Abstract
Balangu is a medicinal plant used in the Iranian traditional medicine to treat nervous, hepatic and renal diseases. To determine the effects of Myco-Root biofertilizer and chitosan nanoparticles (Cs-NPs) on the physiological and biochemical properties of balangu (Lallemantia iberica (M.Bieb.) Fisch. & C.A.Mey.) under different irrigation levels, an experiment was laid out as a factorial based on completely randomized design (CRD) with twelve treatments and three replications. The first factor was represented by different irrigation regimes, including no water deficit (90% FC), mild water deficit (60% FC) and severe water deficit (30% FC); the second factor included control (no Myco-Root and Cs-NPs), inoculation with Myco-Root biofertilizer, foliar application of chitosan nanoparticles (Cs-NPs) and co-application of Cs-NPs along with Myco-Root. The results showed that the highest fresh and dry weight, chlorophyll and carotenoid content, chlorophyll index (SPAD) and fluorescence indices were obtained in 90% FC treated with Cs-NPs+ Myco-Root. In addition, the maximum activity of superoxide dismutase (SOD), ascorbate peroxidase (APX) and peroxidase (POX) was achieved in 60% FC with application of Cs-NPs+ Myco-Root. Moreover, the maximum essential oil content (1.43%) and yield (0.25 g pot−1) were recorded in 60% FC following the application of Cs-NPs+ Myco-Root. Chemical analysis of essential oil showed that germacrene D (31.22–39.77%), (E)-caryophyllene (16.28–19.82%), bicyclogermacrene (7.1–9.22%) and caryophyllene oxide (3.85–6.96%) were the major volatile constituents of balangu. Interestingly, the maximum contents of germacrene D and (E)-caryophyllene were recorded in 60% FC after the application of Cs-NPs+ Myco-Root. Overall, it can be concluded that co-application of Cs-NPs+ Myco-Root could be a sustainable and eco-friendly strategy for improving the essential oil quantity and quality, as well as physiological characteristics, of balangu under water deficit conditions.
1. Introduction
Balangu (Lallemantia iberica (M.Bieb.) Fisch. & C.A.Mey.) is an annual, herbaceous plant belonging to the Lamiaceae family which is distributed in Southwest Asia and Europe [1]. Balangu leaves and seeds are used in the pharmaceutical, cosmetic, health and food industries due to their content of secondary metabolites, fiber, polysaccharides, protein and oil [2,3,4]. This plant is used in the treatment of various disorders, such as neurological, liver, dry cough and kidney diseases [5]. In addition, it is utilized in an extensive range of conventional beverages and industrial products in Iran and Turkey [6]. It was reported that the germacrene D, (E)-caryophyllene, bicyclogermacrene and caryophyllene oxide are the major essential oil (EO) constituents of L. iberica [7].
Drought stress is one of the most common environmental factors that limits crop production in arid and semi-arid regions [8]. Drought stress causes various physiological and metabolic reactions, such as reduction in plant height, total fresh and dry weight and rate of photosynthesis and stomatal closure [9]. Osmotic adjustment in plant cells under water stress is known as a common physiological adaptation. Among these, common solutes such as proline and soluble carbohydrates play an important role in osmotic regulation, stabilize structures of proteins and ultimately protect membranes from damages [10]. Additionally, water deficiency decreases cell turgor and leaf water content, which leads to decline in leaf area, cell growth and, consequently, an imbalance between antioxidants defenses and the number of reactive oxygen species (ROS) [11]. Excessive accumulation of ROS can enhance cell membrane oxidation and cause direct damage to DNA, proteins, pigments, lipids and other essential molecules, resulting in cell death and biomass loss. In this situation, the activity of antioxidant enzymes, such as peroxidase (POX), superoxide dismutase (SOD), ascorbate peroxidase (APX) and catalase (CAT), help to eliminate ROS and protect against stress [12].
In addition to the negative impacts of drought stress on plant productivity, the nutrient use efficiency of chemical fertilizers reduces in water deficit conditions due to the decrease of mass flow and nutrient diffusion, which leads to an increase in production costs [13]. It is worth noting that, in intensive agricultural systems, the application of chemical fertilizers plays a noteworthy role in plant growth and yield. The massive application of chemical inputs in these farming systems, in addition to creating human health hazards, is causing negative impacts on the environmental ecosystems [14]. Moreover, the excessive usage of chemical fertilizer decreases the yield of bioactive compounds in medicinal and aromatic plants [13,15]. Therefore, new, sustainable and eco-friendly strategies are required for improving the EO quantity and quality of medicinal and aromatic plants, especially in arid and semi-arid regions. The application of nanofertilizer and inoculating plants with biofertilizers, such as arbuscular mycorrhiza fungi, are known as an alternative strategy for improving plant productivity and quality under drought stress conditions [16].
Chitosan (Cs) is the second most abundant polymer on earth and is part of the cell wall of many fungi, insects and some algae [17]. Cs can be used as a functional agent for improving the concentrations of plant nutrients, such as copper, iron, manganese, etc., which positively affect plant yield and quality traits [18]. Cs can improve plant tolerance to biotic and abiotic stresses by increasing antioxidant enzyme activity [19,20]. Cs improves stem and root growth and photosynthetic pigments and regulates phenolic and enzymatic activities under drought stress conditions [21,22]. Cs is a natural polymer, environmentally friendly, biodegradable, non-toxic and is classified as a stimulant in plants, triggering genes activating the biosynthetic pathways of secondary metabolites [23,24]. Previous studies reported that the application of Cs-NPs improves EO quantity and quality of medicinal and aromatic plants. For example, Alizadeh et al. [25] reported that Cs-NPs improve the content of total phenol, soluble sugars and proline, as well as increasing antioxidant activity and EO content and quality of summer savory (Satureja hortensis L.) under drought stress.
Arbuscular mycorrhiza fungi (AMF) are the main components of the plant rhizosphere flora in the natural ecosystem. Mycorrhizal fungi can form mutualistic symbiotic associations with more than 80% of plant species which improve plants growth characteristics [26,27]. The positive impacts of AMF symbiosis with plant roots are multiple and variable. Mycorrhizal fungi can enhance water uptake through improvement of the root hydraulic conductivity through a larger surface area of the mycelium [28]. The symbiotic association between plant roots and fungi increases access to nutrients, especially those with low mobility, such as phosphorus, zinc and copper, over time [28,29,30]. Moreover, it was reported that the inoculation of mycorrhizal fungi with host plants improves plant tolerance in the face of biotic and abiotic stresses through increasing the activity of antioxidant enzymes, reducing chlorophyll decomposition rate and promoting chlorophyll synthesis [31,32]. In recent years, the application of mycorrhizal fungi has been used as an eco-friendly method to increase the quantity and quality of medicinal and aromatic plants, especially in stressful conditions. The inoculation of mycorrhizal fungi (Funneliformis mosseae) in thyme plants under drought stress conditions enhanced the EO content and quality by increasing the main EO constituents, such as thymol, p-cymene and γ-terpinen, [14]. The co-application of mycorrhizal fungi and TiO2 nanoparticles enhanced the main EO constituents of sage (Salvia officinalis L.), including cis-thujone, trans-thujone and 1,8-cineole [13]. Another study by Pirzad and Mohammadzadeh [32] showed that mycorrhizal fungi reduce the effects of unfavorable water deficit stress in three Lamiaceae species, including lavender (Lavandula angustifolia Mill.), rosemary (Rosmarinus officinalis L.) and thyme (Thymus vulgaris L.). It was found that mycorrhiza inoculation under drought stress conditions improved growth parameters, grain yield, chlorophyll index and antioxidant activity in two species of balangu plants (Lallemantia iberica L., Lallemantia royleana L.) [2].
Considering the negative effects of drought stress on plants, improving the quantity and quality performance of medicinal plants in these conditions has become a major challenge in the agricultural sector. Research about the effects of nano- and biofertilizer on the morphological, physiological and phytochemical characteristics of balangu under drought stress conditions has not drawn adequate interest to date. Therefore, this study aimed to investigate the effectiveness of Cs-NPs and Myco-Root on balangu morphological and physiological characteristics and EO quantity and quality under drought stress conditions.
2. Materials and Methods
2.1. Plant Material and Experimental Setup
To evaluate the morphological, physiological and biochemical responses of L. iberica to foliar application of Cs-NPs and inoculation with Myco-Root biofertilizer under different irrigation regimes, a factorial experiment was conducted based on a completely randomized design (CRD) in the greenhouse of the Department of Production Engineering and Plant Genetics, Faculty of Agriculture, University of Maragheh, Maragheh, Iran. The first factor was represented by irrigation regimes at three levels, no water deficit (90% FC), moderate (60% FC) and severe (30% FC) water deficit; the second factor was represented by different fertilizer sources: non-fertilizer (control), inoculation with arbuscular mycorrhizal fungi biofertilizer (Myco-Root), chitosan nanoparticles (Cs-NPs) and co-application of Cs-NPs+ Myco-Root.
Balangu seeds were planted in containers including cocopeat and perlite in a ratio of 2:1. Four weeks from planting and in the four-leaf stage, the seedlings were transferred to disposable cups and, after the 15-leaf stage, transplanted to 5 L plastic pots with a diameter of 21 cm containing 50% farm soil, 25% sand, 15% rotted manure and 10% perlite. First, the soil was autoclaved for 1 h at 121 °C in 1.2 atmospheric pressure. Irrigation of pots in the first and second weeks after transplanting was regular and based on the plant needs and soil field capacity (90% FC). After the plants were fully established, water deficit stress was applied until full flowering. The drought stress was applied by weighing the pots daily during 90 days of cultivation. To determine the field capacity (FC), the soil of each pot was weighed and then irrigated up to the saturation level. Subsequently, the pots were covered by a plastic, and, after 24 h, the soils were weighed when the waste water of soil came out. Finally, the soil samples were dried in an oven at 100 °C for 24 h, and their weights were recorded. The FC was calculated from the following equation [33]:
FC: Percentage of field capacity;
Wf: Soil weight in the field capacity;
Wd: Weight of soil dried in the oven.
Biofertilizer (Myco-Root) was obtained from Zist Fanavar Sabz Company, Iran. In Myco-Root treatments, 20 g of three species of arbuscular mycorrhizal fungi (Funneliformis mosseae, Rhizophagus intraradices and Claroideoglomus etunicatum) were added to each pot. Cs-NPs (85% acetylation, Sigma Chem. Co., Street St. Louis, MO, USA) were sprayed three times in 10-leaf growth stage with an interval of 5 days. For this purpose, Cs was dissolved in 3% (v/v) 0.1 M acetic acid by gentle heating at 60 °C and continuous stirring for 12 h and then the pH was adjusted to 5.6 using 1 M NaOH solution. Finally, the Cs nanoparticle was dissolved in sterile, distilled water and sprayed on balangu plants at concentration of 0.5% w/v.
2.2. Photosynthetic Pigments
In flowering stage, 0.5 g fresh tissue of balangu was homogenized in 10 mL of 80% acetone, and the homogenate solution was centrifuged at 12,000 rpm for 15 min. Finally, the absorbance was read at 663 nm, 645 nm and 470 nm by a UV spectrophotometer (UV-1800, Shimadzu, Tokyo, Japan), and the content of chlorophyll a, b, total and carotenoids was calculated in mg g−1 FW based on the following equations [34]:
Chlorophyl a = (19.3 A663 − 0.86 A645) V/100 W
Chlorophyl b = (19.3 A645 − 3.6 A663) V/100 W
Carotenoids = 100 A470 − 3.27 Chlorophyl a − 104 Chlorophyl b/227
2.3. Essential Oil Isolation
Essential oil was obtained from 40 g of the shade-dried balangu leaves for 3 h using a British Pharmacopoeia model Clevenger-type apparatus. Isolated essential oil was dried over anhydrous sodium sulphate sealed in dark vials and kept at 4 °C until analysis [35]. Additionally, the essential oil yield was calculated according to the following equation [36,37]:
Essential oil content (EO%) = (weight of essential oil/40 g) × 100
Essential oil yield = Total dry matter × EO%
2.4. Identification of Essential Oil Compounds
To identify the EO constituents, a gas chromatographic device connected to mass spectrometry (GC-MS) model Agilent 5977A, Stevens Creek Blvd. Santa Clara, CA, USA, with HP-5 MS column (5% phenylmethyl polysiloxane, 30 m length, 0.25 mm internal diameter and 0.1 μm film thickness), was used. In the oven, first the temperature reached 60 °C in 5 min, then gradually increased at a rate of 3 °C min−1 to reach a temperature of 240 °C. It was then kept at this temperature for 20 min. Helium was used as the carrier gas at a flow rate of 1 mL min−1. The ionization voltage was 70 electron volts (EV) in the electron impact (EI) mode, and the temperature of the mass detector was 220 °C. The injection chamber was set in the split mode (split ratio 1:30), and the mass absorption range was from 40 to 400 m z−1. Before injection, the essential oils were diluted in n-hexane (1:100) and then 1 μL of diluted samples was injected into the instrument. In order to calculate the compound inhibition index, a mixture of aliphatic hydrocarbons (C8–C40) was injected into the GC system under the conditions of the temperature analysis program of the essential oil sample. The software used was Chemstation. The EO compounds were identified using their linear inhibition indices and compared with the indices of literature [38] or using the mass spectra overlapping with computer libraries (WILEY275 and NIST 07). Agilent 7990B gas chromatography device, Stevens Creek Blvd. Santa Clara, CA, USA, with flame ionization detector (FID) and VF-5MS column (5% phenyl methylpolysiloxane, 30 m l., 0.25 μm i.d., 0.50 μm f.t.) was used to separate the compounds. The injection and detector temperatures were set at 230 °C and 240 °C, respectively. Helium was the gas carrier at a flow rate of 1 mL min−1 at a ratio of 1:24. The EO samples were diluted 1:100 in hexane gas and injected in 1 μL. Quantification of EO compounds was performed using peak normalization without using correction coefficients [39].
2.5. Malondialdehyde (MDA)
First, 0.5 g of fresh leaf sample was ground in 1500 µL TCA (trichloroacetic acid %1 w/v) and centrifuged at 12,000 rpm (10 min, 4 °C). After that, 1000 µL TBA (thiobarbituric acid %0.1 w/v) was added to 500 μL of the supernatant. The mixture was heated at 95 °C (30 min) and cooled down on ice. Lastly, the absorbance was read at 532 nm and 600 nm. The MDA content was expressed as nmol g−1 FW [40].
2.6. Superoxide Dismutase (SOD) Activity
For determination of SOD activity, the reaction mixture, including 1.5 mM bicarbonate sodium, 100 mM sodium phosphate buffer (pH = 7.6), 3 mM EDTA, 0.2 mM L-Methionine, 60 μM riboflavin, 2.25 mM nitro blue tetrazolium (NBT) and 100 μL of the extract, was prepared. The reaction combination was incubated at 25 °C (15 min) under light, and the absorbance was recorded at 560 nm [41].
2.7. Ascorbate Peroxidase (APX) Activity
The reaction mixture for APX activity comprised 100 mM phosphate buffer (pH = 7.6, EDTA), 2 mM H2O2, 0.5 mM sodium ascorbate and 50 μL of the extract. The absorbance was read at 290 nm (120 s), and APX activity was estimated using the extinction coefficient of ascorbate (2.8 mmol−1 cm−1) and was reported as μmol min−1 mg−1 protein [42].
2.8. Peroxidase (POX) Activity
To measure peroxidase (POX) activity, the reaction mixture, including 2 mL of 0.1 M phosphate buffer (pH 6.8), 1 mL of 0.01 M pyrogallol, 1 mL of 0.005 M H2O2 and 0.5 mL of enzyme extract, was prepared. The solution was incubated for 5 min at 25 °C, after which, the reaction was terminated by adding 1 mL of 2.5 N H2SO4. The absorbance was recorded at 420 nm, and POX activity was expressed in μmol min−1 mg−1 protein [43].
2.9. Proline
Firstly, the reaction mixture, including 2000 µL of extract, 2000 µL of ninhydrin reagent and 2000 µL of glacial acetic acid, was prepared, and the absorbance was determined at 520 nm (UV-1800 Shimadzu, Kyoto, Japan), and proline content was expressed as μmol g−1 FW [44].
2.10. SPAD Index
A relative SPAD index is achieved from the transmittance values that are related to the chlorophyll content in the leaf. Chlorophyll index (SPAD) was recorded in the fully expanded young leaves by a portable chlorophyll meter (Instruments SPAD-502, Osaka, Japan).
2.11. Chlorophyll Fluorescence Parameters
Chlorophyll fluorescence parameters were determined on fully expanded young leaves that were randomly chosen with pulse amplitude modulation fluorometer (PAM-2500, Heinz Walz, Effeltrich, Germany). The chlorophyll fluorescence parameters were measured after adaption of L. iberica in the dark for 20 min, and the data were evaluated by PamWin-3 software, as shown thoroughly by Maxwell and Johnson [45]. Maximal possible fluorescence value (Fm), minimum fluorescence (F0), the difference between F0 and Fm (Fv) and the maximal quantum yield of PSII (Fv/Fm) were calculated by PamWin-3 software [46].
2.12. Statistical Analysis
All obtained data analysis was performed by SAS (version 9.3 CEO James Goodnight, Cary, NC, USA) software, and the mean comparisons were analyzed by least significant difference (LSD) test at the 95% level of probability.
3. Results
3.1. Fresh and Dry Weight (FW and DW)
The FW and DW were significantly impacted by different irrigation regimes, fertilizer application and interaction of the two mentioned factors (Table 1). The highest FW (62.33 g pot−1) and DW (21.63 g pot−1) of balangu plants were observed in 90% FC treated with Cs-NPs+ Myco-Root. In addition, the lowest FW (27.33 g pot−1) and DW (9.60 g pot−1) were recorded in 30% FC without fertilization (control). In comparison with 90% FC, the FW and DW decreased by 14.9 and 15.3% in moderate, and by 33.9 and 34.2% in severe water stress, respectively. Additionally, the application of Cs-NPs+ Myco-Root sharply increased the FW and DW by 43.9 and 44%, respectively, when compared with non-fertilization (Table 1).

Table 1.
Fresh weight, dry weight, essential oil content, essential oil yield, chlorophyll a, chlorophyll b, total chlorophyll and carotenoid content of L. iberica influenced by different fertilizer resources and irrigation levels.
3.2. Chlorophyll a, b and Carotenoid
The content of chlorophyll a, b, total and carotenoid was significantly affected by different irrigation regimes, fertilizer sources and interaction of irrigation levels and fertilizer sources (Table 1). Water stress of 60 and 30% FC significantly reduced the photosynthetic parameters of balangu compared to well-watered plants (90% FC). However, foliar application of Cs-NPs in combination with Myco-Root ameliorated these parameters. The highest content of chlorophyll a (13.31 mg g−1 fresh weight), b (8.87 mg g−1 fresh weight), total chlorophyll (22.19 mg g−1 fresh weight) and carotenoids (5 mg g−1 fresh weight) was found in 90% FC with co-application of Cs-NPs+ Myco-Root. The lowest level of the above-mentioned traits was obtained in 30% FC without Cs-NPs+ Myco-Root (Table 1). In comparison with normal irrigation, the content of chlorophyll a, b, total chlorophylls, and carotenoids under severe water stress decreased by 36.7, 35.4, 36.1, and 28.9% under moderate water stress, and by 53.5, 29.1, 53.1, and 45.4%, respectively. Interestingly, co-application of Cs-NPs+ Myco-Root enhanced the photosynthetic pigments by 36.9, 40.6, 38.3, and 49.8% when compared with non-fertilization, respectively. Like chlorophyll and carotenoid contents, the highest SPAD index was obtained in 90% FC treated with Cs-NPs+ Myco-Root (Figure 1).

Figure 1.
The SPAD index of L. iberica in different fertilizer sources and at different irrigation levels. Different letters indicate significant differences at the 5% level according to LSD test.
3.3. Essential Oil Content (EO) and Essential Oil Yield (EOY)
The results of the interaction effects of irrigation levels and fertilizer sources demonstrated that the highest EO content (1.43%) and EOY (0.25 g pot−1) were found with moderate water stress (60% FC) following integrative application of Cs-NPs + Myco-Root. Among different irrigation regimes, the EO content and yield under moderate stress increased sharply by 283 and 260% in comparison with normal irrigation, respectively. Due to the positive effects of Cs-NPs, Myco-Root and co-application of Cs-NPs+ Myco-Root on the DW and also EO content, the EO yield of balangu plants was enhanced by 50, 83.3, and 150% in comparison with the control, respectively (Table 1).
3.4. EO Compositions
In this research, based on the phytochemical analysis results achieved by GC-FID and GC/MS analyses, 20 components were identified in the balangu essential oil under different treatments, accounting for 85.34–92.77% of the total compositions. Among the main constituents, germacrene D (31.22–39.77%), (E)-caryophyllene (16.28–19.82%), bicyclogermacrene (7.1–9.22%), and caryophyllene oxide (3.85–6.96%) were the most representative. The maximum content of germacrene D and (E)-caryophyllene was recorded in 60% FC after application of Cs-NPs+ Myco-Root. In contrast, the lowest content of the two mentioned constituents was measured in normal irrigation conditions (90% FC) without fertilization. The highest content of bicyclogermacrene was achieved under severe water deficit (30% FC) treated with co-application of Cs-NPs+ Myco-Root (Table 2).

Table 2.
The essential oil constituents of L. iberica affected by different levels of drought stress and fertilizer sources.
3.5. Chlorophyll Fluorescence Indices
Fm, Fv and Fv/Fm ratio was significantly impacted by the interaction of irrigation levels and fertilizer application (Table 3). The highest Fm and Fv and Fv/Fm values were found in 90% FC treated with Cs-NPs+ Myco-Root (Figure 2a–c). Compared with normal irrigation, the Fm, Fv and Fv/Fm values decreased by 15.7, 19.9, and 5.9% in moderate water stress and by 30.9, 37.7, and 10.3% in severe water stress conditions, respectively. In contrast, integrative application of Cs-NPs+ Myco-Root enhanced the Fm and Fv by 15.9 and 17.6% compared with the control, respectively (Figure 2a,b).

Table 3.
Results of variance analysis for the effect of different irrigation levels and fertilizer sources on the physiological characteristics of L. iberica.
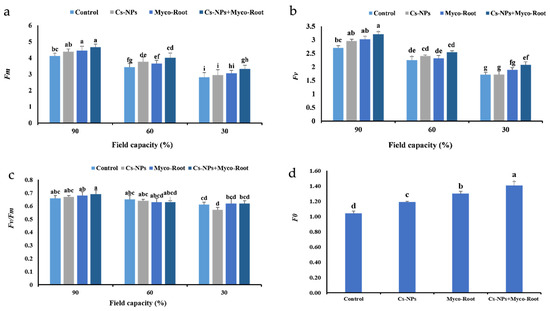

Figure 2.
The Fm (a), Fv (b), Fv/Fm (c) and F0 (d,e) values of L. iberica in different fertilizer sources and irrigation levels. Different letters indicate significant differences at the 5% level according to LSD test.
It is worth noting that the F0 values were significantly impacted by the single effect of irrigation levels and fertilizer application, and interaction of the two mentioned factors did not significantly impact on this trait (Table 3). The F0 value decreased by increasing the water deficit levels. The highest value of F0 was obtained in normal irrigation, which was 54.8 and 131.2% higher than under moderate and severe water deficit, respectively (Figure 2d). In addition, co-application of Cs-NPs+ Myco-Root strongly enhanced the F0 value, which was 35.6% higher than the control (Figure 2e).
3.6. Proline
The results demonstrated that the proline content was noticeably enhanced in the plants grown under moderate and severe stress conditions. The highest (3.04 mg g−1 fresh weight) and lowest (0.79 mg g−1 fresh weight) proline contents were observed when plants were grown under severe water deficit (30% FC) and normal irrigation conditions (90% FC) without fertilization, respectively (Figure 3a).

Figure 3.
The proline (a) and malondialdehyde (MDA) (b) content of L. iberica with different fertilizer sources and irrigation levels. Different letters indicate significant differences at the 5% level according to LSD test.
3.7. Malondialdehyde (MDA)
Similar to proline content, the MDA concentration in balangu was significantly impacted by the interaction of irrigation levels and fertilizer application (Table 3). In comparison with normal irrigation conditions, the MDA concentration increased by 97.2 and 184.4% under moderate and severe water stress, respectively. The highest MDA concentration (6.57 µmol g−1 fresh weight) was achieved under severe water stress (30% FC) without fertilization, while the lowest content of MDA (1.46 µmol g−1 fresh weight) was achieved under normal irrigation (90% FC) following application of Cs-NPs+ Myco-Root (Figure 3b).
3.8. Superoxide Dismutase (SOD), Ascorbate Peroxidase (APX) and Peroxidase (POX) Activity
In this study, the application of fertilizer sources under different irrigation levels had a significant impact on antioxidant enzymes activity (Table 3). The highest SOD (12.96 µmol min−1 mg−1 protein), APX (3.94 µmol min−1 mg−1 protein) and POX (0.37 µmol min−1 mg−1 protein) activities were obtained under moderate water stress (60% FC) treated with Cs-NPs+ Myco-Root. The antioxidant enzymes activity was enhanced by increasing water stress levels. In comparison with normal irrigation, the activity of SOD, APX and POX was enhanced by 176, 220 and 173% under moderate water stress, and by 53, 78 and 73% under severe water stress, respectively. Moreover, co-application of Cs-NPs+ Myco-Root increased the three mentioned enzymes’ activity by 48, 21, and 28% when compared with the control, respectively (Figure 4a–c).
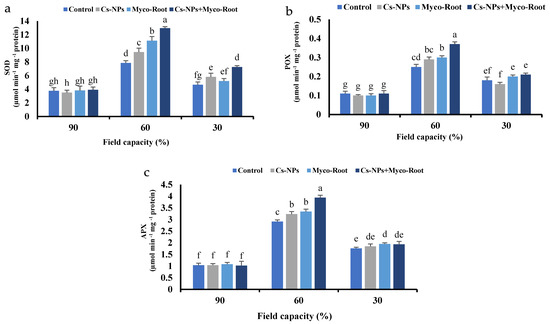
Figure 4.
The superoxide dismutase (SOD) (a), peroxidase (POX) (b) and ascorbate peroxidase (c) activity of L. iberica with different fertilizer sources and irrigation levels. Different letters indicate significant differences at the 5% level according to LSD test.
4. Discussion
The results of the study demonstrated that the fresh and dry weight of balangu decreased noticeably as water deficit stress increased. Under stressful conditions, the decline in plant biomass may be due to the closure of stomata, inhibition of rubisco enzyme and the reduction of chlorophyll content and photosynthetic efficiency [46]. Additionally, the fresh and dry weight reduction in such situations can be due to greater allocation of biomass produced to the roots [25,47,48,49]. In addition, the results relating to the fresh and dry weight of balangu showed that co-application of Cs-NPs and Myco-Root biofertilizer significantly increased the above-mentioned traits relative to the control (non-use of Cs-NPs and Myco-Root) in all irrigation regimes. The improved plant growth characteristics with co-application of Cs-NPs+ Myco-Root could be attributed to the enhancement of nutrient and water uptake which leads to increased leaf area, chlorophyll formation and photosynthetic capacity [50]. In addition, Cs-NPs may increase plant growth due to the uptake of water and essential nutrients by regulating cell osmotic pressure and reducing the accumulation of free radicals by increasing antioxidant and enzymatic activities [51]. On the other hand, the impact of Cs-NPs on dry matter retention during drought stress could be related to the reduction in transpiration induced by the closure of the stomata [18]. Moreover, the inoculation of mycorrhizal fungi with host plant roots improves the plant performance directly through increasing nutrient and water uptake and indirectly by plant phytohormonal secretions of gibberellic acid, cytokinins and jasmonic acid [52]. It was reported that inoculation of mycorrhizal fungi in thyme roots enhanced plant productivity through improving macro- and micro-nutrient uptake under drought stress conditions [14].
One of the main negative effects of drought stress on plants is the reduction of photosynthetic capacity and rate. Drought stress reduces the assimilation of CO2 in the leaves, RUBP enzyme and the quantity of ATP [53]. Our results showed that the chlorophyll content was reduced in water stress conditions. The reduction of photosynthetic pigments under water stress conditions could be explained by chlorophyll decomposition as a result of accumulation of relative oxygen (ROS) compounds and lipid peroxidation. In this situation, the increasing activity of the chlorophyllase enzyme or decreasing activity of enzymes responsible for chlorophyll synthesis negatively affects the chlorophyll concentration in plants [54]. However, in well-watered and drought stress conditions, co-application of Cs-NPs+ Myco-Root sharply increased the chlorophyll content in balangu plants. Since the availability of nutrients, such as nitrogen, iron and magnesium, is required for the synthesis of chlorophyll, it seems that the application of mycorrhizal fungi leads to an increase in the concentration of chlorophyll through the production of extensive, extra-radical mycelia and enhancement of the nutrient’s accessibility [55]. Additionally, Cs-NPs are involved in the increase of chlorophyll content and protection from water deficiency, which can affect leaf chloroplast gene expression and cause changes in chloroplast size and development [56,57]. Similarly, the application of Cs-NPs enhanced the content of chlorophyll in the coffee leaves by 30–50% through a significant increase in the concentration of magnesium and nitrogen in the leaves [58].
Chlorophyll fluorescence parameters are important indicators used to measure the quantum yield of photosystem II (PSII), displaying the plant response to stress and the harmful effects, particularly on photosynthesis and chlorophyll concentrations [31]. Dark adapted measurement of Fv/Fm (ratio of variable to maximal fluorescence) reflects maximum quantum efficiency of PSII photochemistry [59]. In this study, drought stress reduced Fv, Fm, F0 and Fv/Fm values, which clearly indicates the down-regulation of photosynthesis or photo inhibition under stressful conditions [31]. Integrative application of Cs-NPs and Myco-Root alleviated the mentioned fluorescence parameters, indicating that application of the fertilizers had a regulatory impact on water homeostasis and photosynthetic response when balangu plants were subjected to drought stress. It was reported that the exogenous application of chitosan in stressful conditions improves chlorophyll concentration as well as chlorophyll fluorescence parameters (Fv/Fm ratio) [60].
Generally, the accumulation of proline in balangu plants tends to increase when the stress intensity is enhanced. One of the primary responses of plants to drought stress is proline accumulation, which was observed under drought stress in this experiment. Proline, as a potent antioxidant and potential inhibitor of programmed cell death, plays a key role in plant tolerance to water stress [61,62]. Compatible solutes such as proline attenuate osmotic damages by supporting inter-cellular water potential, activating ROS quenching mechanisms and protecting natural arrangements of biological membranes [63]. Similarly, Omidi et al. [4] reported that the concentration of proline in L. iberica is enhanced in drought stress conditions. The concentration of proline decreased after application of Cs-NPs and Myco-Root in drought stress conditions. It seems that application of the mentioned fertilizers, separately and in combination with each other, mitigated the negative impacts of drought stress and accumulation of ROS compounds as a result of improving chlorophylls and carotenoids as well as antioxidant enzymes activity.
In the present study, the amount of MDA increased under moderate and severe water stress. Lipid peroxidation is the result of oxidative stress in plants. Unsaturated fatty acids are the most sensitive part of the membrane to oxidation and degradation by oxidative stress. The accumulation of MDA is due to the breakdown of unsaturated fatty acids in cell membranes by ROS. In addition, MDA is an active, electron-friendly aldehyde that is not normally seen in pure form and is often considered as a biomarker for peroxidation of membrane lipids. MDA accumulation under stress conditions enhances the permeability of the plasma membrane and ion leakage [64]. Notably, the MDA concentration in water stress conditions decreased with application of Cs-NPs, Myco-Root and co-application of the two mentioned fertilizers. The higher activity of antioxidant enzymes mitigates the negative impact of ROS compounds and lipid peroxidation in plant cells. Therefore, the reduction of MDA content could be explained by the role of the mentioned fertilizer increasing the activity of antioxidant enzymes such as SOD, APX and POX.
Our results showed that the activity of antioxidant enzymes was enhanced in water stress conditions, especially in 60% FC. Stressful conditions such as water deficit may have harmful impacts on plant productivity due to lipid peroxidation of membrane cells. Increasing ROS compounds under stressful conditions induces lipid peroxidation, which play a critical role in cell death [65]. The increasing activity of SOD, APX and POX enzymes is known as one of the enzymatic antioxidant defense mechanisms for decreasing ROS level and preventing oxidative damages which lead to stabilization of the cell structure and an improvement of plant growth [55]. In addition, the results showed that co-application of Cs-NPs+ Myco-Root increased the antioxidant enzymes activity. Since enzymes are protein compounds, the availability of nutrients, especially nitrogen, plays an important role in increasing their activity [55]. Therefore, the increasing activity of antioxidant enzymes with co-application of Cs-NPs+ Myco-Root is attributed to the improvement of the availability of nutrients leading to higher synthesis of amino acids, enzymes and their activities [66]. Similarly, Amani Machiani et al. [55] noted that the application of mycorrhiza fungi enhanced antioxidant enzymes SOD and APX by 8.1 and 10.1% under drought stress conditions, respectively.
The synthesis of secondary metabolites in medicinal plants is one of the effective ways to increase the efficiency of these plants in stressful environments. The obtained results showed that the EO content, yield and main EO constituents of balangu plants were enhanced under moderate water stress. Water stress causes a metabolic reaction in plants, such as closure of the stomata and also reduction of CO2 uptake, which leads to decreasing consumption of NADPH+H+ for CO2 fixation via the Calvin cycle. Eventually, metabolic processes are shifted towards the production of secondary metabolites, which increase the synthesis of regenerative compounds such as EOs and phenolic and alkaloid compounds [13]. Additionally, the co-application of Cs-NPs+ Myco-Root improved EO quantity and quality through an increase in the main EO constituents such as germacrene D and (E)-caryophyllene. The increasing photosynthetic efficiency and availability of intermediate (acetyl-CoA, ATP and NADPH) and essential oil precursors play an important role in EO composition [14]. In this study, the integrative application of Cs-NPs+ Myco-Root significantly enhanced the chlorophyll content and chlorophyll fluorescence indices. It seems that the co-application of Cs-NPs+ Myco-Root improves the photosynthetic rate, along with the nutrient accessibility, which have an important role in the production of carbohydrates and also in the development of glandular trichomes, EO channels and secretory ducts [13]. In this situation, the higher photosynthetic rate enhances the concentration of primary metabolites such as pyruvate, erythrose-4-phosphate, phosphoenolpyruvate and glyceraldehyde-3-phosphate, which are used as precursors in the synthesis of EO constituents [52].
5. Conclusions
The results of the study demonstrated that drought stress decreases the chlorophyll concentration, as well as chlorophyll fluorescence parameters, which decrease fresh and dry matter yield in balangu plants. In this situation, integrative application of chitosan nanoparticles, along with arbuscular mycorrhiza fungi biofertilizer (Cs-NPs+ Myco-Root), increases the plant productivity by improving the chlorophyll fluorescence parameters and antioxidant activity. Interestingly, co-application of Cs-NPs+ Myco-Root improved the EO content and its chemical profile by increasing the major EO constituents (germacrene D and (E)-caryophyllene), especially under moderate water stress. Generally, it can be concluded that the co-application of Cs-NPs+ Myco-Root can be regarded as a sustainable and eco-friendly strategy to improve morphological and physiological characteristics and EO quantity and quality in balangu under drought stress conditions.
Author Contributions
Conceptualization, A.J.; data curation, F.R., A.J. and M.A.M.; formal analysis, A.J., M.R.M. and M.A.; methodology, M.R.M. and A.J.; project administration, A.J.; visualization, A.J., M.R.M. and F.R.; writing—original draft, A.J., M.A.M. and F.R.; writing—review and editing, M.R.M., M.A.M. and F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the University of Maragheh, Iran.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We wish to acknowledge the management of research and technology and the central laboratory of University of Maragheh for their help in running the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ursu, B.; Borcean, I. Researches Concerning the Sowing Technology at Lallemantia iberica F.et M. Res. J. Agric. Sci. 2012, 44, 168–171. [Google Scholar]
- Paravar, A.; Maleki Farahani, S.; Rezazadeh, A.R. Lallemantia species response to drought stress and Arbuscular mycorrhizal fungi application. Ind. Crops Prod. 2021, 172, 114002. [Google Scholar] [CrossRef]
- Ghasemi, V.M.; Moghaddam, S.S.; Rahimi, A.; Pourakbar, L.; Popović-Djordjević, J. Winter Cultivation and Nano Fertilizers Improve Yield Components and Antioxidant Traits of Dragon’s Head (Lallemantia iberica (m.b.) Fischer & Meyer). Plants 2020, 9, 252. [Google Scholar] [CrossRef] [Green Version]
- Omidi, H.; Shams, H.; Seif Sahandi, M.; Rajabian, T. Balangu (Lallemantia Sp.) Growth and Physiology under Field Drought Conditions Affecting Plant Medicinal Content. Plant Physiol. Biochem. 2018, 130, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, S.; Aghaei-Gharachorlou, P. Effect of irrigation frequency and planting density on herbage biomass, essential oil production and mucilage yield of dragon’s head (Lallemantia iberica fish. Et mey.). IJAB 2014, 3, 89–94. [Google Scholar]
- Razavi, S.M.A.; Mohammadi, M.T. Influence of different substitution levels of Balangu seed gum on textural characteristics of selected hydrocolloids. Electron. J. Environ. Agric. Food Chem. 2011, 10, 2826–2837. [Google Scholar]
- Yuce, E.; Bagci, E. Study of the essential oil composition of Lallemantia iberica (M. Bieb.) Fisch. and C.A. Mey. (Lamiaceae) from Turkey. Asian J. Chem. 2012, 24, 4817–4818. [Google Scholar]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Xu, J.; Jin, J.; Zhao, H. Drought stress tolerance analysis of Populus ussuriensis clones with different ploidies. J. Res. 2019, 30, 1267–1275. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H.J. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Karimi, S.; Abbaspour, H.; Sinaki, J.M.; Makarian, H. Effects of water deficit and chitosan spraying on osmotic adjustment and soluble protein of cultivars castor bean (Ricinus communis L.). J. Stress Physiol. Biochem. 2012, 28, 160–169. [Google Scholar]
- Dacosta, M.; Huang, B. Changes in antioxidant enzyme activities and lipid peroxidation for bent grass species in response to drought stress. J. Am. Soc. Hortic. Sci. 2007, 132, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Ostadi, A.; Javanmard, A.; Amani Machiani, M.; Sadeghpour, A.; Maggi, F.; Nouraein, M.; Morshedloo, M.R.; Hano, C.; Lorenzo, J.M. Co-Application of TiO2 Nanoparticles and Arbuscular Mycorrhizal Fungi Improves Essential Oil Quantity and Quality of Sage (Salvia officinalis L.) in Drought Stress Conditions. Plants 2022, 11, 1659. [Google Scholar] [CrossRef]
- Amani Machiani, M.; Javanmard, A.; Morshedloo, M.R.; Aghaee, A.; Maggi, F. Funneliformis mosseae inoculation under water deficit stress improves the yield and phytochemical characteristics of thyme in intercropping with soybean. Sci. Rep. 2021, 11, 15279. [Google Scholar] [CrossRef]
- Strzemski, M.; Dzida, K.; Dresler, S.; Sowa, I.; Kurzepa, J.; Szymczak, G.; Wójciak, M. Nitrogen fertilisation decreases the yield of bioactive compounds in Carlina acaulis L. grown in the field. Ind. Crops Prod. 2021, 170, 113698. [Google Scholar] [CrossRef]
- Rasouli, F.; Amini, T.; Asadi, M.; Hassanpouraghdam, M.B.; Aazami, M.A.; Ercisli, S.; Skrovankova, S.; Mlcek, J. Growth and Antioxidant Responses of Lettuce (Lactuca sativa L.) to Arbuscular Mycorrhiza Inoculation and Seaweed Extract Foliar Application. Agronomy 2022, 12, 401. [Google Scholar] [CrossRef]
- Palacio-Márquez, A.; Ramírez-Estrada, C.A.; Gutiérrez-Ruelas, N.J.; Sánchez, E.; Ojeda-Barrios, D.L.; Chávez-Mendoza, C.; Sida-Arreola, J.P. Efficiency of foliar application of zinc oxide nanoparticles versus zinc nitrate complexed with chitosan on nitrogen assimilation, photosynthetic activity, and production of green beans (Phaseolus vulgaris L.). Sci. Hortic. 2021, 288, 110297. [Google Scholar] [CrossRef]
- Golkar, P.; Taghizadeh, M.; Yousefian, Z. The effects of chitosan and salicylic acid on elicitation of secondary metabolites and antioxidant activity of safflower under in vitro salinity stress. Plant Cell Tissue Organ Cult. 2019, 137, 575–585. [Google Scholar] [CrossRef]
- Farouk, S.; Amany, R. Improving growth and yield of cowpea by foliar application of chitosan under water stress. Egypt. J. Biol. Pest. Control 2012, 14, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Hassan, F.A.S.; Ali, E.; Gaber, A.; Fetouh, M.I.; Mazrou, R. Chitosan Nanoparticles Effectively Combat Salinity Stress by Enhancing Antioxidant Activity and Alkaloid Biosynthesis in Catharanthus roseus (L.) G. Don. Plant Physiol. Biochem. 2021, 162, 291–300. [Google Scholar] [CrossRef]
- Tourian, N.; Sinaki, J.M.; Hasani, N.; Madani, H. Change in photosynthetic pigment concentration of wheat grass (Agropyron repens) cultivars response to drought stress and foliar application with chitosan. Int. J. Agron. Plant Prod. 2013, 4, 1084–1091. [Google Scholar]
- El-Serafy, R.S. Phenotypic Plasticity, Biomass allocation, and biochemical analysis of cordyline seedlings in response to oligo-chitosan foliar spray. Soil Sci. Plant Nutr. 2020, 20, 1503–1514. [Google Scholar] [CrossRef]
- Emami Bistgani, Z.; Siadat, S.A.; Bakhshandeh, A.; Ghasemi Pirbalouti, A.; Hashemi, M. Interactive effects of drought stress and chitosan application on physiological characteristics and essential oil yield of Thymus daenesis Celak. Crop J. 2017, 5, 407–415. [Google Scholar] [CrossRef]
- Yin, H.; Frette, X.C.; Christensen, L.P.; Grevsen, K. Chitosan oligosaccharides promote the content of polyphenols in Greek oregano (Origanum vulgare ssp. hirtum). J. Agric. Food Chem. 2012, 60, 136–143. [Google Scholar] [CrossRef]
- Alizadeh, A.; Moghaddam, M.; Asgharzade, A.; Mahmoodi Sourestani, M. Phytochemical and physiological response of Satureja hortensis L. to different irrigation regimes and chitosan application. Ind. Crops Prod. 2020, 158, 112990. [Google Scholar] [CrossRef]
- Panwar, J.; Tarafdar, J.C. Arbuscular mycorrhizal fungal dynamics under Mitragyna parvifolia (Roxb.) Korth. in thar desert. Appl. Soil Ecol. 2006, 34, 200–208. [Google Scholar] [CrossRef]
- Abdollahi, A.A.; Feizian, M.; Mehdipourian, G.; Khojasteh, D.N. Arbuscular mycorrhizal fungi inoculation improve essential oil and physiological parameters and nutritional values of Thymus daenensis Celak and Thymus vulgaris L. under normal and drought stress conditions. Eur. J. Soil Biol. 2020, 100, 103217. [Google Scholar] [CrossRef]
- Hashem, A.; Kumar, A.; Al-Dbass, A.M.; Alqarawi, A.A.; Al-Arjani, A.B.F.; Singh, G.; Farooq, M.; Abd_Allah, E.F. Arbuscular mycorrhizal fungi and biochar improves drought tolerance in chickpea. Saudi J. Biol. Sci. 2019, 26, 614–624. [Google Scholar] [CrossRef]
- Silva, V.C.; Casaes Alves, F.A.; Oliveira, R.A.; Jesus, R.M.; Bomfim Costa, L.C.; Gross, E. Influence of arbuscular mycorrhizal fungi on growth, mineral composition and production of essential oil in Mentha × piperita L. var. citrata (Ehrh.) Briq. under two phosphorus levels. J. Med. Plant Res. 2014, 8, 1321–1332. [Google Scholar] [CrossRef]
- Benaffari, W.; Boutasknit, A.; Anli, M.; Ait-El-Mokhtar, M.; Ait-Rahou, Y.; Ben-Laouane, R.; Ahmed, H.; Mitsui, T.; Baslam, M.; Meddich, A. The native arbuscular mycorrhizal fungi and vermicompost-based organic amendments enhance soil fertility, growth performance, and the drought stress tolerance of quinoa. Plants 2022, 11, 393. [Google Scholar] [CrossRef]
- Mathur, S.; Tomar, R.S.; Jajoo, A. Arbuscular mycorrhizal fungi (AMF) protects photosynthetic apparatus of wheat under drought stress. Photosynth. Res. 2019, 139, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Pirzad, A.; Mohammadzadeh, S.J. Water use efficiency of three mycorrhizal Lamiaceae species (Lavandula officinalis, Ros-ma-rinus officinalis and Thymus vulgaris). Agric. Water Manag. 2018, 204, 1–10. [Google Scholar] [CrossRef]
- Khorasaninejad, S.; Mousavi, A.; Soltanloo, H.; Hemmati, K.; Khalighi, A. The effect drought stress on growth parameters, essential oil yield and constituent of pepprmint (Mentha piperita L.). J. Med. Plant Res. 2011, 22, 5360–5365. [Google Scholar]
- Arnon, A. Method of extraction of chlorophyll in the plants. Agron. J. 1967, 23, 112–121. [Google Scholar] [CrossRef]
- Morshedloo, M.R.; Craker, L.E.; Salami, A.; Nazeri, V.; Sang, H.; Maggi, F. Effect of prolonged water stress on essential oil content, compositions and gene expression patterns of mono-and sesquiterpene synthesis in two oreganos (Origanum vulgare L.) subspecies. Plant Physiol. Biochem. 2017, 111, 119–128. [Google Scholar] [CrossRef]
- Safaei, L.; Sharifi Ashorabadi, E.; Afyouni, D. The effects npk, chemical and manure fertilizers investigation on the phenolic yield and essential oil components in Thymus daenensis L. EJMP 2017, 17, 1–15. [Google Scholar]
- Poshtdar, A.; Mashhadie, A.R.A.; Moradi, F.; Siadat, S.A.; Bakhshandeh, A. Effect of source and rate of nitrogen fertilizer on yield and water and nitrogen use efficiency of peppermint (Mentha piperita L.). Iran. J. Crop Sci. 2016, 18, 14–31. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectrometry; Allured Publishing Corporation: Carroll Stream, IL, USA, 2007; Volume 456, pp. 544–545. [Google Scholar]
- Morshedloo, M.R.; Maggi, F.; Neko, H.T.; Aghdam, M.S. Sumac (Rhus coriaria L.) fruit: Essential oil variability in iranian populations. Ind. Crops Prod. 2018, 111, 1–7. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Kumar, K.B.; Khan, P.A. Peroxidase & Polyphenol Oxidase in Excised Ragi (Eleusine Corocana Cv PR 202) Leaves during Senescence. Indian J. Exp. Biol. 1982, 20, 412–416. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Vargas-Ortiz, E.; Ramírez-Tobias, H.M.; González-Escobar, J.L.; Gutiérrez-García, A.K.; Bojórquez-Velázquez, E.; Espitia-Rangel, E.; Barba de la Rosa, A.P. Biomass, chlorophyll fluorescence, and osmoregulation traits let differentiation of wild and cultivated Amaranthus under water stress. J. Photochem. Photobiol. B Biol. 2021, 220, 112210. [Google Scholar] [CrossRef]
- Albouchi, A.; Bejaoui, Z.; El Aouni, M.H. Influence of moderate or severe water stress on the growth of Casuarina glauca Sieb. Seedlings. Sécheresse 2003, 14, 137–142. [Google Scholar]
- Bahreininejad, B.; Razmjoo, J.; Mirza, M. Influence of water stress on morphophysiological and phytochemical traits in (Thymus daenensis). Int. J. Plant Prod. 2013, 7, 152–166. [Google Scholar]
- Tabrizi, L.; Koocheki, A.; Rezvani Moghaddam, P.; Nasiri Mahallati, M.; Bannayan, M. Effect of irrigation and organic manure on Khorasan thyme (Thymus transcaspicus Klokov). Arch. Agron. Soil Sci. 2011, 57, 317–326. [Google Scholar] [CrossRef]
- Abd El-Azeim, M.M.; Sherif, M.A.; Hussien, M.S.; Tantawy, I.A.A.; Bashandy, S.O. Impacts of nano- and non-nanofertilizers on potato quality and productivity. Acta Ecol. Sin. 2020, 40, 388–397. [Google Scholar] [CrossRef]
- Ghasemi Pirbalouti, A.; Malekpoor, F.; Salimi, A.; Golparvar, A. Exogenous application of chitosan on biochemical and physiological characteristics, phenolic content and antioxidant activity of two species of basil (Ocimum ciliatum and Ocimum basilicum) under reduced irrigation. Sci. Hortic. 2017, 217, 114–122. [Google Scholar] [CrossRef]
- Zhao, Y.; Cartabia, A.; Lalaymia, I.; Declerck, S. Arbuscular mycorrhizal fungi and production of secondary metabolites in medicinal plants. Mycorrhiza 2022, 32, 221–256. [Google Scholar] [CrossRef]
- Minaei, A.; Hassani, A.; Nazemiyeh, H.; Besharat, S. Effect of drought stress on some morphophysiological and phytochemical characteristics of oregano (Origanum vulgare L. ssp. gracile). IJMAPR 2019, 35, 252–265. [Google Scholar]
- Gholinezhad, E.; Darvishzadeh, R. Influence of arbuscular mycorrhiza fungi and drought stress on fatty acids profile of sesame (Sesamum indicum L.). Field Crops Res. 2021, 262, 108035. [Google Scholar] [CrossRef]
- Amani Machiani, M.; Javanmard, A.; Morshedloo, M.R.; Janmohammadi, M.; Maggi, F. Funneliformis mosseae Application Improves the Oil Quantity and Quality and Ecophysiological Characteristics of Soybean (Glycine max L.) Under Water Stress Conditions. J. Plant Nutr. Soil Sci. 2021, 21, 3076–3090. [Google Scholar] [CrossRef]
- Limpanavech, P.; Chaiyasuta, S.; Vongpromek, R.; Pichyangkura, R.; Khunwasi, C.; Chadchawan, S. Chitosan effects on floral production, gene expression, and anatomical changes in the Dendrobium orchid. Sci. Hortic. 2008, 116, 65–72. [Google Scholar] [CrossRef]
- Ali, E.F.; El-Shehawi, A.M.; Ibrahim, O.H.M.; Abdul-Hafeez, E.Y.; Moussa, M.M.; Hassan, F.A.S. A vital role of chitosan nanoparticles in improvisation the drought stress tolerance in Catharanthus roseus (L.) through biochemical and gene expression modulation. Plant Physiol. Biochem. 2021, 161, 166–175. [Google Scholar] [CrossRef]
- Van, S.; Dinh Minh, H.; Nguyen Anh, D. Study on chitosan nanoparticles on biophysical characteristics and growth of Robusta coffee in greenhouse. Biocatal. Agric. Biotechnol. 2013, 2, 289–294. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annul. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Tian, Y.; Zhang, B.; Hassan, M.J.; Li, Z.; Zhu, Y. Chitosan (CTS) Alleviates Heat-Induced Leaf Senescence in Creeping Bentgrass by Regulating Chlorophyll Metabolism, Antioxidant Defense, and the Heat Shock Pathway. Molecules 2021, 26, 5337. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, S.; Wang, B.; Zhao, J. Physiological and biochemical changes in different drought-tolerant alfalfa (Medicago sativa L.) varieties under PEG-induced drought stress. Acta Physiol. Plant 2018, 40, 25. [Google Scholar] [CrossRef]
- Dutta, T.; Neelapu, N.R.; Wani, S.H.; Challa, S. Compatible Solute Engineering of Crop Plants for Improved Tolerance toward Abiotic Stresses. Biochemical, Physiological and Molecular Avenues for Combating Abiotic Stress Tolerance in Plants; Academic Press: Cambridge, MA, USA, 2018; pp. 221–254. [Google Scholar]
- Pryor, W.A.; Stanley, J.P. A suggested mechanism for the production of malonaldehyde during the antioxidation of polyunsaturated fatty acids, nonenzymatic production of prostaglandin endoperoxides during autoxidation. Organells 1975, 40, 3615–3617. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Yu, H.Y.; Yang, M.M.; Kong, D.S.; Zhang, Y.J. Effect of Drought Stress on Lipid Peroxidation, Osmotic Adjustment and Antioxidant Enzyme Activity of Leaves and Roots of Lycium ruthenicum Murr. Seedling. Russ. J. Plant Physiol. 2018, 65, 244–250. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).