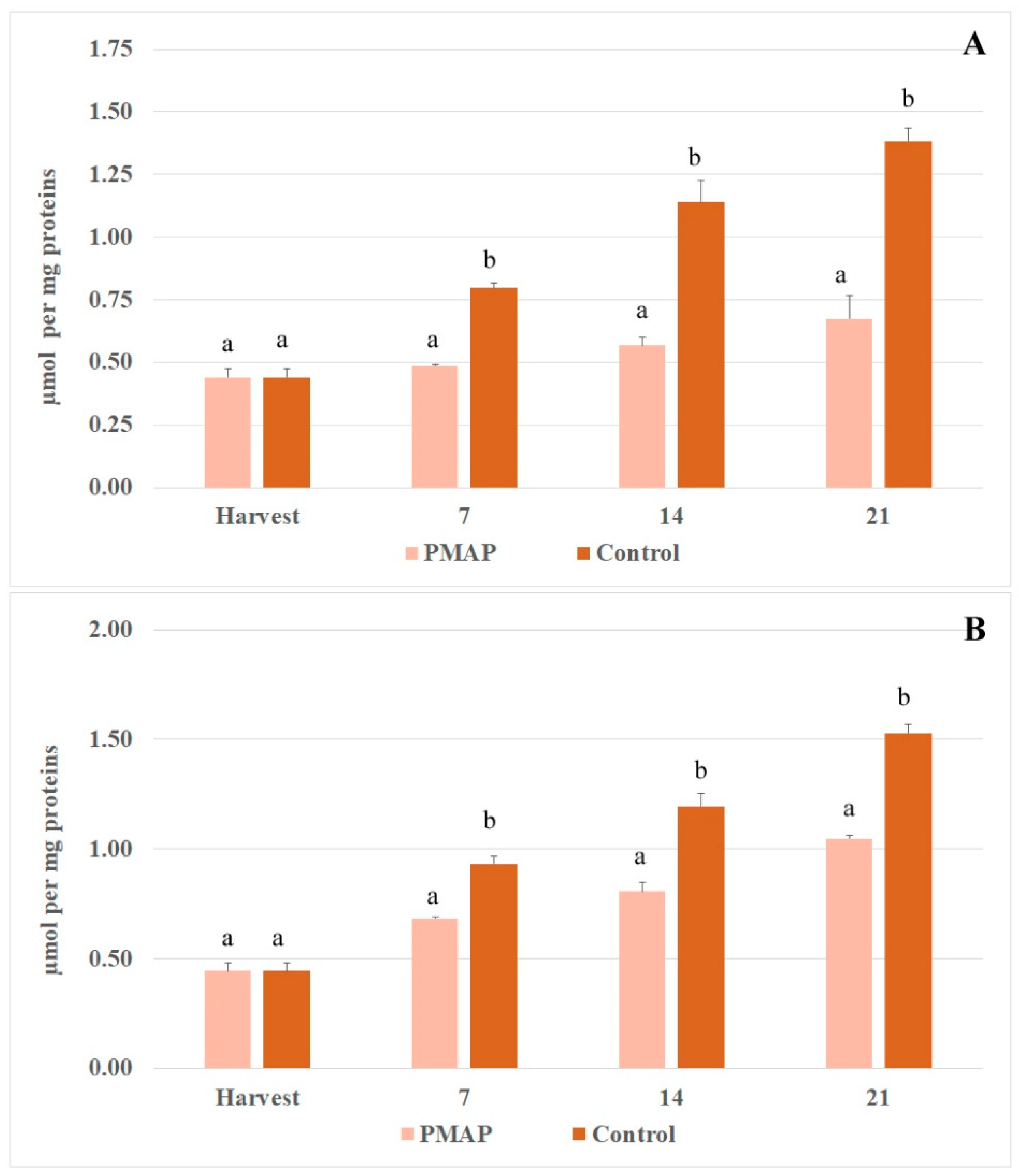
3.3. Enzymatic Antioxidant System and Enzymatic Browning
3.4. Response of Multivariate Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stover, E.W.; Aradhya, M.K.; Crisosto, C.; Ferguson, L. The fig: Overview of an ancient fruit. HortScience 2007, 42, 1083–1087. [Google Scholar] [CrossRef]
- Russo, F.; Caporaso, N.; Paduano, A.; Sacchi, R. Phenolic Compounds in Fresh and Dried Figs from Cilento (Italy), by Considering Breba Crop and Full Crop, in Comparison to Turkish and Greek Dried Figs. J. Food Sci. 2014, 79, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burfield, T. Burfield Large Fig Crop Expected. 2020. Available online: https://www.thepacker.com/markets/marketing-news/produce-crops/large-fig-crop-expected (accessed on 14 June 2022).
- Crisosto, C.H.; Bremer, V.; Ferguson, L.; Crisosto, G.M. Evaluating quality attributes of four fresh fig (Ficus carica L.) cultivars harvested at two maturity stages. HortScience 2010, 45, 707–710. [Google Scholar] [CrossRef] [Green Version]
- Yemis, O.; Bakkalbas, E.; Artık, N. Changes in pigment profile and surface colour of fig (Ficus carica L.) during drying. Int. J. Food Sci. 2012, 47, 1710–1719. [Google Scholar] [CrossRef]
- Solomon, A.; Golubowicz, S.; Yablowicz, Z.; Grossman, S.; Bergman, M.; Gottlieb, H.; Altman, A.; Kerem, Z.; Flaishman, M.A. Antioxidant activities and anthocyanin content of fresh fruit of common fig (Ficus carica L.). J. Agric. Food Chem. 2006, 54, 7717–7723. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, O.; Polat, A.A. Phytochemical and antioxidant properties of selected fig (Ficus carica L.) accessions from the eastern Mediterranean region of Turkey. Sci. Hortic. 2011, 128, 473–478. [Google Scholar] [CrossRef]
- Caliskan, O. Mediterranean Figs (Ficus carica L.) Functional Food Properties. In The Mediterranean Diet: An Evidence-Based Approach, 1st ed.; Preedy, V.R., Watson, R.R., Eds.; Academic Press: London, UK, 2015; pp. 629–637. [Google Scholar]
- Kong, M.; Lampinen, B.; Shackel, K.; Crisosto, C.H. Fruit skin side cracking and ostiole end splitting shorten postharvest life in fresh figs (Ficus carica L.), but are reduced by deficit irrigation. Postharvest Biol. Technol. 2013, 85, 154–161. [Google Scholar] [CrossRef]
- Afsah-Hejri, L.; Toudeshki, A.; Homayouni, T.; Mehrazi, S.; Pareh, A.G.; Gordon, P.; Ehsani, R. Potential of ozonated-air (OA) application to reduce the weight and volume loss in fresh figs (Ficus carica L.). Postharvest Biol. Technol. 2021, 180, 111631. [Google Scholar] [CrossRef]
- Bouzo, C.A.; Travaercicdelo, M.; Gariglio, N.F. Effect of different packaging materials on postharvest quality of fresh fig fruit. Int. J. Agric. Biol. 2012, 14, 821–825. [Google Scholar]
- Allegra, A.; Gallotta, A.; Carimi, F.; Mercati, F.; Inglese, P.; Martinelli, F. Metabolic profiling and post-harvest behavior of “Dottato” fig (Ficus carica L.) fruit covered with an edible coating from O. ficus-indica. Front. Plant Sci. 2018, 9, 1321. [Google Scholar] [CrossRef]
- Allegra, A.; Sortino, G.; Inglese, P.; Settanni, L.; Todaro, A.; Gallotta, A. The effectiveness of Opuntia ficus-indica mucilage edible coating on postharvest maintenance of ‘Dottato’ fig (Ficus carica L.) fruit. Food Packag. Shelf Life 2017, 12, 135–141. [Google Scholar] [CrossRef]
- Adiletta, G.; Zampella, L.; Coletta, C.; Petriccione, M. Chitosan coating to preserve the qualitative traits and improve antioxidant system in fresh figs (Ficus carica L.). Agriculture 2019, 9, 84. [Google Scholar] [CrossRef] [Green Version]
- Hamanaka, D.; Norimura, N.; Baba, N.; Mano, K.; Kakiuchi, M.; Tanaka, F.; Uchino, T. Surface decontamination of fig fruit by combination of infrared radiation heating with ultraviolet irradiation. Food Control 2011, 22, 375–380. [Google Scholar] [CrossRef]
- Song, C.; Li, A.; Chai, Y.; Li, Q.; Lin, Q.; Duan, Y. Effects of 1-Methylcyclopropene Combined with Modified Atmosphere on Quality of Fig (Ficus carica L.) during Postharvest Storage. J. Food Qual. 2019, 2019, 2134924. [Google Scholar] [CrossRef] [Green Version]
- Crisosto, C.H.Y.; Kader, A.A. Figs. Postharvest Quality Maintenance Guidelines Postharvest Information for fruit and Nuts. 2007. Available online: http://www.uckac.edu/postharv (accessed on 14 June 2022).
- Tsantili, E.; Paraskos, G.; Pontikis, C. Storage of fresh figs in low oxygen atmosphere. Hort. Sci. Biotechnol. 2003, 78, 56–60. [Google Scholar] [CrossRef]
- Villalobos, M.C.; Serradilla, M.J.; Martín, A.; Ruiz-Moyano, S.; Pereira, C.; Córdoba, M.G. Use of equilibrium modified atmosphere packaging for preservation of ‘San Antonio’ and ‘Banane’ breba crops (Ficus carica L.). Postharvest Biol. Technol. 2014, 98, 14–22. [Google Scholar] [CrossRef]
- Villalobos, M.C.; Serradilla, M.J.; Martín, A.; Ruiz-Moyano, S.; Pereira, C.; Córdoba, M.G. Synergism of defatted soybean meal extract and modified atmosphere packaging to preserve the quality of figs (Ficus carica L.). Postharvest Biol. Technol. 2016, 111, 264–273. [Google Scholar] [CrossRef]
- Adiletta, G.; Petriccione, M.; Liguori, L.; Pizzolongo, F.; Romano, R.; Di Matteo, M. Study of pomological traits and physico-chemical quality of pomegranate (Punica granatum L.) genotypes grown in Italy. Eur. Food Res. Technol. 2018, 244, 1427–1438. [Google Scholar] [CrossRef]
- Adiletta, G.; Senadeera, W.; Liguori, L.; Crescitelli, A.; Albanese, D.; Russo, P. The Influence of Abrasive Pretreatment on Hot Air Drying of Grape. Nutr. Food Sci. 2015, 6, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Adiletta, G.; Di Matteo, M.; Albanese, D.; Farina, V.; Cinquanta, L.; Corona, O.; Magri, A.; Petriccione, M. Changes in physico-chemical traits and enzymes oxidative system during cold storage of ‘Formosa’ papaya fresh cut fruits grown in the Mediterranean area (Sicily). Ital. J. Food. Sci. 2020, 32, 845–857. [Google Scholar]
- Bradford, M.M. A dye binding assay for protein. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Pasquariello, M.S.; Di Patre, D.; Mastrobuoni, F.; Zampella, L.; Scortichini, M.; Petriccione, M. Influence of postharvest chitosan treatment on enzymatic browning and antioxidant enzyme activity in sweet cherry fruit. Postharvest Biol. Technol. 2015, 109, 45–56. [Google Scholar] [CrossRef]
- Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.S.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of chitosan coating on the postharvest quality and antioxidant enzyme system response of strawberry fruit during cold storage. Foods 2015, 4, 501–523. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Li, D.; Xu, W.; Fu, Y.; Liao, R.; Shi, J.; Chen, Y. Application of passive modified atmosphere packaging in the preservation of sweet corns at ambient temperature. LWT 2021, 136, 110295. [Google Scholar] [CrossRef]
- Dogan, A. Effects of different oxygen levels with high-carbon dioxide atmosphere on postharvest quality of fresh fig under palliflex storage systems. Horticulturae 2022, 8, 353. [Google Scholar] [CrossRef]
- Belay, Z.A.; Caleb, O.J.; Opara, U.L. Influence of initial gas modification on physicochemical quality attributes and molecular changes in fresh and fresh-cut fruit during modified atmosphere packaging. Food Packag. Shelf Life 2019, 21, 100359. [Google Scholar] [CrossRef]
- Allegra, A.; Sortino, G.; Miciletta, G.; Riotto, M.; Fasciana, T.; Inglese, P. The influence of harvest period and fruit ripeness at harvest on minimally processed cactus pears (Opuntia ficus-indica L. Mill.) stored under passive atmosphere. Postharvest Biol. Technol. 2015, 104, 57–62. [Google Scholar] [CrossRef]
- Chen, J.; Du, J.; Ge, Z.Z.; Zhu, W.; Nie, R.; Li, C.M. Comparison of sensory and compositions of five selected persimmon cultivars (Diospyros kaki L.) and correlations between chemical components and processing characteristics. J. Food Sci. Technol. 2016, 53, 1597–1607. [Google Scholar] [CrossRef] [Green Version]
- Ayhan, Z.; Kara Cay, E. Preservation of the ‘Bursa siyahı’ fresh fig under modified atmosphere packaging (MAP) and cold storage. Int. J. Agric. Sci. 2011, 1, 1–9. [Google Scholar]
- Díaz-Mula, H.M.; Martínez-Romero, D.; Castillo, S.; Serrano, M.; Valero, D. Modified atmosphere packaging of yellow and purple plum cultivars.1. Effect on organoleptic quality. Postharvest Biol. Technol. 2011, 6, 103–109. [Google Scholar] [CrossRef]
- Durán-Soria, S.; Pott, D.M.; Osorio, S.; Vallarino, J.G. Sugar Signaling During Fruit Ripening. Front Plant Sci. 2020, 11, 564917. [Google Scholar] [CrossRef]
- Aljane, F.; Neily, M.H.; Msaddak, A. Phytochemical Characteristics and Antioxidant Activity of Several Fig (Ficus carica L.) Ecotypes. Ital. J. Food Sci. 2020, 32, 755–768. [Google Scholar]
- Ma, J.; Li, D.; Yang, D.; Xu, W.; Fu, Y.; Liao, R.; Shi, J.; Wang, J.; Wang, Y.; He, X. Effects of packaging designs with multiple pieces of function films on the quality of figs stored at ambient temperature. Sci. Hortic. 2019, 251, 32–38. [Google Scholar] [CrossRef]
- Brizzolara, S.; Manganaris, G.A.; Fotopoulos, V.; Watkins, C.B.; Tonutti, P. Primary metabolism in fresh fruit during storage. Front. Plant Sci. 2020, 11, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Shao, X.F.; Gong, Y.F.; Zhu, Y.; Wang, H.F.; Zhang, X.L.; Yu, D.; Yu, F.; Qiu, Z.; Lu, H. The metabolism of soluble carbohydrates related to chilling injury in peach fruit exposed to cold stress. Postharvest Biol. Technol. 2013, 86, 53–61. [Google Scholar] [CrossRef]
- Wang, L.; Shan, T.M.; Xie, B.; Ling, C.; Shao, S.; Jin, P.; Zheng, Y. Glycine betaine reduces chilling injury in peach fruit by enhancing phenolic and sugar metabolisms. Food Chem. 2019, 272, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Pande, G.; Akoh, C.C. Organic acids, antioxidant capacity, phenolic content and lipid characterisation of Georgia-grown underutilized fruit crops. Food Chem 2010, 120, 1067–1075. [Google Scholar] [CrossRef]
- Palmeira, L.; Pereira, C.; Dias, M.I.; Abreu, R.M.V.; Corrêa, R.C.G.; Pires, T.C.S.P.; Ferreira, I.C.F.R. Nutritional, chemical and bioactive profiles of different parts of a Portuguese common fig (Ficus carica L.) variety. Food Res. Int. 2019, 108572. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.P.; Valentão, P.; Pereira, J.A.; Silva, B.M.; Tavares, F.; Andrade, P.B. Ficus carica L.: Metabolic and biological screening. Food Chem. Toxicol. 2009, 47, 2841–2846. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Silva, R.L.; Andrade, P.B.; Valentão, P.; Silva, B.M.; Pereira, J.A.; de Pinho, D.G. Determination of low molecular weight volatiles in Ficus carica using HSSPME and GC/FID. Food Chem. 2010, 121, 1289–1295. [Google Scholar] [CrossRef]
- Islam, A.; Acıkalın, R.; Ozturk, B.; Aglar, E.; Kaiser, C. Combined effects of Aloe vera gel and modified atmosphere packaging treatments on fruit quality traits and bioactive compounds of jujube (Ziziphus jujuba Mill.) fruit during cold storage and shelf life. Postharvest Biol. Technol. 2022, 187, 111855. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.; Cao, J.; Ma, L. A combination of 1-methylcyclopropene treatment and intermittent warming alleviates chilling injury and affects phenolics and antioxidant activity of peach fruit during storage. Sci. Hortic. 2018, 229, 175–181. [Google Scholar] [CrossRef]
- Adiletta, G.; Liguori, L.; Albanese, D.; Russo, P.; Di Matteo, M.; Crescitelli, A. Soft-Seeded Pomegranate (Punica granatum L.) Varieties: Preliminary Characterization and Quality Changes of Minimally Processed Arils during Storage. Food Bioprocess. Technol. 2017, 10, 1631–1641. [Google Scholar] [CrossRef]
- Adiletta, G.; Magri, A.; Albanese, D.; Liguori, L.; Sodo, M.; Di Matteo, M.; Petriccione, M. Overall quality and oxidative damage in packaged freshly shelled walnut kernels during cold storage. J. Food Meas. Charact. 2020, 14, 3483–3492. [Google Scholar] [CrossRef]
- Mirshekari, A.; Madani, B.; Wall, M.; Biggs, A.R. Aloe vera coatings maintain antioxidants of fig (Ficus carica L.) fruit during storage. Adv. Hortic. Sci. 2020, 34, 205–212. [Google Scholar]
- Hssaini, L.; Hernandez, F.; Viuda-Martos, M.; Charafi, J.; Razouk, R.; Houmanat, K.; Hanine, H. Survey of phenolic acids, flavonoids and in vitro antioxidant potency between fig peels and pulps: Chemical and chemometric approach. Molecules 2021, 26, 2574. [Google Scholar] [CrossRef]
- Singh, J.P.; Singh, B.; Kaur, A. Polyphenols in fig: A review on their characterisation, biochemistry during ripening, antioxidant activity and health benefits. Int. J. Food Sci. 2022, 57, 3333–3342. [Google Scholar] [CrossRef]
- Veberic, R.; Mikulic-Petkovsek, M. Phytochemical composition of common figs (Ficus carica L.) cultivars. In Nutrional Composition of Fruit Cultivars, 1st ed.; Simmonds, M.S.J., Preedy, V.R., Eds.; American Press: London, UK, 2016; pp. 235–255. [Google Scholar]
- Baraiya, N.S.; Rao, T.V.R.; Thakkar, V.R. Improvement of postharvest quality and storability of jamun fruit (Syzygium cumini L. var. Paras) by zein coating enriched with antioxidants. Food Bioprocess. Technol. 2015, 11, 2225–2234. [Google Scholar] [CrossRef]
- Meitha, K.; Pramesti, Y.; Suhandono, S. Reactive Oxygen Species and Antioxidants in Postharvest Vegetables and fruits. Int. Food Sci. 2020, 2020, 8817778. [Google Scholar] [CrossRef]
- Pétriacq, P.; López, A.; Luna, E. Fruit decay to diseases: Can induced resistance and priming help? Plants 2018, 7, 77. [Google Scholar] [CrossRef] [Green Version]
- Adiletta, G.; Di Matteo, M.; Petriccione, M. Multifunctional Role of Chitosan Edible Coatings on Antioxidant Systems in Fruit Crops: A Review. Int. J. Mol. Sci. 2021, 22, 2633. [Google Scholar] [CrossRef]
- Modesti, M.; Zampella, L.; Petriccione, M. Chitosan mono- and bilayer edible coatings for preserving postharvest quality of fresh fruit. In Polymers for Agri-Food Applications; Gutiérrez, T.J., Ed.; Editorial Springer International Publishing: Cham, Switzerland, 2019; pp. 465–486. [Google Scholar]
- Adiletta, G.; Petriccione, M.; Liguori, L.; Zampella, L.; Mastrobuoni, F.; Di Matteo, M. Overall quality and antioxidant enzymes of ready-to-eat ‘Purple Queen’ pomegranate arils during cold storage. Postharvest Biol. Technol. 2019, 155, 20–28. [Google Scholar] [CrossRef]
- De Gara, L.; Paciolla, C.; De Tullio, M.C.; Motto, M.; Arrigoni, O. Ascorbate-dependent hydrogen peroxide detoxification and ascorbate regeneration during germination of a highly productive maize hybrid: Evidence of an improved detoxification mechanism against reactive oxygen species. Physiol. Plant. 2000, 109, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Sheikhi, A.; Mirdehghan, S.H.; Karimi, H.R.; Ferguson, L. Effects of Passive- and Active-Modified Atmosphere Packaging on Physio-Chemical and Quality Attributes of Fresh In-Hull Pistachios (Pistacia vera L. cv. Badami). Foods 2019, 8, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byeon, S.; Lee, J. Fruit maturity differentially affect fruit quality and responses of targeted metabolites in cold-stored figs (Ficus carica L.). J. Sci. Food Agric. 2021, 101, 673–683. [Google Scholar] [CrossRef]
- Cozzolino, R.; Cefola, M.; Laurino, C.; Pellicano, M.P.; Palumbo, M.; Stocchero, M.; Pace, B. Electronic-Nose as non-destructive tool to discriminate “Ferrovia” sweet cherries cold stored in air or packed in high CO2 modified atmospheres. Front. Nutr. 2021, 8, 671. [Google Scholar] [CrossRef]


| Days | RS | pH | TA | WL | H |
|---|---|---|---|---|---|
| Control | |||||
| Harvest | 19.1 c | 5.2 b | 0.12 a | - | 85.0 c |
| 7 | 19.8 cd | 5.1 b | 0.12 a | 7.0 b | 77.6 abc |
| 14 | 20.6 de | 5.3 b | 0.11 a | 14.5 d | 73.9 ab |
| 21 | 21.8 e | 5.3 b | 0.09 a | 22.3 e | 70.3 a |
| PMAP | |||||
| Harvest | 19.1 c | 5.2 b | 0.12 a | - | 85.0 c |
| 7 | 18.6 bc | 5.0 ab | 0.12 a | 3.2 a | 82.4 c |
| 14 | 17.6 b | 5.0 ab | 0.11 a | 6.2 b | 81.7 bc |
| 21 | 15.7 a | 4.8 a | 0.12 a | 10.1 c | 82.3 c |
| Days | MA | CA | OA | AA | Fru | Glu | Suc |
|---|---|---|---|---|---|---|---|
| Control | |||||||
| Harvest | 0.12 ab | 0.17 b | 0.03 a | 0.01 a | 42.13 bc | 54.87 c | 1.47 a |
| 7 | 0.14 b | 0.14 ab | 0.03 a | 0.01 a | 40.71 bc | 42.90 ab | 1.86 b |
| 14 | 0.11 ab | 0.13 ab | 0.03 a | 0.01 a | 41.72 bc | 43.68 ab | 2.28 c |
| 21 | 0.08 a | 0.11 a | 0.02 a | 0.01 a | 42.71 c | 45.55 b | 2.67 d |
| PMAP | |||||||
| Harvest | 0.12 ab | 0.17 b | 0.03 a | 0.01 a | 42.13 bc | 54.87 c | 1.47 a |
| 7 | 0.12 ab | 0.14 ab | 0.03 a | 0.01 a | 35.74 a | 41.47 a | 1.33 a |
| 14 | 0.11 ab | 0.14 ab | 0.02 a | 0.01 a | 37.96 ab | 41.24 a | 1.47 a |
| 21 | 0.12 ab | 0.15 ab | 0.03 a | 0.01 a | 38.76 abc | 42.34 ab | 2.32 c |
| Days | POL | FLAV | ANN |
|---|---|---|---|
| Control | |||
| Harvest | 112.29 f | 42.91 f | 72.71 e |
| 7 | 90.17 d | 28.50 d | 51.13 c |
| 14 | 66.40 c | 16.81 b | 38.79 b |
| 21 | 33.32 a | 10.03 a | 27.88 a |
| PMAP | |||
| Harvest | 112.29 f | 42.91 f | 72.71 e |
| 7 | 101.64 e | 34.30 e | 61.80 d |
| 14 | 80.67 d | 22.79 c | 48.40 c |
| 21 | 51.27 b | 18.39 bc | 36.36 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adiletta, G.; Petriccione, M.; Di Matteo, M. Effects of Passive Modified Atmosphere Packaging on Physico-Chemical Traits and Antioxidant Systems of ‘Dottato’ Fresh Fig. Horticulturae 2022, 8, 709. https://doi.org/10.3390/horticulturae8080709
Adiletta G, Petriccione M, Di Matteo M. Effects of Passive Modified Atmosphere Packaging on Physico-Chemical Traits and Antioxidant Systems of ‘Dottato’ Fresh Fig. Horticulturae. 2022; 8(8):709. https://doi.org/10.3390/horticulturae8080709
Chicago/Turabian StyleAdiletta, Giuseppina, Milena Petriccione, and Marisa Di Matteo. 2022. "Effects of Passive Modified Atmosphere Packaging on Physico-Chemical Traits and Antioxidant Systems of ‘Dottato’ Fresh Fig" Horticulturae 8, no. 8: 709. https://doi.org/10.3390/horticulturae8080709
APA StyleAdiletta, G., Petriccione, M., & Di Matteo, M. (2022). Effects of Passive Modified Atmosphere Packaging on Physico-Chemical Traits and Antioxidant Systems of ‘Dottato’ Fresh Fig. Horticulturae, 8(8), 709. https://doi.org/10.3390/horticulturae8080709







