Key Determinants of the Physiological and Fruit Quality Traits in Sweet Cherries and Their Importance in a Breeding Programme
Abstract
:1. Introduction
2. Phenotyping Based on Agronomic and Physiological Traits in Sweet Cherries
2.1. Agronomic Traits
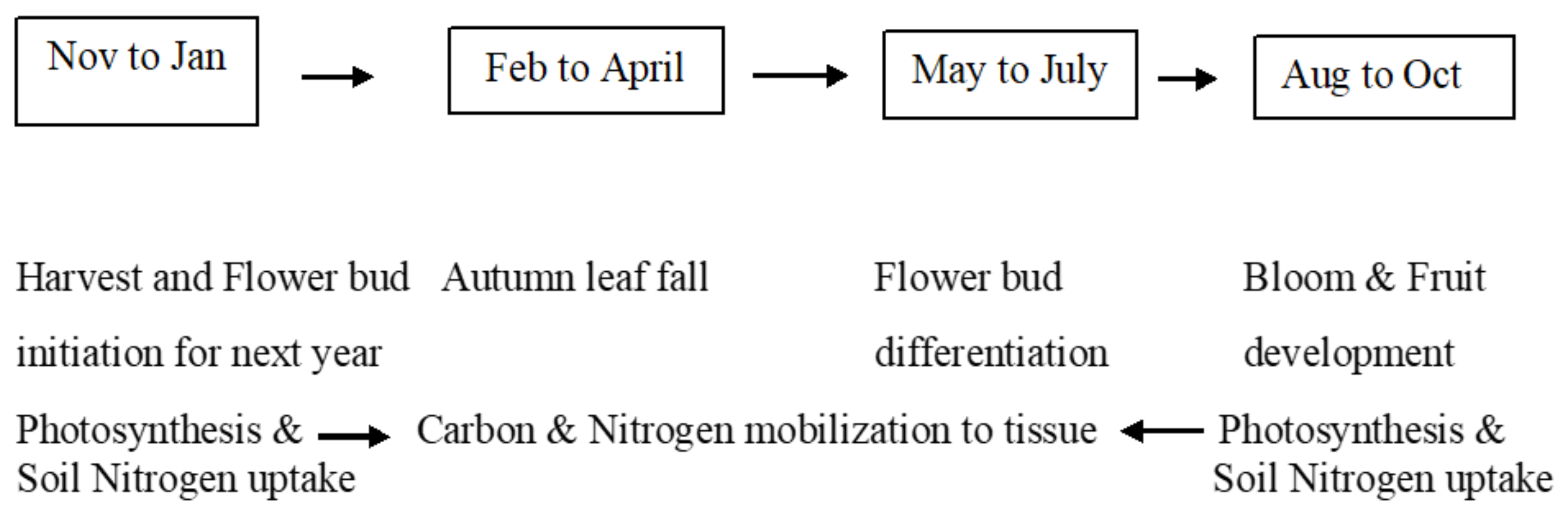
2.2. Physiological Traits
2.3. Impact of Environmental Factors in Agronomic, Physiological and Reproductive Traits
3. Phenotyping Based on Fruit Quality Traits in Sweet Cherries
4. Postharvest Physiology in Sweet Cherry
5. Sweet Cherry Genetic Resources for Improving Yield Genetic Gains
6. QTLs Associated with Physiological and Fruit Traits in Sweet Cherries
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benková, M.; Èièová, D.I.; Benedikova, L.; Mendel, L.; Glasa, M. Variability of old sweet cherries found in Slovak regions and their preservation. Proc. Latv. Acad. Sci. 2017, 71, 184–189. [Google Scholar] [CrossRef] [Green Version]
- James, P. Australian Cherry Production guide. Cherry Growers of Australia Inc. 2011. Available online: www.cherrygrowers.org.au (accessed on 28 January 2022).
- FAO. The State of Food and Agriculture 2020. In Overcoming Water Challenges in Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Cherry growers Australia Inc. 2021, Varieties and Management, Cherry Quality. Available online: http://cherrygrowers.org.au (accessed on 21 May 2022).
- Cherries, Australian Horticulture Statistics Handbook. 2020, pp. 92–97. Available online: www.horticulture.com.au (accessed on 20 April 2022).
- Binns, M.; Hoffmann, A.; Heldon, M.V.; Heddle, T.; Umina, P. Earwigs—Latest Research on These Damaging Pests. 2019. Available online: https://grdc.com.au/resources-and-publications (accessed on 20 February 2022).
- Quarrell, S.R.; Corkrey, R.; Allen, G.R. Predictive thresholds for forecasting the compatibility of Forficula auricularia and Aphelinus mali as biological control agents against woolly apple aphid in apple orchards. BioControl 2017, 62, 243–256. [Google Scholar] [CrossRef]
- Hardner, C.M.; Hayes, B.J.; Kumar, S.; Vanderzande, S.; Cai, L.; Piaskowski, J.; Quero-Garcia, J.; Campoy, J.A.; Barreneche, T.; Giovannini, D.; et al. Prediction of genetic values of sweet cherry fruit maturity among environments using a 6K SNP array. Hortic. Res. 2019, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.R.; Gallardo, K.; Luby, J.J.; Rihn, A.L.; McFerson, J.R.; McCracken, V.; Oraguzie, N.; Weebadde, C.; Sebolt, A.; Iezzoni, A. An evaluation of U.S. tart and sweet cherry producer’s trait prioritization: Evidence from audience surveys. HortScience 2014, 49, 931–937. [Google Scholar] [CrossRef] [Green Version]
- Quero-Garcia, J.; Fodor, A.; Reignier, A.; Capdeville, G.; Joly, J.; Tauzin, Y.; Fouilhaux, L.; Direlwanger, E. QTL detection of important agronomic traits for sweet cherry production. Acta Hortic. 2014, 1020, 57–64. [Google Scholar] [CrossRef]
- Etienne, C.; Rothan, C.; Moing, A.; Plomion, C. Candidate genes and QTLs for sugar and organic acid content in peach [Prunus persica (L.) Batsch]. Theor. Appl. Genet. 2002, 105, 145–159. [Google Scholar] [CrossRef]
- Salazar, J.A.; Ruiz, D.; Campoy, J.A.; Tartarini, S.; Dondini, L.; Martinez-Gomez, P. Inheritance of reproductive phenology traits and related QTL identification in apricot. Tree Genet. Genomes 2016, 12, 71. [Google Scholar] [CrossRef]
- Tukey, H.B. Growth of the peach embryo in relation to growth of fruit and season of ripening. Proc. Amer. Soc. Hort. Sci. 1933, 30, 208–218. [Google Scholar]
- Tukey, H.B. Embryo abortion in early-ripening varieties of Prunus avium. Bot. Gaz. 1933, 94, 433–468. [Google Scholar] [CrossRef]
- Braak, J.P. The effect of flowering date and temperature on embryo development in sweet cherry Prunus avium L. Neth. J. Agric. Sei. 1978, 26, 13–30. [Google Scholar] [CrossRef]
- Marti, A.F.; Athanson, B.; Koepke, T.; Font-Forcada, C.; Dhingra, A.; Oraguzie, N. Genetic diversity and relatedness of sweet cherry (Prunus avium L.) cultivars based on single nucleotide polymorphic markers. Front. Plant Sci. 2012, 3, 116. [Google Scholar] [CrossRef] [Green Version]
- Azarenko, A.N.; Chozinski, A.; Brewer, L.J. Fruit growth curve analysis of seven sweet cherry cultivars. Acta Hortic. 2008, 795, 561–565. [Google Scholar] [CrossRef]
- Brunt, C. Cherry Cultivar Selection: Chill Hours and Climate Change; Horticulture Australia Ltd.: North Sydney, Australia, 2012. [Google Scholar]
- Prasad, P.V.V.; Djanaguiraman, M. Response of floret fertility and individual grain weight of wheat to high temperature stress: Sensitive stages and thresholds for temperature and duration. Funct. Plant Biol. 2014, 41, 1261–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devasirvatham, V.; Gaur, P.M.; Mallikarjuna, N.; Raju, T.N.; Trethowan, R.M.; Tan, D.K.Y. Effect of high temperature on the reproductive development of chickpea genotypes under controlled environments. Funct. Plant Biol. 2012, 39, 1009–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, P.V.V.; Craufurd, P.Q.; Summerfield, R.J. Sensitivity of peanut to timing of heat stress during reproductive development. Crop Sci. 1999, 39, 1352–1359. [Google Scholar] [CrossRef]
- Dordevic, B.; Durovic, D.B.; Zec, G.N.; Boscov, D.D. The influence of rootstocks on the sensitivity of flower buds to frost and the main properties of the Carmen sweetcherry cultivar. J. Agri. Sci. 2021, 66, 351–358. [Google Scholar] [CrossRef]
- Dziedzic, E.; Bieniasz, M.; Kowalczyk, B. Morphological and physiological features of sweet cherry floral organ affecting the potential fruit crop in relation to the rootstock. Sci. Hortic. 2019, 251, 127–135. [Google Scholar] [CrossRef]
- Baji, M.E.; Hafida, H.; En-Nahli, S.; Company, R.S.; Kodad, O. Morphological and Pomological Characteristics of Sweet Cherry (Prunus avium L.) Grown In-situ under South Mediterranean Climate in Morocco. Int. J. Fruit Sci. 2021, 21, 52–65. [Google Scholar] [CrossRef]
- Rakonjac, V.; Mratinic, E.; Jovkovic, R.; Aksic, F.M. Analysis of morphological variability in wild cherry (Prunus avium L.) genetic resources from Central Serbia. J. Agric. Sci. Tech. 2014, 16, 151–162. [Google Scholar]
- Ganopoulos, I.; Moysiadis, T.A.; Xanthopoulou, M.; Ganopoulou, E.; Avramidou, F.A.; Aravanopoulos, E.; Tani, P.; Madesis, A.; Tsaftaris, A.; Kazantzis, K. Diversity of morpho-physiological traits in worldwide sweet cherry cultivars of Gene Bank collection using multivariate analysis. Sci. Hortic. 2015, 197, 381–391. [Google Scholar] [CrossRef]
- Bondarenko, P. Physiological basis of sweet cherry productivity depending on rootstocks, interstems and plant density. Open Agric. 2019, 6, 267–274. [Google Scholar] [CrossRef]
- Roper, T.R.; Keller, J.D.; Loescher, W.H.; Rom, C.R. Photosynthesis and carbohydrate partitioning in sweet cherry: Fruiting effects. Physi. Plan. 2006, 71, 42–47. [Google Scholar] [CrossRef]
- Demirsoy, H.; Demirsoy, L. A study on the relationships between some fruit characteristics in cherries. Fruits 2004, 59, 219–223. [Google Scholar] [CrossRef]
- Kappel, F.; Fisher-Fleming, B.; Hogue, E. Fruit characteristics and sensory attributes of an ideal sweet cherry. HortScience 1996, 31, 443–446. [Google Scholar] [CrossRef] [Green Version]
- Ayala, M.; Lang, G.A. 13C-Photoassimilate partitioning in sweet cherry (Prunus avium) during early spring. Sci. Agr. Res. 2015, 42, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Ayala, M.; Lang, G.A. Examining the influence of different leaf populations in sweet cherry fruit quality. Acta Hortic. 2004, 636, 481–488. [Google Scholar] [CrossRef]
- Ayala, M.; Mora, L.; Torreblanca, J. Effect of pre-bloom pruning on 13C and 15N distribution during early spring in sweet cherry. HortScience 2018, 53, 805–809. [Google Scholar] [CrossRef]
- Macit, I.; Lang, G.A.; DemIrsoy, H. Bud management affects fruit wood, growth, and precocity of cherry trees. Turk. J. Agr. For. 2017, 41, 42–49. [Google Scholar] [CrossRef]
- Goncalves, B.; Moutinho-Pereira, J.; Santos, A.; Silva, A.P.; Bacelar, E.; Correia, C.; Rosa, E. Scion-rootstock interaction affects the physiology and fruit quality of sweet cherry. Tree Physiol. 2006, 26, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Xie, Z.; Zhang, A.; Xu, W.; Zhang, C.; Liu, Q.; Liu, C.; Wang, S. Tree growth characteristics and flower bud differentiation of sweet cherry (Prunus avium) under different climate conditions in China. Hortic. Sci. 2010, 27, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Guler, Y.; Dikmen, F. Potential bee pollinators of sweet cherry in inclement weather conditions. J. Ento. Res. Soc. 2013, 15, 9–19. Available online: https://www.researchgate.net/publication/286268023 (accessed on 20 May 2022).
- Predieri, S.; Dris, R.; Sekse, L.; Rapparini, F. Influence of environmental factors and orchard management on yield and quality of sweet cherry. Food Agri. Envi. 2003, 1, 263–266. Available online: https://www.researchgate.net/publication/266226841 (accessed on 20 May 2022).
- Beppu, K.; Ikeda, T.; Kataoka, I. Effect of high temperature exposure time during flower bud formation on the occurrence of double pistils in ‘Satohnishiki’ sweet cherry. Sci. Hortic. 2001, 87, 77–84. [Google Scholar] [CrossRef]
- Blanco, V.; Blayo-rose, P.J.; Torres-Sánchez, R.; Domingo, R. Influence of regulated deficit irrigation and environmental conditions on reproductive response of sweet cherry trees. Plants 2020, 9, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zang, L.; Ferguson, L.; Whiting, M.D. Temperature effect on pistil viability and fruit set in sweet cherry. Sci. Hortic. 2018, 241, 8–17. [Google Scholar] [CrossRef]
- Juhasz, A.; Sepsi, P.; Nagy, Z.; Tokei, L.; Hrotko, K. Water consumption of sweet cherry trees estimated by sap flow measurement. Sci. Hortic. 2013, 164, 41–49. [Google Scholar] [CrossRef]
- Ranney, T.G.; Bassuk, N.L.; Whitlow, T.H. Osmotic adjustment and solute constituents in leaves and roots of water-stressed cherry (Prunus) Trees. J. Am. Soc. Hortic. Sci. 1991, 116, 684–688. [Google Scholar] [CrossRef]
- Blanco, V.; Martínez-Hernández, G.B.; Artés-Hernández, F.; Blaya-Ros, P.J.; Torres-Sánchez, R.; Domingo, R. Water relations and quality changes throughout fruit development and shelf life of sweet cherry grown under regulated deficit irrigation. Agric. Water Manag. 2019, 217, 243–254. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, M.C.; Domingo, R.; Castel, J.R. Review. Deficit irrigation in fruit trees and vines in Spain. Span. J. Agric. Res. 2010, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Vosnjak, M.; Mrzlic, D.; Hudina, M.; Usenik, V. The effect of water supply on sweet cherry phytochemicals in buds, leaf and fruit. Plants 2021, 10, 1131. [Google Scholar] [CrossRef] [PubMed]
- Romano, G.S.; Cittadini, E.D.; Pugh, B.; Schouten, R. Sweet cherry quality in the horticultural production chain. Stewart Post Harv. Rev. 2006, 6, 2. [Google Scholar] [CrossRef]
- Kazantzis, K.; Chatzicharissis, I.; Papachatzis, A.; Sotiropoulos, T.; Kalorizou, H.; Koutinas, N. Evaluation of sweet cherry cultivars introduced in Greece. J. Univ. Kraiova Ser. Hortic. 2011, 16, 293–296. Available online: http://pomologyinstitute.gr (accessed on 28 March 2022).
- Clayton, M.; Biasi, W.V.; Agar, I.T.; Southwick, S.M.; Mitcham, E.I. Sensory quality of Bing sweet cherries following preharvest treatment with hydrogen cyanamide, calcium ammonium nitrate or gibberellic acid. HortScience 2006, 41, 745–748. [Google Scholar] [CrossRef] [Green Version]
- Zang, C.; Whitting, M. Plant growth regulators improve sweet cherry fruit quality without reducing endocarp growth. Sci. Hortic. 2011, 150, 73–79. [Google Scholar] [CrossRef]
- Kaiser, C.; Long, L.; Brewer, L. Understanding and Preventing Sweet Cherry Cracking. Oregan State University EM 9227. 2019. Available online: https://www.researchgate.net/publication/331358391 (accessed on 15 February 2022).
- Looney, N.E.; Lidster, P.D. Some growth regulator effects on fruit quality, mesocarp composition, and susceptibility to postharvest surface marking of sweet cherries. J. Amer. Soc. Hortic. Sci. 1980, 105, 130–134. [Google Scholar]
- Kappel, F.; MacDonald, I.K. Gibberellic acid increases fruit firmness, fruit size, and delays maturity of ‘sweetheart’ sweet cherry. Isr. J. Plant Sci. 2002, 56, 219–222. [Google Scholar]
- Einhorn, T.C.; Wang, Y.; Turner, J. Sweet cherry fruit firmness and postharvest quality of late maturing cultivars are improved with low rate and single application of Gibberellic Acid. HortScience 2013, 48, 1010–1017. [Google Scholar] [CrossRef]
- Christensen, J.V. Rain-induced cracking of sweet cherries: Its causes and prevention. In Cherries: Crop Physiology, Production and Uses; Webster, A.D., Looney, N.E., Eds.; CAB International: Wallingford, UK, 1996; pp. 297–330. ISBN 9780851989365. [Google Scholar]
- Christensen, J.V. Cracking in cherries. III. Determination of cracking susceptibility. Acta Agric. Scand. 1972, 22, 128–136. [Google Scholar] [CrossRef]
- Measham, P.F.; Bound, S.A.; Gracie, A.J.; Wilson, S.J. Incidence and type of cracking in sweet cherry (Prunus avium L.) are affected by genotype and season. Crop Pasture Sci. 2009, 60, 1002–1008. [Google Scholar] [CrossRef]
- Winkler, A.; Knoche, M. Calcium and the physiology of sweet cherries: A review. Sci. Hortic. 2019, 245, 107–115. [Google Scholar] [CrossRef]
- Winkler, A.; Fiedler, B.; Knoche, M. Calcium physiology of sweet cherry fruits. Trees 2020, 34, 1157–1167. [Google Scholar] [CrossRef]
- Amarante, C.V.T.; Miqueloto, A.; Stefens, C.A.; Dos Santos, A.; Argenta, L.C. Changes in xylem functionality during apple fruit development: Implications on calcium concentration and incidence of bitter pit. Acta Hortic. 2013, 1012, 135–140. [Google Scholar] [CrossRef]
- Xiloyannis, C.; Celano, G.; Montanaro, G.; Dichio, B.; Sebastiani, L.; Minnocci, A. Water relations, calcium and potassium concentration in fruits and leaves during annual growth in mature kiwifruit plants. Acta Hortic. 2001, 564, 129–134. [Google Scholar] [CrossRef]
- Windt, C.W.; Gerkema, E.; Van As, H. Most water in the tomato truss is imported through the Xylem, not the Phloem: A nuclear magnetic resonance flow imaging study. Plant Phsiol. 2009, 151, 830–842. [Google Scholar] [CrossRef] [Green Version]
- Winkler, A.; Peschel, S.; Kohrs, K.; Knoche, M. Rain cracking in sweet cherry is not due to excess water but localized skin phenomena. J. Am. Soc. Hortic. Sci. 2016, 141, 653–660. [Google Scholar] [CrossRef]
- Blanco, V.; Zoffoli, J.P.; Ayala, M. Influence of high tunnel microclimate on fruit quality and calcium concentration in Santina sweet cherries in a Maditerranean climate. Agronomy 2021, 11, 1186. [Google Scholar] [CrossRef]
- Esti, M.; Cinquanta, L.; Silesio, F.; Moneta, E.; Di Matteo, M. Physicochemical and sensory fruit characteristics of two sweet cherry cultivars after cool storage. Food Chem. 2002, 76, 399–405. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M.; Metheney, P. Consumer acceptance of ‘Brooks’ and ‘Bing’ cherries is mainly dependent on fruit SSC and visual skin colour. Postharvest Biol. Technol. 2003, 28, 159–165. [Google Scholar] [CrossRef]
- Golding, J. Review of international best practice for postharvest management of sweet cherries. 2018. Available online: www.horticulture.com.au (accessed on 1 July 2022).
- Dziedzic, E.; Blaszczyk, J. Evaluation of sweet cherry fruit quality after short term storage in relation to the rootstock. Hortic. Environ. Biotechnol. 2019, 60, 925–934. [Google Scholar] [CrossRef] [Green Version]
- Long, L.E.; Kaiser, C. Sweet Cherry Rootstocks. 2010. Available online: https://catalog.extension.oregonstate.edu/pnw619 (accessed on 25 January 2022).
- Lanauskas, J.; Uselis, N.; Kviklys, D.; Kviklienė, N.; Buskiene, L. Rootstock effect on the performance of sweet cherry cv. Lapins. Hortic. Sci. 2012, 39, 55–60. [Google Scholar]
- Sansavini, S.; Lugli, S. Sweet cherry breeding programmes in Europe and Asia. Acta Hortic. 2008, 795, 41–58. [Google Scholar] [CrossRef] [Green Version]
- Dirlewanger, E.; Quero-Garcı’a, J.; Le Dantec, L.; Lambert, P.; Ruiz, D.; Dondini, L.; Illa, E.; Quilot-Turion, B.; Audergon, J.-M.; Tartarini, S.; et al. Comparison of the genetic determinism of two key phenological traits, flowering and maturity dates, in three Prunus species: Peach, apricot and sweet cherry. Heredity 2012, 109, 280–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piaskowski, J.; Hardner, C.; Cai, L.; Zhao, Y.; Iezzoni, A.; Peace, C. Genomic heritability estimates in sweet cherry reveal non-additive genetic variance is relevant for industry-prioritized traits. BMC Genet. 2018, 19, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariette, S.; Tavaud, M.; Arunyawat, U.; Capdeville, G.; Millan, M.; Salin, F. Population structure and genetic bottleneck in sweet cherry estimated with SSRs and the gametophytic self-incompatibility locus. BMC Genet. 2010, 11, 77. Available online: http://www.biomedcentral.com/1471-2156/11/7 (accessed on 10 November 2021). [CrossRef] [PubMed] [Green Version]
- Gratez, D. Evaluation of High-Quality Australian Bred Sweet Cherries for Export and Domestic Markets; 2014. Available online: http://www.horticulture.com.au (accessed on 10 November 2021).
- Measham, P.F.; Long, L.E.; Aglar, E.; Kaiser, C. Efficacy of anti-transpirant sprays on fruit cracking and on fruit Quality at harvest and post-harvest storage in sweet cherry. Int. J. Agric. Wildl. Sci. 2020, 6, 141–151. [Google Scholar] [CrossRef]
- Cai, L.; Quero-García, J.; Barreneche, T.; Dirlewanger, E.; Saski, C.; Iezzon, A. A fruit firmness QTL identified on linkage group 4 in sweet cherry (Prunus avium L.) is associated with domesticated and bred germplasm. Nature 2019, 9, 5008. [Google Scholar] [CrossRef] [Green Version]
- Calle, A.; Wunsch, A. Multiple-population QTL mapping of maturity and fruit-quality traits reveals LG4 region as a breeding target in sweet cherry (Prunus avium L.). Hortic. Res. 2020, 7, 127. [Google Scholar] [CrossRef]
- Panda, S.; Martin, J.P.; Aguinagalde, I.; Mohanty, A. Chloroplast DNA variation in cultivated and wild Prunus avium L.: A comparative study. Plant Breed. 2003, 122, 92–94. [Google Scholar] [CrossRef]
- Xanthopoulou, A.; Manioudaki, M.; Bazakos, C.; Kissoudis, C.; Farsakoglou, A.; Karagiannis, E.; Michailidis, M.; Polychroniadou, C.; Zambounis, A.; Kazantzis, K.; et al. Whole genome re-sequencing of sweet cherry (Prunus avium L.) yields insights into genomic diversity of a fruit species. Hortic. Rese. 2020, 7, 60. [Google Scholar] [CrossRef]
- Bhat, J.A.; Yu, D.; Bohra, A.; Ganie, S.A.; Varshney, R.K. Features and application of haplotypes in crop breeding. Commun. Biol. 2021, 4, 1266. [Google Scholar] [CrossRef]
- Vergara, R.; Olivares, F.; Olmedo, B.; Toro, C.; Muñoz, M.; Zúñiga, C.; Mora, R.; Plantat, P.; Miccono, M.; Loyola, R.; et al. Gene editing in Prunus Spp.: The challenge of adapting regular gene transfer procedures for precision breeding. In Punnes Spp.: Recent Advances; Kuden, A.B., Kuden, A., Eds.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devasirvatham, V.; Tan, D.K.Y. Key Determinants of the Physiological and Fruit Quality Traits in Sweet Cherries and Their Importance in a Breeding Programme. Horticulturae 2022, 8, 694. https://doi.org/10.3390/horticulturae8080694
Devasirvatham V, Tan DKY. Key Determinants of the Physiological and Fruit Quality Traits in Sweet Cherries and Their Importance in a Breeding Programme. Horticulturae. 2022; 8(8):694. https://doi.org/10.3390/horticulturae8080694
Chicago/Turabian StyleDevasirvatham, Viola, and Daniel K. Y. Tan. 2022. "Key Determinants of the Physiological and Fruit Quality Traits in Sweet Cherries and Their Importance in a Breeding Programme" Horticulturae 8, no. 8: 694. https://doi.org/10.3390/horticulturae8080694





