Establishment of an Efficient In Vitro Propagation Method for a Sustainable Supply of Plectranthus amboinicus (Lour.) and Genetic Homogeneity Using Flow Cytometry and SPAR Markers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Shoot Materials and Explants Preparation
2.2. Culture Media and Growth Condition
2.3. Growth Regulators, Shoot Induction, and Proliferation
2.4. Rooting of Shootlets and Acclimation
2.5. Flow Cytometric Profile of Plants
2.6. DNA Extraction and Molecular Characterization
2.7. Statistical Analysis
3. Results
3.1. Effects of Cytokinin on Shoot Induction
3.2. Effects of Auxin with Optimal Concentration of BA
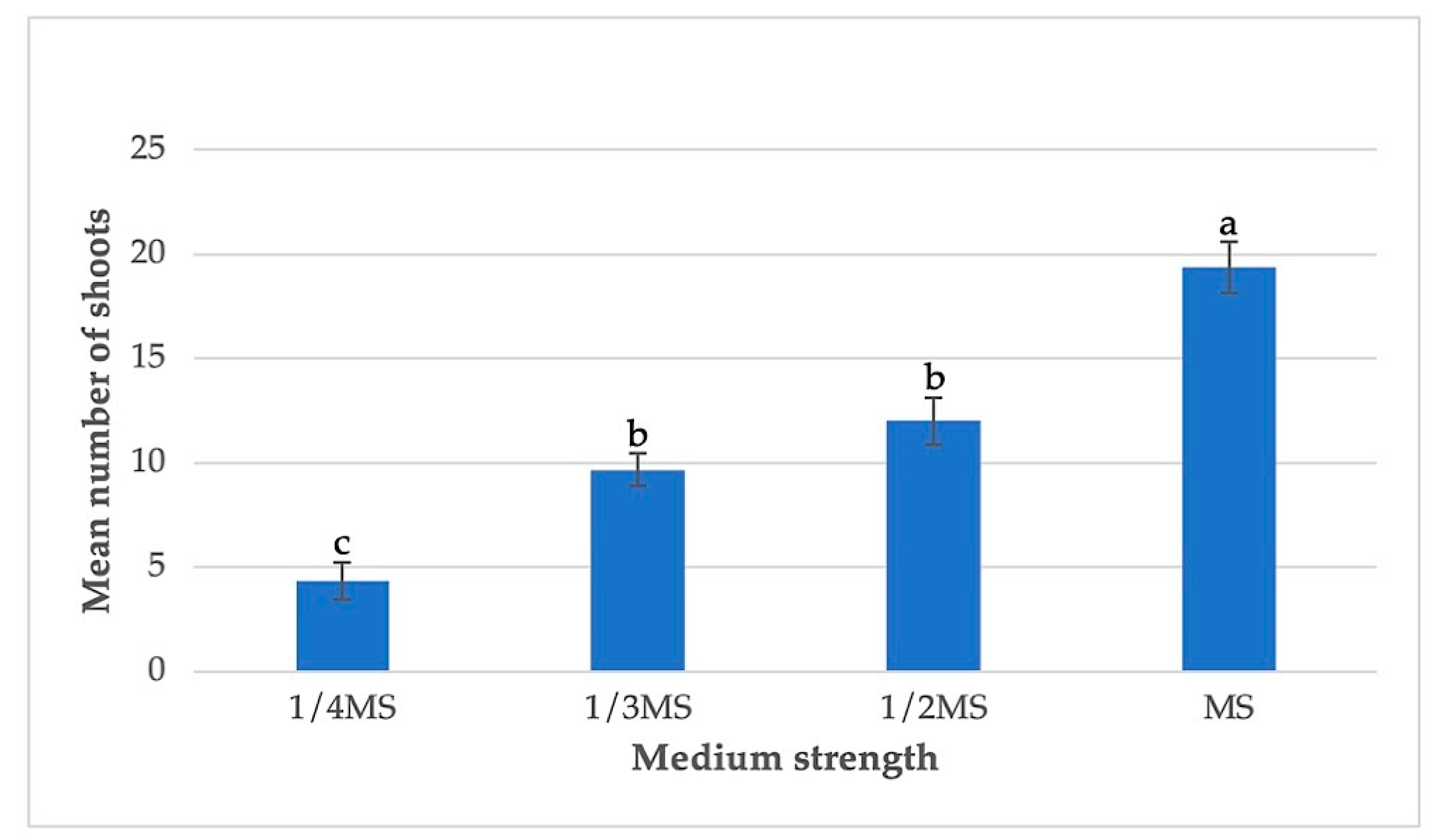
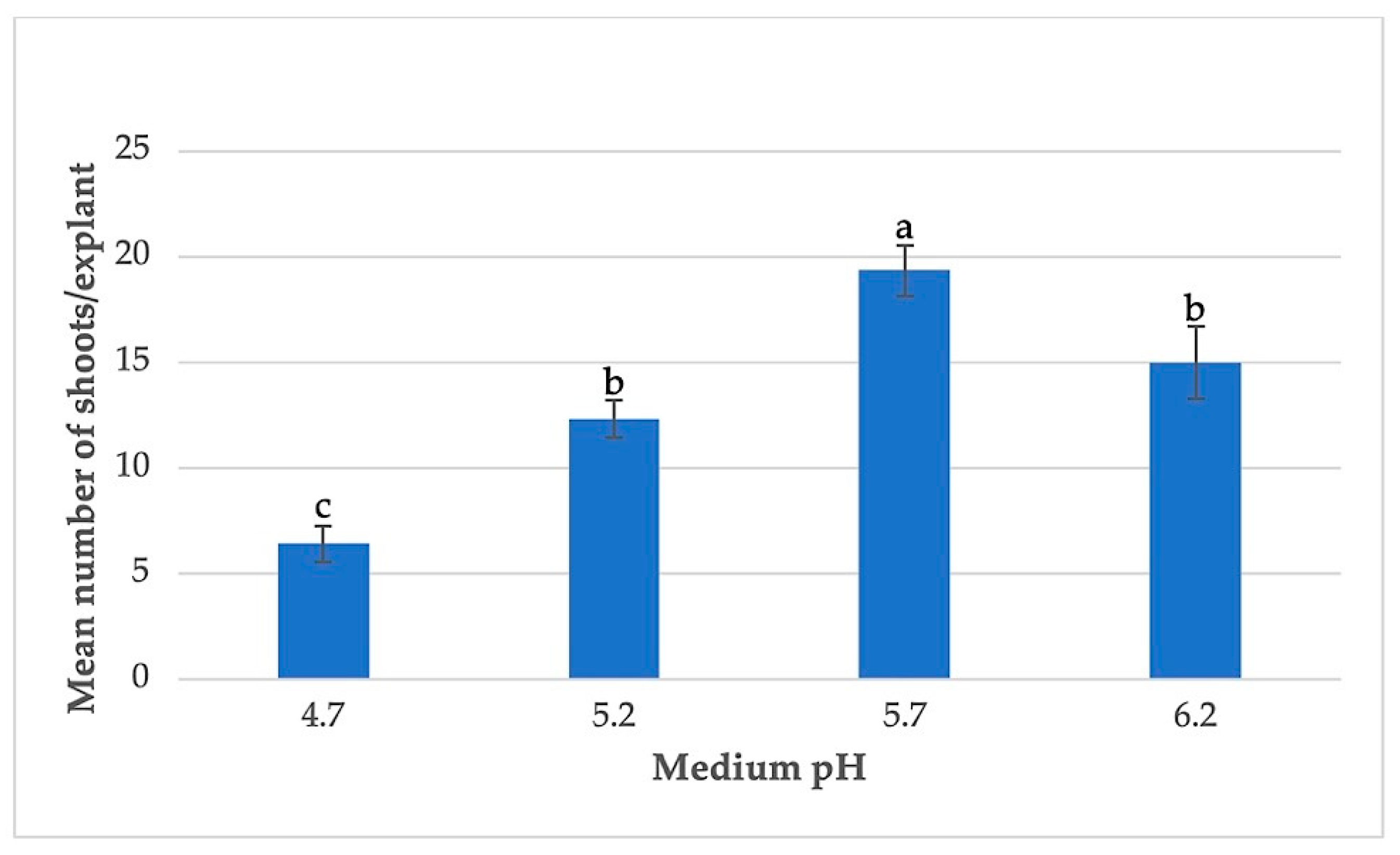
3.3. Effects of Basal Medium Strength and pH
3.4. Rooting of Microshoots and Acclimation
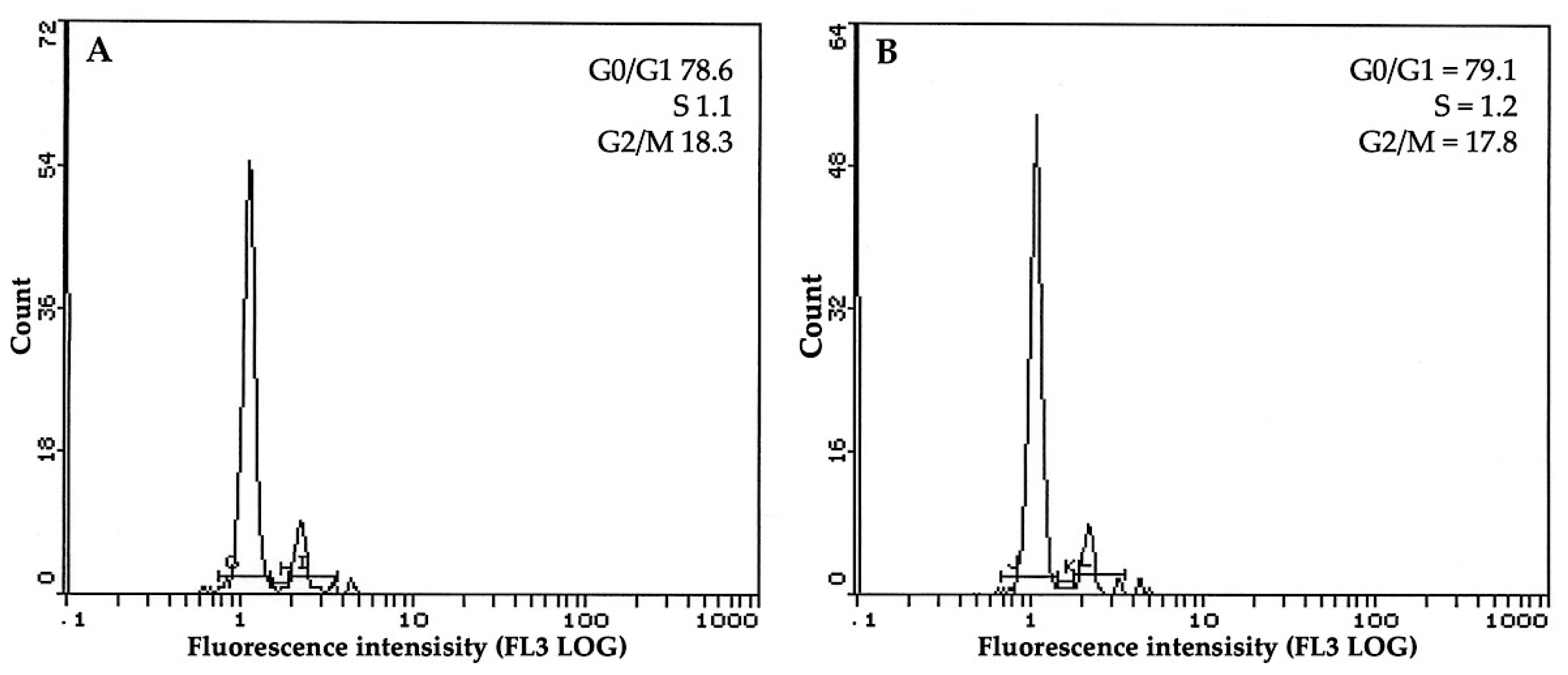
3.5. Flow Cytometric Profile of Plants
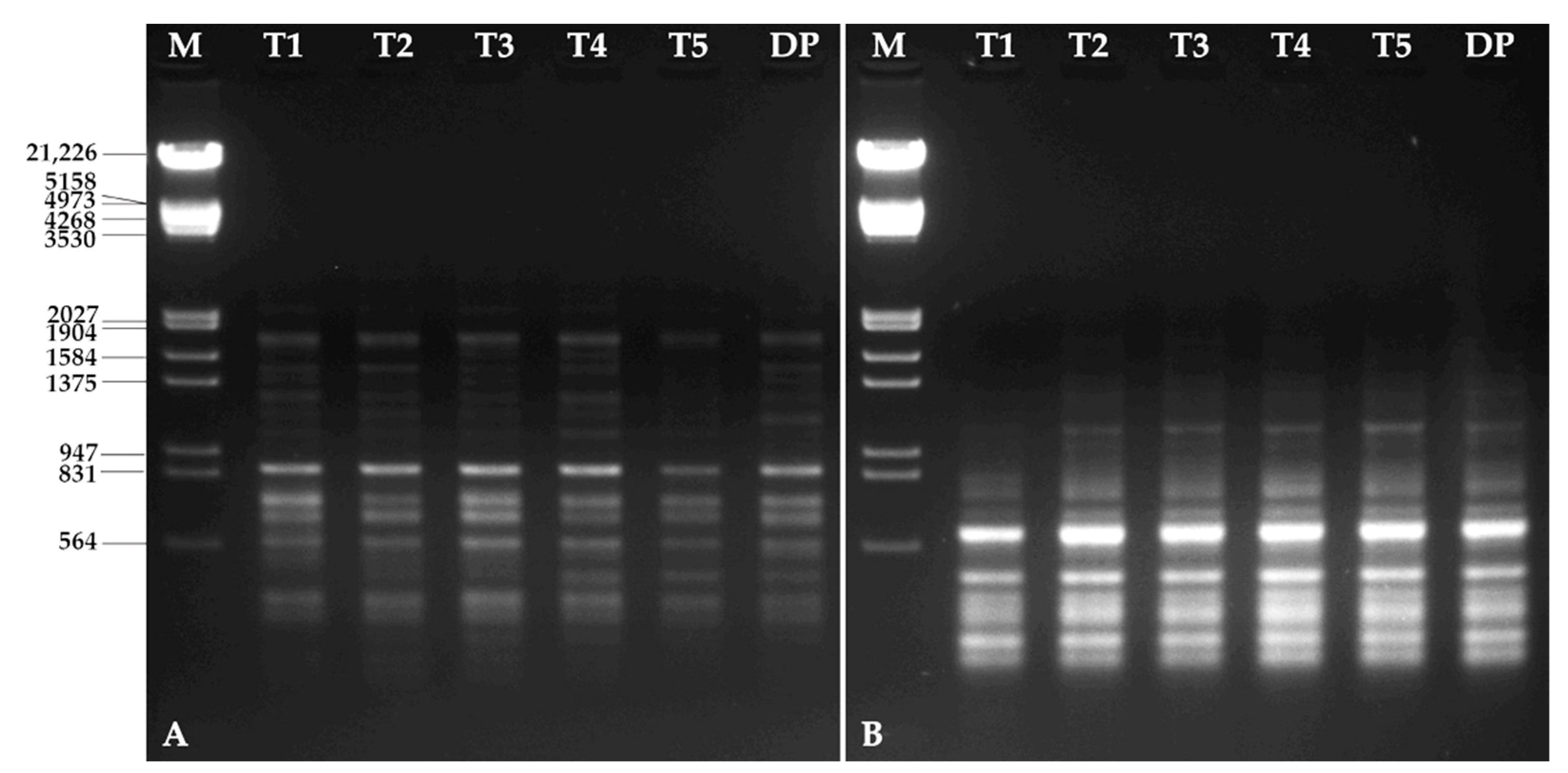
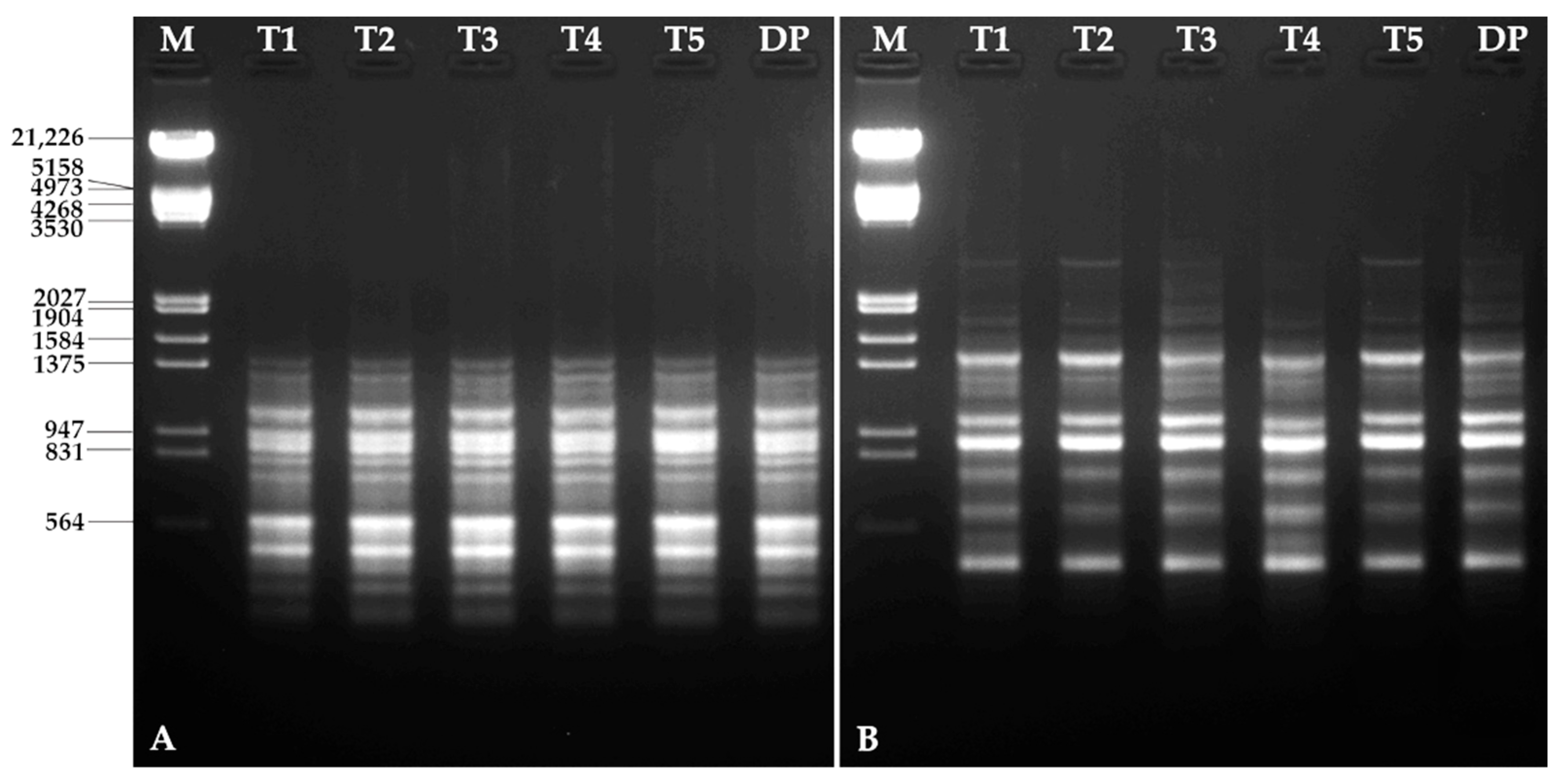
3.6. Molecular Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arumugam, G.; Swamy, M.K.; Sinniah, U.R. Plectranthus amboinicus (Lour.) Spreng: Botanical, Phytochemical, Pharmacological and Nutritional Significance. Molecules 2016, 21, 369. [Google Scholar] [CrossRef]
- Lukhoba, C.W.; Simmonds, M.S.J.; Paton, A.J. Plectranthus: A review of ethnobotanical uses. J. Ethnopharmacol. 2006, 103, 1–24. [Google Scholar] [CrossRef]
- Hsu, K.-P.; Ho, C.-L. Antimildew Effects of Plectranthus amboinicus Leaf Essential Oil on Paper. Nat. Prod. Commun. 2019, 14, 1–6. [Google Scholar] [CrossRef]
- Senthilkumar, A.; Venkatesalu, V. Chemical composition and larvicidal activity of the essential oil of Plectranthus amboinicus (Lour.) Spreng against Anopheles stephensi: A malarial vector mosquito. Parasitol. Res. 2010, 107, 1275–1278. [Google Scholar] [CrossRef]
- Khare, R.S.; Kundu, S. Coleus aromaticus Benth-A Nutritive Medicinal Plant Of Potential Therapeutic Value. Int. J. Pharma Bio. Sci. 2011, 2, 488–500. [Google Scholar]
- Menéndez Castillo, R.A.; Pavón González, V. Plecthranthus amboinicus (Lour.) spreng. Rev. Cuba. Plantas Med. 1999, 4, 110–115. [Google Scholar]
- Singh, G.; Singh, O.P.; Prasad, Y.; De Lampasona, M.; Catalan, C. Studies on essential oils, Part 33: Chemical and insecticidal investigations on leaf oil of Coleus amboinicus Lour. Flavour Fragr. J. 2002, 17, 440–442. [Google Scholar] [CrossRef]
- Murthy, P.S.; Ramalakshmi, K.; Srinivas, P. Fungitoxic activity of Indian borage (Plectranthus amboinicus) volatiles. Food Chem. 2009, 114, 1014–1018. [Google Scholar] [CrossRef]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef]
- Aguiar, J.J.S.; Sousa, C.P.B.; Araruna, M.K.A.; Silva, M.K.N.; Portelo, A.C.; Lopes, J.C.; Carvalho, V.R.A.; Figueredo, F.G.; Bitu, V.C.N.; Coutinho, H.D.M.; et al. Antibacterial and modifying-antibiotic activities of the essential oils of Ocimum gratissimum L. and Plectranthus amboinicus L. Eur. J. Integr. Med. 2015, 7, 151–156. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Vinoj, G.; Malaikozhundan, B.; Shanthi, S.; Vaseeharan, B. Plectranthus amboinicus leaf extract mediated synthesis of zinc oxide nanoparticles and its control of methicillin resistant Staphylococcus aureus biofilm and blood sucking mosquito larvae. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 886–891. [Google Scholar] [CrossRef]
- Oliveira, R.d.A.G.d.; Lima, E.d.O.; Souza, E.L.d.; Vieira, W.L.; Freire, K.R.; Trajano, V.N.; Lima, I.O.; Silva-Filho, R.N. Interference of Plectranthus amboinicus (Lour.) Spreng essential oil on the anti-Candida activity of some clinically used antifungals. Rev. Bras. Farmacogn. 2007, 17, 186–190. [Google Scholar] [CrossRef]
- Asiimwe, S.; Borg-Karlsson, A.-K.; Azeem, M.; Mugisha, K.M.; Namutebi, A.; Gakunga, N.J. Chemical composition and toxicological evaluation of the aqueous leaf extracts of Plectranthus amboinicus Lour. Spreng. Int. J. Pharm. Sci. Invent. 2014, 3, 19–27. [Google Scholar]
- Hattori, M.; Nakabayashi, T.; Lim, Y.A.; Miyashiro, H.; Kurokawa, M.; Shiraki, K.; Gupta, M.P.; Correa, M.; Pilapitiya, U. Inhibitory effects of various ayurvedic and Panamanian medicinal plants on the infection of herpes simplex virus-1 in vitro and in vivo. Phytother. Res. 1995, 9, 270–276. [Google Scholar] [CrossRef]
- Kusumoto, I.T.; Nakabayashi, T.; Kida, H.; Miyashiro, H.; Hattori, M.; Namba, T.; Shimotohno, K. Screening of various plant extracts used in ayurvedic medicine for inhibitory effects on human immunodeficiency virus type 1 (HIV-1) protease. Phytother. Res. 1995, 9, 180–184. [Google Scholar] [CrossRef]
- Hamidi, J.A.; Ismaili, N.; Ahmadi, F.; Lajisi, N.H. Antiviral and cytotoxic activities of some plants used in Malaysian indigenous medicine. Pertanika. J. Trop. Agric. Sci. 1996, 19, 129–136. [Google Scholar]
- Bhattacharjee, P.; Majumder, P. Investigation of phytochemicals and anti-convulsant activity of the plant Coleus amboinicus (Lour.). Int. J. Green Pharm. 2013, 7, 211. [Google Scholar]
- Gurgel, A.P.A.D.; da Silva, J.G.; Grangeiro, A.R.S.; Oliveira, D.C.; Lima, C.M.P.; da Silva, A.C.P.; Oliveira, R.A.G.; Souza, I.A. In vivo study of the anti-inflammatory and antitumor activities of leaves from Plectranthus amboinicus (Lour.) Spreng (Lamiaceae). J. Ethnopharmacol. 2009, 125, 361–363. [Google Scholar] [CrossRef]
- Ramalakshmi, P.; Subramanian, N.; Saravanan, R.; Mohanakrishnan, H.; Muthu, M. Anticancer effect of Coleus amboinicus (Karpooravalli) on human lung cancer cell line (A549). Int. J. Dev. Res. 2014, 4, 2442–2449. [Google Scholar]
- Silitonga, M.; Ilyas, S.; Hutahaean, S.; Sipahutar, H. Levels of apigenin and immunostimulatory activity of leaf extracts of bangunbangun (Plectranthus amboinicus Lour.). Int. J. Biol. 2015, 7, 46. [Google Scholar] [CrossRef]
- Chiu, Y.-J.; Huang, T.-H.; Chiu, C.-S.; Lu, T.-C.; Chen, Y.-W.; Peng, W.-H.; Chen, C.-Y. Analgesic and antiinflammatory activities of the aqueous extract from Plectranthus amboinicus (Lour.) Spreng. Both in vitro and in vivo. Evid. Based Complement. Alternat. Med. 2012, 2012, 508137. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, A. Antioxidant and free radical scavenging activity of an aqueous extract of Coleus aromaticus. Food Chem. 2006, 97, 109–114. [Google Scholar] [CrossRef]
- Praveena, B.; Pradeep, S.N. Antioxidant and antibacterial activities in the leaf extracts of Indian borage (Plectranthus amboinicus). Food Sci. Nutr. 2012, 2012, 146–152. [Google Scholar] [CrossRef]
- Cartaxo, S.L.; Souza, M.M.d.A.; de Albuquerque, U.P. Medicinal plants with bioprospecting potential used in semi-arid northeastern Brazil. J. Ethnopharmacol. 2010, 131, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Hole, R.C.; Juvekar, A.R.; Roja, G.; Eapen, S.; D’Souza, S.F. Positive inotropic effect of the leaf extracts of parent and tissue culture plants of Coleus amboinicus on an isolated perfused frog heart preparation. Food Chem. 2009, 114, 139–141. [Google Scholar] [CrossRef]
- Santos, F.A.V.; Serra, C.G.; Bezerra, R.J.A.C.; Figueredo, F.G.; Edinardo; Matias, F.F.; Menezes, I.R.A.; Costa, J.G.M.; Coutinho, H.D.M. Antibacterial activity of Plectranthus amboinicus Lour. (Lamiaceae) essential oil against Streptococcus mutans. Eur. J. Integr. Med. 2016, 8, 293–297. [Google Scholar] [CrossRef]
- Shubha, J.R.; Bhatt, P. Plectranthus amboinicus leaves stimulate growth of probiotic L. plantarum: Evidence for ethnobotanical use in diarrhea. J. Ethnopharmacol. 2015, 166, 220–227. [Google Scholar] [CrossRef]
- Patel, R.; Mahobia, N.K.; Gendle, R.; Kaushik, B.; Singh, S.K. Diuretic activity of leaves of Plectranthus amboinicus (Lour.) Spreng in male albino rats. Pharmacognosy Res. 2010, 2, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Dixit, A.; Mehta, S.C. Wound healing activity of aqueous extracts of leaves and roots of Coleus aromaticus in rats. Acta Pol. Pharm. 2012, 69, 1119–1123. [Google Scholar]
- Bhat, P.; Hegde, G.; Hegde, G.R. Ethnomedicinal practices in different communities of Uttara Kannada district of Karnataka for treatment of wounds. J. Ethnopharmacol. 2012, 143, 501–514. [Google Scholar] [CrossRef]
- Selvakumar, P.; naveena, B.E.; prakash, S.D. Studies on the antidandruff activity of the essential oil of Coleus amboinicus and Eucalyptus globulus. Asian Pac. J. Trop. Dis. 2012, 2, S715–S719. [Google Scholar] [CrossRef]
- Bhojwani, S.S.; Dantu, P.K. Micropropagation. In Plant Tissue Culture: An Introductory Text; Bhojwani, S.S., Dantu, P.K., Eds.; Springer: New Delhi, India, 2013. [Google Scholar] [CrossRef]
- Faisal, M.; Ahmad, N.; Anis, M.; Alatar, A.A.; Qahtan, A.A. Auxin-cytokinin synergism in vitro for producing genetically stable plants of Ruta graveolens using shoot tip meristems. Saudi J. Biol. Sci. 2018, 25, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Sreedevi, E.; Anuradha, M.; Pullaiah, T. Plant regeneration from leaf-derived callus in Plectranthus barbatus Andr.[Syn.: Coleus forskohlii (Wild.) Briq.]. Afr. J. Biotechnol. 2013, 12, 2441–2448. [Google Scholar] [CrossRef]
- Thaniarasu, R.; Kumar, T.S.; Rao, M. In vitro Propagation of Plectranthus bourneae Gamble? An Endemic Red Listed Plant. Plant Tissue Cult. Biotechnol. 2015, 25, 273–284. [Google Scholar] [CrossRef]
- Thaniarasu, R.; Senthil Kumar, T.; Rao, M.V. Mass propagation of Plectranthus bourneae Gamble through indirect organogenesis from leaf and internode explants. Physiol. Mol. Biol. Plants 2016, 22, 143–151. [Google Scholar] [CrossRef]
- Belete, K.; Balcha, A. Micropropagation of Plectranthus edulis (Vatke) Agnew from shoot tip and nodal explants. Afr. J. Agric. Res. 2015, 10, 6–13. [Google Scholar] [CrossRef]
- Tsegaw, M.; Feyissa, T. Micropropagation of Plectranthus edulis (Vatke) Agnew from meristem cultur. Afr. J. Biotechnol. 2014, 13, 3682–3688. [Google Scholar] [CrossRef]
- Rahman, Z.A.; Noor, E.S.M.; Ali, M.S.M.; Mirad, R.; Othman, A.N. In Vitro Micropropagation of a Valuable Medicinal Plant, Plectranthus amboinicus. Am. J. Plant Sci. 2015, 6, 7. [Google Scholar] [CrossRef]
- Arumugam, G.; Sinniah, U.R.; Swamy, M.K.; Lynch, P.T. Micropropagation and essential oil characterization of Plectranthus amboinicus (Lour.) Sprengel, an aromatic medicinal plant. In Vitro Cell. Dev. Biol. Plant 2020, 56, 491–503. [Google Scholar] [CrossRef]
- Mahmoud, D.S.; Sayed, L.M.; Diab, M.; Fahmy, E.M. In Vitro Propagation Of Plectranthus barbatus Andrews As Important Medicinal Plant. Arab Univ. J. Agri. Sci. 2019, 27, 511–517. [Google Scholar] [CrossRef]
- Fonseka, D.; Wickramaarachchi, W.; Situge, C. Mass production of Plectranthus zeylanicus—A valuable medicinal and aromatic plant with a future value. Int. J. Minor. Fruits Med. Aromat. Plants 2019, 5, 15–20. [Google Scholar]
- Kujeke, G.T.; Chitendera, T.C.; Masekesa, R.T.; Mazarura, U.; Ngadze, E.; Rugare, J.T.; Matikiti, A. Micropropagation of Livingstone Potato (Plectranthus esculentus N.E.Br). Adv. Agric. 2020, 2020, 8364153. [Google Scholar] [CrossRef]
- Faisal, M.; Alatar, A.A.; El-Sheikh, M.A.; Abdel-Salam, E.M.; Qahtan, A.A. Thidiazuron induced in vitro morphogenesis for sustainable supply of genetically true quality plantlets of Brahmi. Ind. Crops Prod. 2018, 118, 173–179. [Google Scholar] [CrossRef]
- Faisal, M.; Alatar, A.A.; Ahmad, N.; Anis, M.; Hegazy, A.K. An Efficient and Reproducible Method for in vitro Clonal Multiplication of Rauvolfia tetraphylla L. and Evaluation of Genetic Stability using DNA-Based Markers. Appl. Biochem. Biotechnol. 2012, 168, 1739–1752. [Google Scholar] [CrossRef]
- Faisal, M.; Alatar, A.A.; Hegazy, A.K.; Alharbi, S.A.; El-Sheikh, M.; Okla, M.K. Thidiazuron induced in vitro multiplication of Mentha arvensis and evaluation of genetic stability by flow cytometry and molecular markers. Ind. Crops Prod. 2014, 62, 100–106. [Google Scholar] [CrossRef]
- Thakur, J.; Dwivedi, M.D.; Sourabh, P.; Uniyal, P.L.; Pandey, A.K. Genetic Homogeneity Revealed Using SCoT, ISSR and RAPD Markers in Micropropagated Pittosporum eriocarpum Royle- An Endemic and Endangered Medicinal Plant. PLoS ONE 2016, 11, e0159050. [Google Scholar] [CrossRef]
- Jena, S.; Ray, A.; Sahoo, A.; Sahoo, S.; Dash, B.; Kar, B.; Nayak, S. Rapid plant regeneration in industrially important Curcuma zedoaria revealing genetic and biochemical fidelity of the regenerants. 3 Biotech. 2019, 10, 17. [Google Scholar] [CrossRef]
- Rohela, G.K.; Jogam, P.; Bylla, P.; Reuben, C. Indirect Regeneration and Assessment of Genetic Fidelity of Acclimated Plantlets by SCoT, ISSR, and RAPD Markers in Rauwolfia tetraphylla L.: An Endangered Medicinal Plant. BioMed Res. Int. 2019, 2019, 3698742. [Google Scholar] [CrossRef]
- Qahtan, A.A.; Faisal, M.; Alatar, A.A.; Abdel-Salam, E.M. High-Frequency Plant Regeneration, Genetic Uniformity, and Flow Cytometric Analysis of Regenerants in Rutachalepensis, L. Plants 2021, 10, 2820. [Google Scholar] [CrossRef]
- Sirikonda, A.; Jogam, P.; Ellendula, R.; Kudikala, H.; Mood, K.; Allini, V.R. In vitro micropropagation and genetic fidelity assesment in Flemingia macrophylla (Willd.) Merr: An ethnomedicinal plant. Vegetos 2020, 33, 286–295. [Google Scholar] [CrossRef]
- Thakur, M.; Rakshandha; Sharma, V.; Chauhan, A. Genetic fidelity assessment of long term in vitro shoot cultures and regenerated plants in Japanese plum cvs Santa Rosa and Frontier through RAPD, ISSR and SCoT markers. S. Afr. J. Bot. 2021, 140, 428–433. [Google Scholar] [CrossRef]
- Faisal, M.; Abdel-Salam, E.M.; Alatar, A.A.; Qahtan, A.A. Induction of somatic embryogenesis in Brassica juncea L. and analysis of regenerants using ISSR-PCR and flow cytometer. Saudi, J. Biol. Sci. 2021, 28, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Abdolinejad, R.; Shekafandeh, A.; Jowkar, A.; Gharaghani, A.; Alemzadeh, A. Indirect regeneration of Ficus carica by the TCL technique and genetic fidelity evaluation of the regenerated plants using flow cytometry and ISSR. Plant. Cell Tissue Organ. Cult. 2020, 143, 131–144. [Google Scholar] [CrossRef]
- Parzymies, M.; Pogorzelec, M.; Głębocka, K.; Śliwińska, E. Genetic Stability of the Endangered Species Salix lapponum L. Regenerated In Vitro during the Reintroduction Process. Biology 2020, 9, 378. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Galbraith, D.W. Simultaneous flow cytometric quantification of plant nuclear DNA contents over the full range of described angiosperm 2C values. Cytometry A 2009, 75, 692–698. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Tan, S.N.; Tee, C.S.; Wong, H.L. Multiple shoot bud induction and plant regeneration studies of Pongamia pinnata. Plant. Biotechnol. 2018, 35, 325–334. [Google Scholar] [CrossRef]
- El-Shafey, N.; Sayed, M.; Ahmed, E.; Hammouda, O.; Khodary, S.E. Effect of growth regulators on micropropagation, callus induction and callus flavonoid content of Rumex pictus Forssk. Egypt, J. Bot. 2019, 59, 293–302. [Google Scholar] [CrossRef]
- Vaidya, B.N.; Asanakunov, B.; Shahin, L.; Jernigan, H.L.; Joshee, N.; Dhekney, S.A. Improving micropropagation of Mentha × piperita L. using a liquid culture system. In Vitro Cell. Dev. Biol. Plant 2019, 55, 71–80. [Google Scholar] [CrossRef]
- Rajput, S.; Agrawal, V. Micropropagation of Atropa acuminata Royle ex Lindl. (a critically endangered medicinal herb) through root callus and evaluation of genetic fidelity, enzymatic and non-enzymatic antioxidant activity of regenerants. Acta Physiol. Plant. 2020, 42, 160. [Google Scholar] [CrossRef]
- Wróbel, T.; Dreger, M.; Wielgus, K.; Słomski, R. Modified Nodal Cuttings and Shoot Tips Protocol for Rapid Regeneration of Cannabis sativa L. J. Nat. Fibers 2022, 19, 536–545. [Google Scholar] [CrossRef]
- Machado, J.S.; Degenhardt, J.; Maia, F.R.; Quoirin, M. Micropropagation of Campomanesia xanthocarpa O. Berg (Myrtaceae), a medicinal tree from the Brazilian Atlantic Forest. Trees 2020, 34, 791–799. [Google Scholar] [CrossRef]
- Zahid, N.A.; Jaafar, H.Z.E.; Hakiman, M. Micropropagation of Ginger (Zingiber officinale Roscoe) ‘Bentong’ and Evaluation of Its Secondary Metabolites and Antioxidant Activities Compared with the Conventionally Propagated Plant. Plants 2021, 10, 630. [Google Scholar] [CrossRef]
- Kumari, P.; Singh, S.; Yadav, S.; Tran, L.S.P. Pretreatment of seeds with thidiazuron delimits its negative effects on explants and promotes regeneration in chickpea (Cicer arietinum L.). Plant Cell Tissue Organ. Cult. (PCTOC) 2018, 133, 103–114. [Google Scholar] [CrossRef]
- Kumari, P.; Singh, S.; Yadav, S.; Tran, L.-S.P. Influence of different types of explants in chickpea regeneration using thidiazuron seed-priming. J. Plant Res. 2021, 134, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Clapa, D.; Hârța, M. Establishment of an Efficient Micropropagation System for Humulus lupulus L. cv. Cascade and Confirmation of Genetic Uniformity of the Regenerated Plants through DNA Markers. Agronomy 2021, 11, 2268. [Google Scholar] [CrossRef]
- Faisal, M.; Ahmad, N.; Anis, M. An efficient micropropagation system for Tylophora indica: An endangered, medicinally important plant. Plant. Biotechnol. Rep. 2007, 1, 155–161. [Google Scholar] [CrossRef]
- Sahoo, N.; Thirunavoukkarasu, M.; Behera, P.R.; Satpathy, G.B.; Panda, P.K. Direct shoot organogenesis from hypocotyl explants of J. atropha curcas L. an important bioenergy feedstock. GCB Bioenergy 2012, 4, 234–238. [Google Scholar] [CrossRef]
- Panigrahi, J.; Gantait, S.; Patel, I.C. An Efficient In Vitro Approach for Direct Regeneration and Callogenesis of Adhatoda vasica Nees, a Potential Source of Quinazoline Alkaloids. Natl. Acad. Sci. Lett. 2017, 40, 319–324. [Google Scholar] [CrossRef]
- Jinyan, H. A high throughput plant regeneration system from shoot stems of Sapium sebiferum Roxb., a potential multipurpose bioenergy tree. Ind. Crops Prod. 2020, 154, 112653. [Google Scholar] [CrossRef]
- Hussain, S.A.; Anis, M.; Alatar, A.A. Efficient In Vitro Regeneration System for Tecoma stans L., Using Shoot Tip and Assessment of Genetic Fidelity Among Regenerants. Proc. Indian Natl. Sci. Acad. (B Biol. Sci.) 2020, 90, 171–178. [Google Scholar] [CrossRef]
- Wei, Q.; Cao, J.; Qian, W.; Xu, M.; Li, Z.; Ding, Y. Establishment of an efficient micropropagation and callus regeneration system from the axillary buds of Bambusa ventricosa. Plant Cell Tissue Organ. Cult. 2015, 122, 1–8. [Google Scholar] [CrossRef]
- Fatima, N.; Ahmad, N.; Anis, M. Enhanced in vitro regeneration and change in photosynthetic pigments, biomass and proline content in Withania somnifera L. (Dunal) induced by copper and zinc ions. Plant. Physiol. Biochem. 2011, 49, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Swain, D.; Lenka, S.; Hota, T.; Rout, G.R. Micro-propagation of Hypericum gaitii Haines, an endangered medicinal plants: Assessment of genetic fidelity. Nucleus 2016, 59, 7–13. [Google Scholar] [CrossRef]
- Ahmed, M.R.; Anis, M.; Alatar, A.A.; Faisal, M. In vitro clonal propagation and evaluation of genetic fidelity using RAPD and ISSR marker in micropropagated plants of Cassia alata L.: A potential medicinal plant. Agrofor. Syst. 2017, 91, 637–647. [Google Scholar] [CrossRef]
- Bolyard, M. In vitro regeneration of Artemisia abrotanum L. by means of somatic organogenesis. In Vitro Cell. Dev. Biol. Plant 2018, 54, 127–130. [Google Scholar] [CrossRef]
- Naaz, A.; Hussain, S.A.; Naz, R.; Anis, M.; Alatar, A.A. Successful plant regeneration system via de novo organogenesis in Syzygium cumini (L.) Skeels: An important medicinal tree. Agrofor. Syst. 2019, 93, 1285–1295. [Google Scholar] [CrossRef]
- Chinnadurai, V.; Viswanathan, P.; Kalimuthu, K.; Vanitha, A.; Ranjitha, V.; Pugazhendhi, A. Comparative studies of phytochemical analysis and pharmacological activities of wild and micropropagated plant ethanol extracts of Manihot esculenta. Biocatal. Agric. Biotechnol. 2019, 19, 101166. [Google Scholar] [CrossRef]
- Upadhyay, A.; Shahzad, A.; Ahmad, Z. In vitro propagation and assessment of genetic uniformity along with chemical characterization in Hildegardia populifolia (Roxb.) Schott & Endl.: A critically endangered medicinal tree. In Vitro Cell. Dev. Biol. Plant 2020, 56, 803–816. [Google Scholar] [CrossRef]
- Kim, Y.-G.; Okello, D.; Yang, S.; Komakech, R.; Rahmat, E.; Kang, Y. Histological assessment of regenerating plants at callus, shoot organogenesis and plantlet stages during the in vitro micropropagation of Asparagus cochinchinensis. Plant. Cell Tissue Organ. Cult. 2021, 144, 421–433. [Google Scholar] [CrossRef]
- Kumar, S.S.; Giridhar, P. In vitro micropropagation of Basella rubra L. through proliferation of axillary shoots. Plant Cell Tissue Organ Cult. 2021, 144, 477–483. [Google Scholar] [CrossRef]
- De Klerk, G.-J. Rooting of microcuttings: Theory and practice. In Vitro Cell. Dev. Biol. Plant 2002, 38, 415–422. [Google Scholar] [CrossRef]
- Yadav, V.; Shahzad, A.; Ahmad, Z.; Sharma, S.; Parveen, S. Synthesis of nonembryonic synseeds in Hemidesmus indicus R. Br.: Short term conservation, evaluation of phytochemicals and genetic fidelity of the regenerants. Plant. Cell Tissue Organ. Cult. 2019, 138, 363–376. [Google Scholar] [CrossRef]
- Hussain, S.A.; Ahmad, N.; Anis, M. Synergetic effect of TDZ and BA on minimizing the post-exposure effects on axillary shoot proliferation and assessment of genetic fidelity in Rauvolfia tetraphylla (L.). Rend. Lincei Sci. Fis. Nat. 2018, 29, 109–115. [Google Scholar] [CrossRef]
- Jogam, P.; Sandhya, D.; Shekhawat, M.S.; Alok, A.; Manokari, M.; Abbagani, S.; Allini, V.R. Genetic stability analysis using DNA barcoding and molecular markers and foliar micro-morphological analysis of in vitro regenerated and in vivo grown plants of Artemisia vulgaris L. Ind. Crops Prod. 2020, 151, 112476. [Google Scholar] [CrossRef]
- Dilkalal, A.; AS, A.; TG, U. In vitro regeneration, antioxidant potential, and genetic fidelity analysis of Asystasia gangetica (L.) T. Anderson. In Vitro Cell. Dev. Biol. Plant 2021, 57, 447–459. [Google Scholar] [CrossRef]
- Faisal, M.; Alatar, A.A.; Ahmad, N.; Anis, M.; Hegazy, A.K. Assessment of Genetic Fidelity in Rauvolfia serpentina Plantlets Grown from Synthetic (Encapsulated) Seeds Following In Vitro Storage at 4 °C. Molecules 2012, 17, 5050–5061. [Google Scholar] [CrossRef]
- Devi, S.P.; Kumaria, S.; Rao, S.R.; Tandon, P. Single primer amplification reaction (SPAR) methods reveal subsequent increase in genetic variations in micropropagated plants of Nepenthes khasiana Hook. f. maintained for three consecutive regenerations. Gene 2014, 538, 23–29. [Google Scholar] [CrossRef]
- Parab, A.R.; Lynn, C.B.; Subramaniam, S. Assessment of genetic stability on in vitro and ex vitro plants of Ficus carica var. black jack using ISSR and DAMD markers. Mol. Biol. Rep. 2021, 48, 7223–7231. [Google Scholar] [CrossRef]

| Primers | Sequence (5′ → 3′) | Annealing Temperature (°C) | Size Range (bp) | Number of Bands |
|---|---|---|---|---|
| HBV3 | GCTCCTCCCCTCCT | 53 | 2500–500 | 9 |
| HBV5 | GGTGAAGCACAGGTG | 56 | 2000–300 | 11 |
| HVR | GGTGTAGAGAGGGGT | 50 | 20,000–400 | 17 |
| M13 | GAGGGTGGCGGTTCT | 57 | 1500 | 1 |
| 33.6 | GGAGGTGGGCA | 47 | 3500–400 | 10 |
| Total = | 48 | |||
| Average band per primer = | 9.6 | |||
| Primers | Sequence (5′ → 3′) | Annealing Temperature (°C) | Size Range (bp) | Number of Bands |
|---|---|---|---|---|
| UBC-811 | (GA)8C | 49 | 3000–400 | 11 |
| UBC-825 | (AC)8T | 46 | 3000–500 | 14 |
| UBC-827 | (AC)8G | 50 | 3000–500 | 9 |
| UBC-834 | (AG)8YT | 50 | 2000–300 | 10 |
| UBC-841 | (GA)8YC | 50 | 3000–400 | 11 |
| UBC-855 | (AC)8YT | 50 | 20,000–500 | 12 |
| UBC-866 | (CTC)6GT | 55 | 20,000–500 | 13 |
| UBC-868 | (GAA)6 | 46 | 5000–700 | 12 |
| UBC-880 | (GGAGA)3 | 50 | 5000–800 | 11 |
| Total = | 150 | |||
| Average band per primer = | 11.4 | |||
| Plant Growth Regulators (µM) | Regeneration % | Shoot Number per Explant | |
|---|---|---|---|
| BA | Kin | ||
| 0.0 | 0.0 | 23.01 ± 1.15 f | 1.26 ± 0.15 g |
| 0.5 | 46.67 ± 2.02 e | 2.13 ± 0.20 f | |
| 2.5 | 61.31 ± 2.31 cd | 4.73 ± 0.45 c | |
| 5.0 | 79.37 ± 2.30 a | 7.57 ± 0.30 a | |
| 7.5 | 70.66 ± 1.88 b | 5.06 ± 0.35 c | |
| 10 | 62.10 ± 1.51 cd | 3.23 ± 0.15 e | |
| 0.5 | 41.10 ± 1.73 e | 2.03 ± 0.14 f | |
| 2.5 | 55.33 ± 1.20 d | 4.13 ± 0.21 cde | |
| 5.0 | 69.43 ± 2.61 bc | 6.36 ± 0.26 b | |
| 7.5 | 62.00 ± 1.73 cd | 4.27 ± 0.15 cd | |
| 10 | 42.67 ± 1.45 e | 3.37 ± 0.23 de | |
| Plant Growth Regulators (µM) | Regeneration % | Shoots Number per Explant | |||
|---|---|---|---|---|---|
| BA | Kin | NAA | IAA | ||
| 5.0 | 79.37 ± 2.30 cde | 7.53 ± 0.50 gh | |||
| 5.0 | 69.43 ± 2.61 ghi | 6.27 ± 0.46 h | |||
| 5.0 | 0.1 | 81.01 ± 2.70 cde | 12.43 ± 1.14 cd | ||
| 5.0 | 0.5 | 85.04 ± 2.01 bcd | 14.70 ± 1.15 bc | ||
| 5.0 | 2.5 | 97.10 ± 2.16 a | 19.37 ± 1.21 a | ||
| 5.0 | 5.0 | 72.76 ± 1.45 efgh | 7.97 ± 0.53 fgh | ||
| 5.0 | 0.1 | 80.33 ± 2.53 cde | 11.43 ± 0.97 de | ||
| 5.0 | 0.5 | 80.71 ± 2.04 cde | 10.63 ± 0.87 def | ||
| 5.0 | 2.5 | 92.67 ± 3.10 ab | 15.36 ± 1.32 b | ||
| 5.0 | 5.0 | 67.31 ± 1.65 hi | 6.98 ± 0.42 gh | ||
| 5.0 | 0.1 | 77.67 ± 1.45 defg | 11.70 ± 0.75 de | ||
| 5.0 | 0.5 | 85.10 ± 1.53 bcd | 12.20 ± 1.05 cd | ||
| 5.0 | 2.5 | 90.43 ± 1.45 ab | 16.27 ± 1.16 b | ||
| 5.0 | 5.0 | 70.31 ± 1.98 fgh | 7.01 ± 0.93 gh | ||
| 5.0 | 0.1 | 73.44 ± 1.83 efgh | 9.30 ± 0.77 efg | ||
| 5.0 | 0.5 | 80.11 ± 1.45 cde | 8.53 ± 0.50 fgh | ||
| 5.0 | 2.5 | 88.33 ± 1.54 abc | 12.27 ± 0.67 cd | ||
| 5.0 | 5.0 | 61.01 ± 1.73 i | 5.97 ± 0.56 h | ||
| IBA (µM) | Regeneration % | Roots Number per Shootlet |
|---|---|---|
| 0.0 | 36.67 ± 2.02 d | 0.73 ± 2.02 c |
| 0.25 | 93.76 ± 2.71 a | 5.30 ± 0.44 a |
| 0.5 | 87.10 ± 2.33 a | 4.13 ± 0.45 ab |
| 1.0 | 72.36 ± 2.30 b | 3.87 ± 0.30 b |
| 2.0 | 60.50 ± 1.88 c | 1.06 ± 0.35 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faisal, M.; Alatar, A.A. Establishment of an Efficient In Vitro Propagation Method for a Sustainable Supply of Plectranthus amboinicus (Lour.) and Genetic Homogeneity Using Flow Cytometry and SPAR Markers. Horticulturae 2022, 8, 693. https://doi.org/10.3390/horticulturae8080693
Faisal M, Alatar AA. Establishment of an Efficient In Vitro Propagation Method for a Sustainable Supply of Plectranthus amboinicus (Lour.) and Genetic Homogeneity Using Flow Cytometry and SPAR Markers. Horticulturae. 2022; 8(8):693. https://doi.org/10.3390/horticulturae8080693
Chicago/Turabian StyleFaisal, Mohammad, and Abdulrahman A. Alatar. 2022. "Establishment of an Efficient In Vitro Propagation Method for a Sustainable Supply of Plectranthus amboinicus (Lour.) and Genetic Homogeneity Using Flow Cytometry and SPAR Markers" Horticulturae 8, no. 8: 693. https://doi.org/10.3390/horticulturae8080693
APA StyleFaisal, M., & Alatar, A. A. (2022). Establishment of an Efficient In Vitro Propagation Method for a Sustainable Supply of Plectranthus amboinicus (Lour.) and Genetic Homogeneity Using Flow Cytometry and SPAR Markers. Horticulturae, 8(8), 693. https://doi.org/10.3390/horticulturae8080693







