Effect of White LED Light on the Growth of Apple Seedlings in Controlled Environment System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Preliminary Temperature Treatment
2.3. Treatment (Light Effect)
2.4. Measurement of Plant Growth Parameters
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global warming, climate change, and environmental pollution: Recipe for a multifactorial stress combination disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef]
- Anderson, J.T.; Song, B.H. Plant adaptation to climate change—Where are we? J. Syst. Evol. 2020, 58, 533–545. [Google Scholar] [CrossRef]
- Borrelli, P.; Robinson, D.A.; Panagos, P.; Lugato, E.; Yang, J.E.; Alewell, C.; Wuepper, D.; Montanarella, L.; Ballabio, C. Land use and climate change impacts on global soil erosion by water (2015–2070). Proc. Natl. Acad. Sci. USA 2020, 117, 21994–22001. [Google Scholar] [CrossRef]
- Alexandratos, N.; Bruinsma, J. World Agriculture Towards 2030/2050: The 2012 Revision. 2012. Available online: https://ageconsearch.umn.edu/record/288998 (accessed on 16 December 2021).
- Niu, G.; Masabni, J. Plant production in controlled environments. Horticulturae 2018, 4, 28. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Hinkle, A.F. South Korea: 2017 Apple Report-Revised. Gain Report No. KS1733. 2017. Available online: https://www.fas.usda.gov/data/south-korea-2017-apple-report-revised (accessed on 23 November 2018).
- Yoon, T.M.; Kim, K.R.; Uhm, J.Y.; Byun, J.K. The extension results of the cultivation systems for dwarf apple trees and following tasks. In Proceedings of the 23rd Apple Festival for the Establishment of Dwarf Apple Orchards, North Kyungsang Province and Daegu Kyungbuk Apple Cooperative, Daegu, Korean, 21 December 2021; pp. 9–235. [Google Scholar]
- Hajnajari, H.; Chashnidel, B.; Vahdati, K.; Ebrahimi, M.; Nabipour, A.; Fallahi, E. Heritability of morphological traits in apple early-ripening full-sib and half-sib offspring and its potential use for assisted selection. HortScience 2012, 47, 328–333. [Google Scholar] [CrossRef]
- Merkle, S.A.; Nairn, C.J. Hardwood tree biotechnology. In Vitro Cell. Dev. Biol. Plant. 2005, 41, 602–619. [Google Scholar] [CrossRef]
- Chandra, S.; Bandopadhyay, R.; Kumar, V.; Chandra, R. Acclimatization of tissue cultured plantlets: From laboratory to land. Biotechnol. Lett. 2010, 32, 1199–1205. [Google Scholar] [CrossRef]
- Son, K.H.; Oh, M.M. Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. HortScience 2013, 48, 988–995. [Google Scholar] [CrossRef]
- Slattery, R.A.; VanLoocke, A.; Bernacchi, C.J.; Zhu, X.G.; Ort, D.R. Photosynthesis, light use efficiency, and yield of reduced-chlorophyll soybean mutants in field conditions. Front. Plant Sci. 2017, 8, 549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, Z.; Jiang, N.; Grundy, S.; Lu, C. Uncovering LED light effects on plant growth: New angles and perspectives-LED light for improving plant growth, nutrition and energy-use efficiency. Acta Hortic. 2017, 1227, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Yeh, N.; Chung, J.P. High-brightness LEDs—Energy efficient lighting sources and their potential in indoor plant cultivation. Renew. Sustain. Energy Rev. 2009, 13, 2175–2180. [Google Scholar] [CrossRef]
- Dutta Gupta, S.; Jatothu, B. Fundamentals and applications of light-emitting diodes (LEDs) in vitro plant growth and morphogenesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- Manivannan, A.; Soundararajan, P.; Halimah, N.; Ko, C.H.; Jeong, B.R. Blue LED light enhances growth, phytochemical contents, and antioxidant enzyme activities of Rehmannia glutinosa cultured in vitro. Hortic. Environ. Biotechnol. 2015, 56, 105–113. [Google Scholar] [CrossRef]
- Shin, Y.-S.; Lee, M.-J.; Lee, E.-S.; Ahn, J.-H.; Do, H.-W.; Choi, D.-W.; Jeong, J.-D.; Lee, J.-E.; Kim, M.-K.; Park, J.-U.; et al. Effect of light emitting diodes treatment on growth and mineral contents of lettuce (Lactuca sativa L. ‘Chung Chi Ma’). Korean J. Org. Agric. 2013, 21, 659–668. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E.; Barickman, T.C.; Morrow, R.C. Sprouting broccoli accumulate higher concentrations of nutritionally important metabolites under narrow-band light-emitting diode lighting. J. Am. Soc. Hortic. Sci. 2014, 139, 469–477. [Google Scholar] [CrossRef]
- Choi, M.-K.; Chang, M.-S.; Eom, S.-H.; Min, K.-S.; Kang, M.-H. Physicochemical composition of buckwheat microgreens grown under different light conditions. J. Korean Soc. Food Sci. Nutr. 2015, 44, 709–715. [Google Scholar] [CrossRef]
- Gerovac, J.R.; Craver, J.K.; Boldt, J.K.; Lopez, R.G. Light intensity and quality from sole-source light emitting diodes impact growth, morphology, and nutrient content of Brassica microgreens. HortScience 2016, 51, 497–503. [Google Scholar] [CrossRef] [Green Version]
- Sams, C.; Kopsell, D.; Morrow, R. Light quality impacts on growth, flowering, mineral uptake and petal pigmentation of marigold. Acta Hortic. 2016, 1134, 139–146. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E. Increases in shoot tissue pigments, glucosinolates, and mineral elements in sprouting broccoli after exposure to short-duration blue light from light emitting diodes. J. Am. Soc. Hortic. Sci. 2013, 138, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Liang, Y.; Yang, M. Effects of composite LED light on root growth and antioxidant capacity of Cunninghamia lanceolata tissue culture seedlings. Sci. Rep. 2019, 9, 9766. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Miliauskienė, J.; Vaštakaitė-Kairienė, V.; Sutulienė, R.; Laužikė, K.; Duchovskis, P.; Małek, S. Effect of different ratios of blue and red LED light on brassicaceae microgreens under a controlled environment. Plants 2021, 10, 801. [Google Scholar] [CrossRef]
- Islam, M.; Ryu, B.R.; Azad, M.; Kalam, O.; Rahman, M.; Rana, M.; Kang, C.W.; Lim, J.D.; Lim, Y.S. Comparative growth, photosynthetic pigments, and osmolytes analysis of hemp (Cannabis sativa L.) seedlings under an aeroponics system with different LED light sources. Horticulturae 2021, 7, 239. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Kjaer, K.H.; Adnan, M.; Naznin, M.T.; Lim, J.D.; Sung, I.J.; Park, C.H.; Lim, Y.S. The evaluation of growth performance, photosynthetic capacity, and primary and secondary metabolite content of leaf lettuce grown under limited irradiation of blue and red LED light in an urban plant factory. Agriculture 2020, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- So, E.H.; An, J.H.; Kim, J.K.; Yoon, Y.J.; Park, S.J.; Na, J.K.; Choi, K.Y. Effect of artificial light sources on the growth of apple rootstock m.9 seedling. Prot. Hort. Plant Fac. 2018, 27, 341–348. [Google Scholar] [CrossRef]
- Kang, J.H.; KrishnaKumar, S.; Atulba, S.L.S.; Jeong, B.R.; Hwang, S.J. Light intensity and photoperiod influence the growth and development of hydroponically grown leaf lettuce in a closed-type plant factory system. Hortic. Environ. Biotechnol. 2013, 54, 501–509. [Google Scholar] [CrossRef]
- Rezazadeh, A.; Harkess, R.L.; Telmadarrehei, T. The effect of light intensity and temperature on flowering and morphology of potted red firespike. Horticulturae 2018, 4, 36. [Google Scholar] [CrossRef] [Green Version]
- Robinson, T.; Hoying, S.; Sazo, M.M.; DeMarree, A.; Dominguez, L. A vision for apple orchard systems of the future. N. Y. Fruit Quar. 2013, 21, 11–16. [Google Scholar]
- Zavala, J.A.; Ravetta, D.A. Allocation of photoassimilates to biomass, resin and carbohydrates in Grindelia chiloensis as affected by light intensity. Field Crops Res. 2001, 69, 143–149. [Google Scholar] [CrossRef]
- Grossnickle, S.C. Why seedlings survive: Influence of plant attributes. New For. 2012, 43, 711–738. [Google Scholar] [CrossRef]
- Rose, R.; Ketchum, J.S. Interaction of initial seedling diameter, fertilization and weed control on douglas-fir growth over the first four years after planting. Ann. For. Sci. 2003, 60, 625–635. [Google Scholar] [CrossRef] [Green Version]
- Morrissey, R.C.; Jacobs, D.F.; Davis, A.S.; Rathfon, R.A. Survival and competitiveness of quercus rubra regeneration associated with planting stocktype and harvest opening intensity. New For. 2010, 40, 273–287. [Google Scholar] [CrossRef]
- Fan, X.X.; Xu, Z.G.; Liu, X.Y.; Tang, C.M.; Wang, L.W.; Han, X.L. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Gelderen, K.V.; Kang, C.; Pierik, R. Light signaling, root development, and plasticity. Plant Physiol. 2018, 176, 1049–1060. [Google Scholar] [CrossRef] [Green Version]
- Durand, M.; Mainson, D.; Porcheron, B.; Maurousset, L.; Lemoine, R.; Pourtau, N. Carbon source–sink relationship in Arabidopsis thaliana: The role of sucrose transporters. Planta 2018, 247, 587–611. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.K.; Kang, H.M.; Na, J.K.; Choi, K.Y. Changes in growth characteristics and functional components of Lactuca indica L. ‘sunhyang’ baby leaf vegetable by light Intensity and cultivation period. Hortic. Sci. Technol. 2019, 5, 579–588. [Google Scholar]
- Xu, Y.; Yang, M.; Cheng, F.; Liu, S.; Liang, Y. Effects of LED photoperiods and light qualities on in vitro growth and chlorophyll fluorescence of Cunninghamia lanceolata. BMC Plant Biol. 2020, 20, 269. [Google Scholar] [CrossRef]
- Ciganda, V.S.; Gitelson, A.A.; Schepers, J. How deep does a remote sensor sense? Expression of chlorophyll content in a maize canopy. Remote Sens. Environ. 2012, 126, 240–247. [Google Scholar] [CrossRef]
- Martin, M.; Noarbe, D.M.; Serrot, P.H.; Sabater, B. The rise of the photosynthetic rate when light intensity increases is delayed in ndh gene-defective tobacco at high but not at low CO2 concentrations. Front. Plant Sci. 2015, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Weston, E.; Thorogood, K.; Vinti, G.; López-Juez, E. Light quantity controls leaf-cell and chloroplast development in Arabidopsis thaliana wild type and blue-light-perception mutants. Planta 2000, 211, 807–815. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef] [Green Version]
- Prado, S.A.; Cabrera-Bosquet, L.; Grau, A.; Coupel-Ledru, A.; Millet, E.J.; Welcker, C.; Tardieu, F. Phenomics allows identification of genomic regions affecting maize stomatal conductance with conditional effects of water deficit and evaporative demand. Plant Cell Environ. 2018, 41, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Shawon, M.R.A.; An, J.H.; Yun, Y.J.; Park, S.J.; Na, J.K.; Choi, K.Y. Influence of substrate composition and container size on the growth of tissue culture propagated apple rootstock plants. Agronomy 2021, 11, 2450. [Google Scholar] [CrossRef]
- Dinh, T.H.; Takaragawa, H.; Watanabe, K.; Nakabaru, M.; Kawamitsu, Y. Leaf photosynthesis response to change of soil moisture content in sugarcane. Sugar Tech. 2019, 21, 949–958. [Google Scholar] [CrossRef]
- Yamori, W.; Kusumi, K.; Iba, K.; Terashima, I. Increased stomatal conductance induces rapid changes to photosynthetic rate in response to naturally fluctuating light conditions in rice. Plant Cell Environ. 2020, 43, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
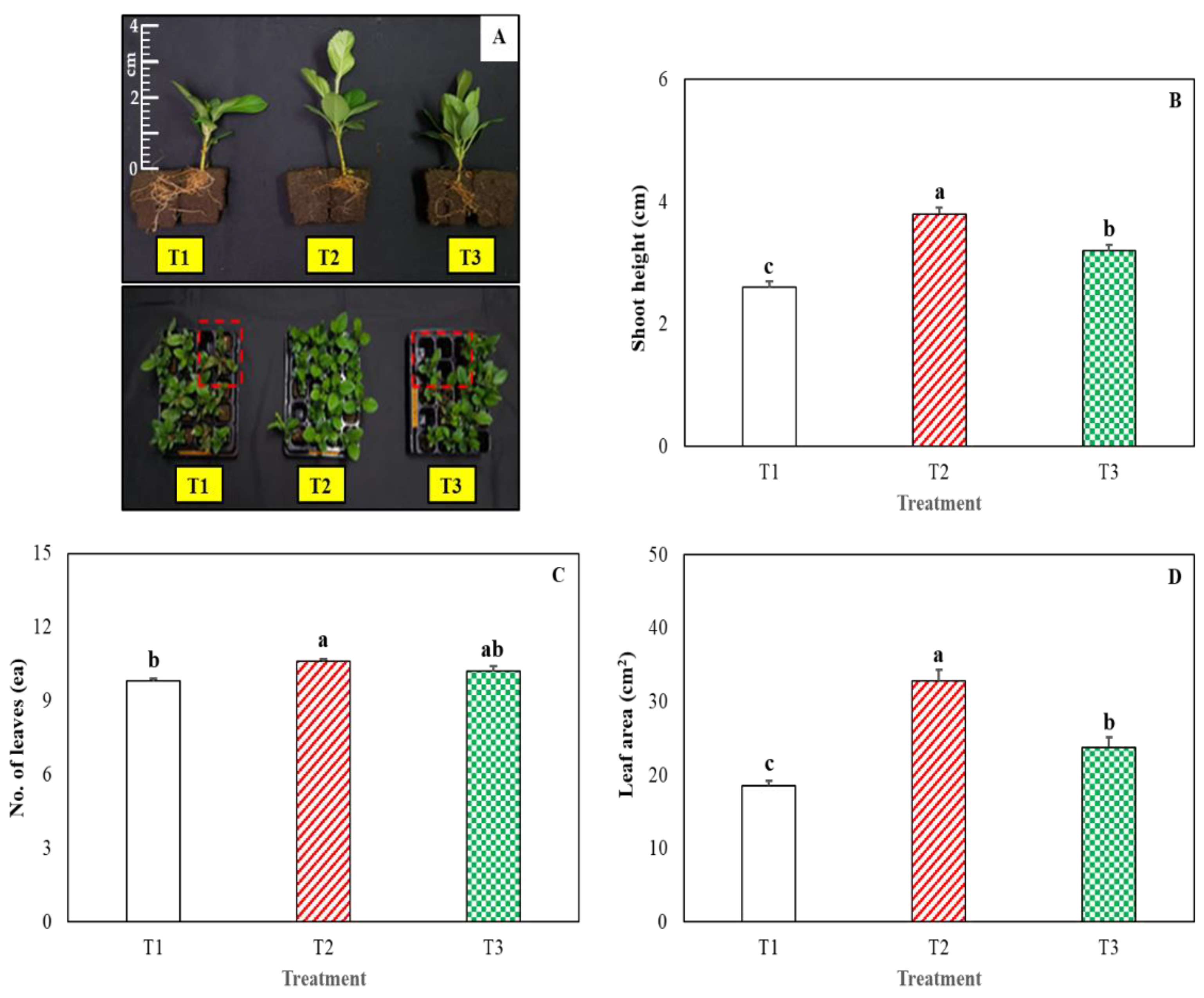
| Treatment | Survival Rate (%) | ||
|---|---|---|---|
| 0 DAT | 7 DAT | 20 DAT | |
| T1 | 100 z a y | 95.0 b | 95.0 a |
| T2 | 100 a | 100 a | 97.4 a |
| T3 | 100 a | 92.1 b | 84.0 c |
| Treatment | Leaf (cm) | Stem Diameter (mm) | SPAD (Value) | Fresh Weight (g) | ||
|---|---|---|---|---|---|---|
| Length | Width | Shoot | Root | |||
| T1 | 3.0 z b y | 1.8 b | 1.74 a | 30.1 a | 2.93 a | 0.38 a |
| T2 | 3.7 a | 2.4 a | 1.73 a | 29.6 a | 2.99 a | 0.37 a |
| T3 | 3.5 a | 2.4 a | 1.56 b | 31.6 a | 2.98 a | 0.38 a |
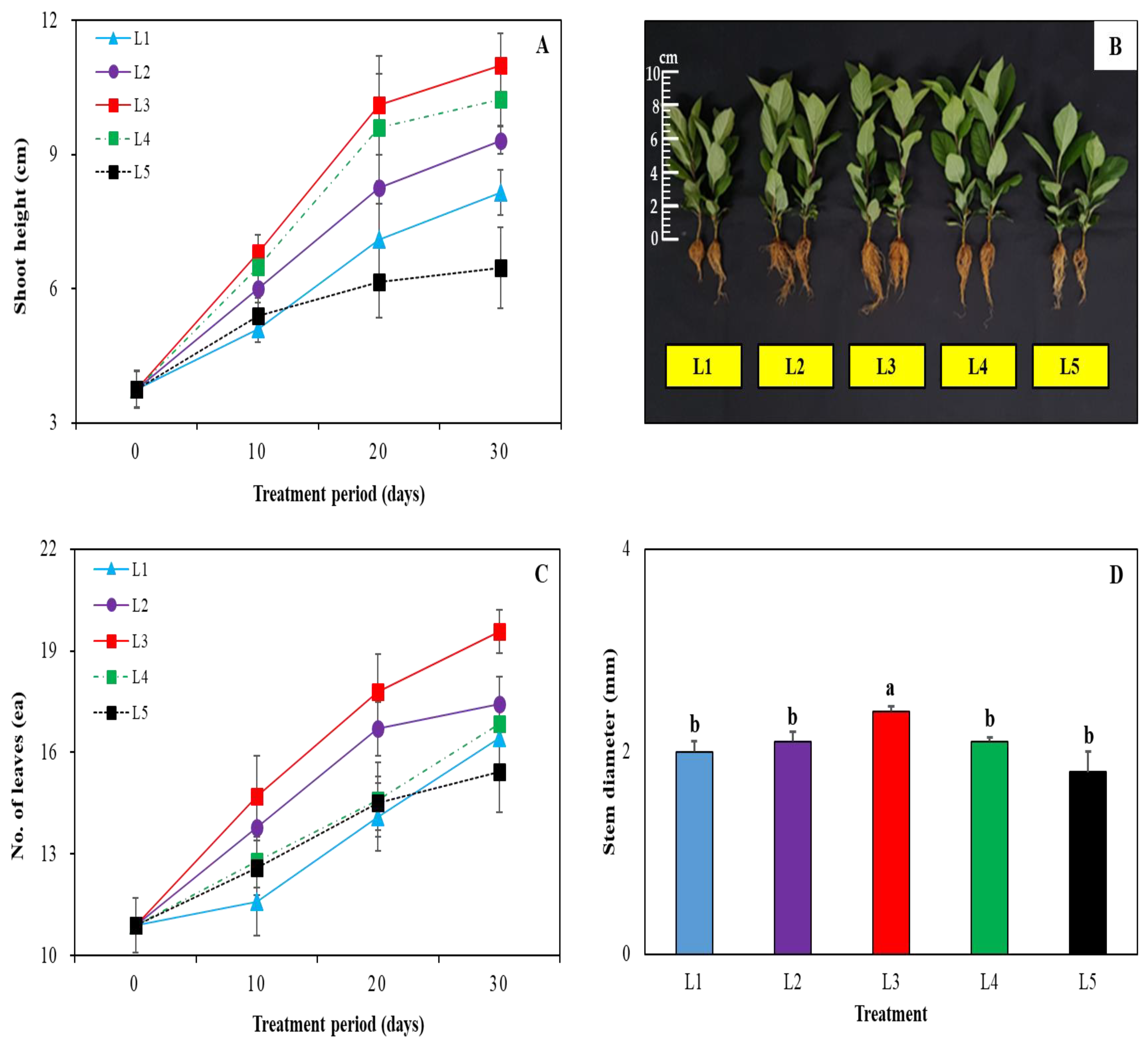
| Treatment | PPFD (μmol·m−2·s−1) | ||
|---|---|---|---|
| 0–10 Days | 11–20 Days | 21–30 Days | |
| L1 | 100 | 250 | 500 |
| L2 | 250 | 500 | 500 |
| L3 | 500 | 500 | 500 |
| L4 | 250 | 250 | 250 |
| L5 | 100 | 100 | 100 |
| Treatment | Leaf (cm) | Root Length (cm) | Fresh Weight (g/Plant) | Dry Weight (g/Plant) | |||
|---|---|---|---|---|---|---|---|
| Length | Width | Shoot | Root | Shoot | Root | ||
| L1 | 5.4 z b y | 3.3 a | 8.6 b | 1.5 c | 0.3 b | 0.42 c | 0.05 c |
| L2 | 5.6 b | 3.3 a | 9.2 b | 1.9 bc | 0.4 b | 0.62 b | 0.08 ab |
| L3 | 5.7 b | 3.4 a | 10.0 a | 2.6 a | 0.6 a | 0.85 a | 0.12 a |
| L4 | 6.4 a | 3.7 a | 10.2 a | 2.1 b | 0.3 b | 0.64 b | 0.07 bc |
| L5 | 5.3 b | 3.3 a | 9.1 b | 1.1 d | 0.2 b | 0.32 c | 0.04 c |
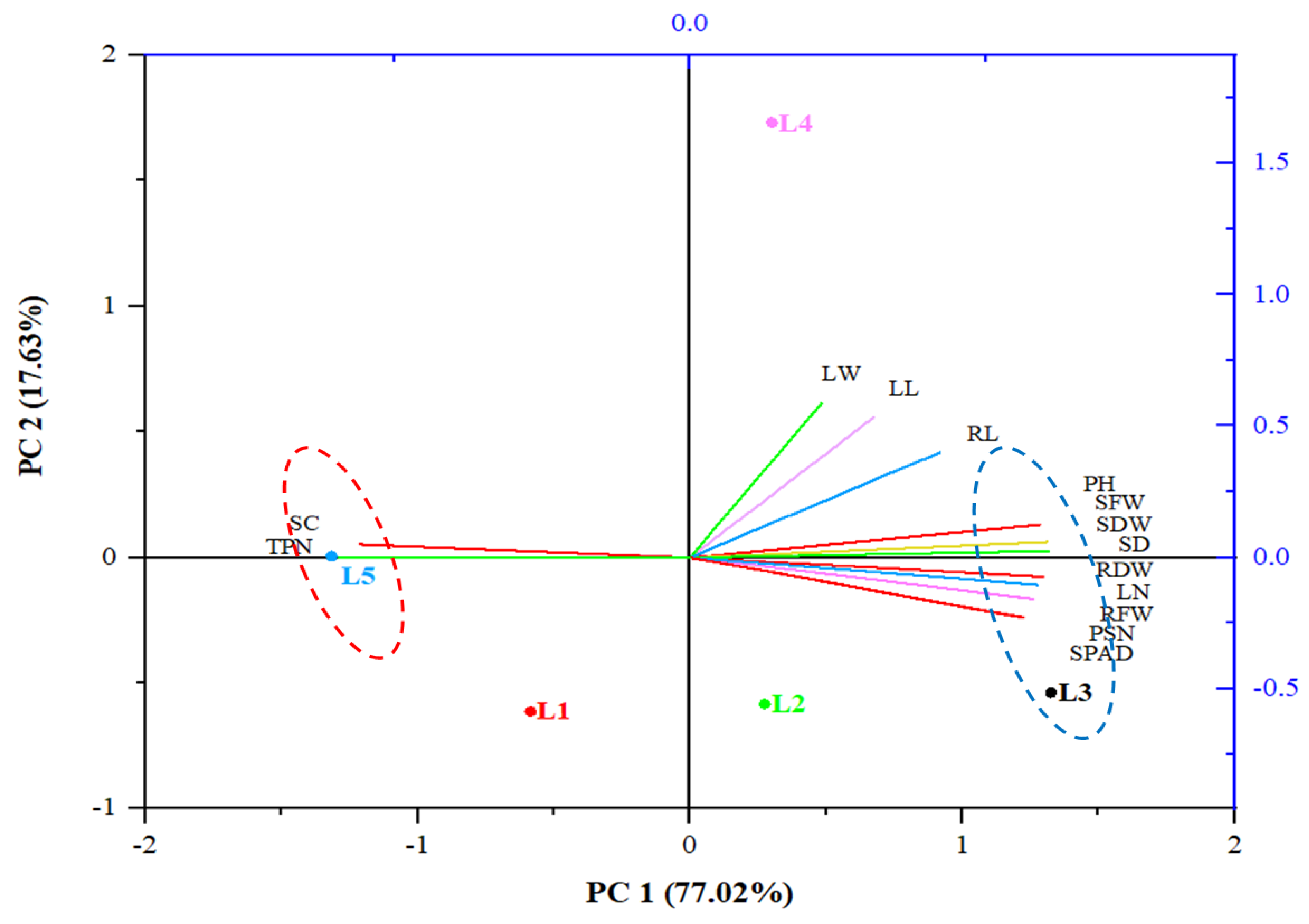
| Treatment | SPAD (Value) | Photosynthesis (µmol·CO2·m−2·s−1) | Conductance (mol·H2O·m−2·s−1) | Transpiration (mol·H2O·m−2·s−1) |
|---|---|---|---|---|
| L1 | 32.6 z b y | 8.55 ab | 0.18 a | 3.12 a |
| L2 | 34.9 ab | 9.17 a | 0.16 a | 2.79 a |
| L3 | 37.9 a | 9.88 a | 0.16 a | 2.67 a |
| L4 | 32.8 b | 7.54 b | 0.17 a | 2.87 a |
| L5 | 30.9 b | 4.24 c | 0.20 a | 3.23 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, K.Y.; Shawon, M.R.A.; Kim, J.K.; Yoon, Y.J.; Park, S.J.; Na, J.K. Effect of White LED Light on the Growth of Apple Seedlings in Controlled Environment System. Horticulturae 2022, 8, 692. https://doi.org/10.3390/horticulturae8080692
Choi KY, Shawon MRA, Kim JK, Yoon YJ, Park SJ, Na JK. Effect of White LED Light on the Growth of Apple Seedlings in Controlled Environment System. Horticulturae. 2022; 8(8):692. https://doi.org/10.3390/horticulturae8080692
Chicago/Turabian StyleChoi, Ki Young, Md. Rayhan Ahmed Shawon, Jae Kyung Kim, Yeo Joong Yoon, Soo Jeong Park, and Jong Kuk Na. 2022. "Effect of White LED Light on the Growth of Apple Seedlings in Controlled Environment System" Horticulturae 8, no. 8: 692. https://doi.org/10.3390/horticulturae8080692
APA StyleChoi, K. Y., Shawon, M. R. A., Kim, J. K., Yoon, Y. J., Park, S. J., & Na, J. K. (2022). Effect of White LED Light on the Growth of Apple Seedlings in Controlled Environment System. Horticulturae, 8(8), 692. https://doi.org/10.3390/horticulturae8080692





